Low-Temperature Magnetothermodynamics Performance of Tb1-xErxNi2 Laves-Phases Compounds for Designing Composite Refrigerants
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Heat Capacity Measurements
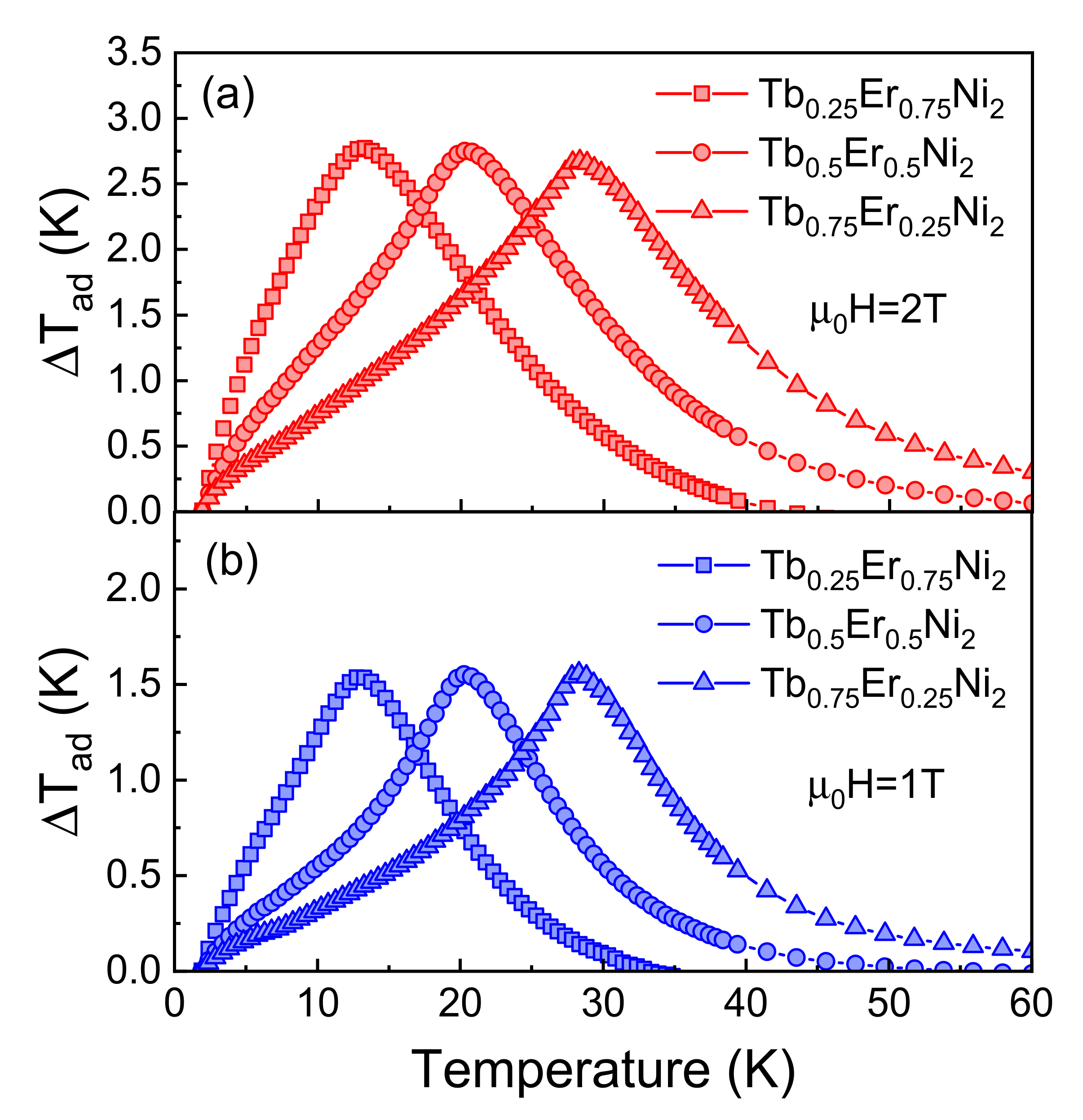
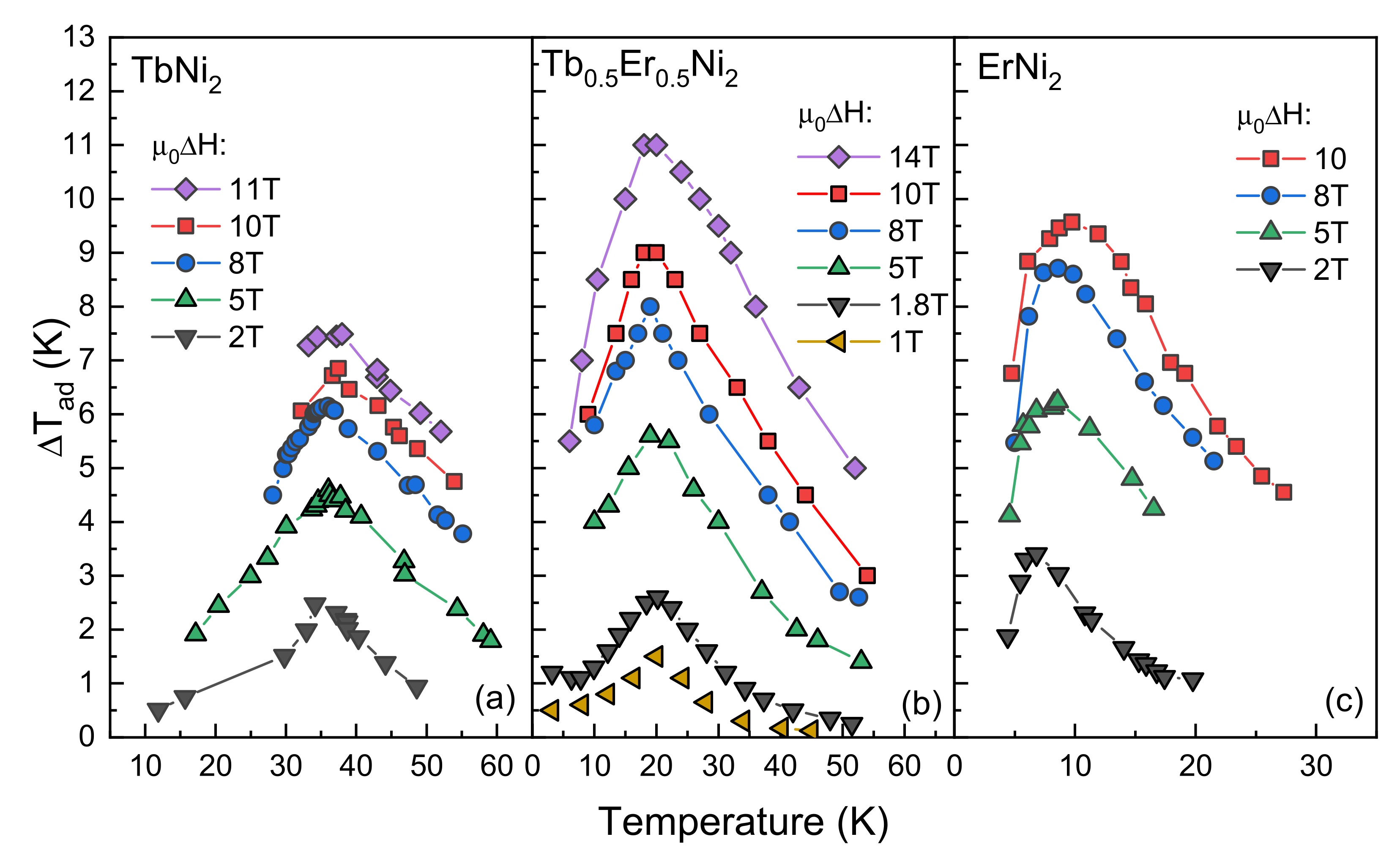
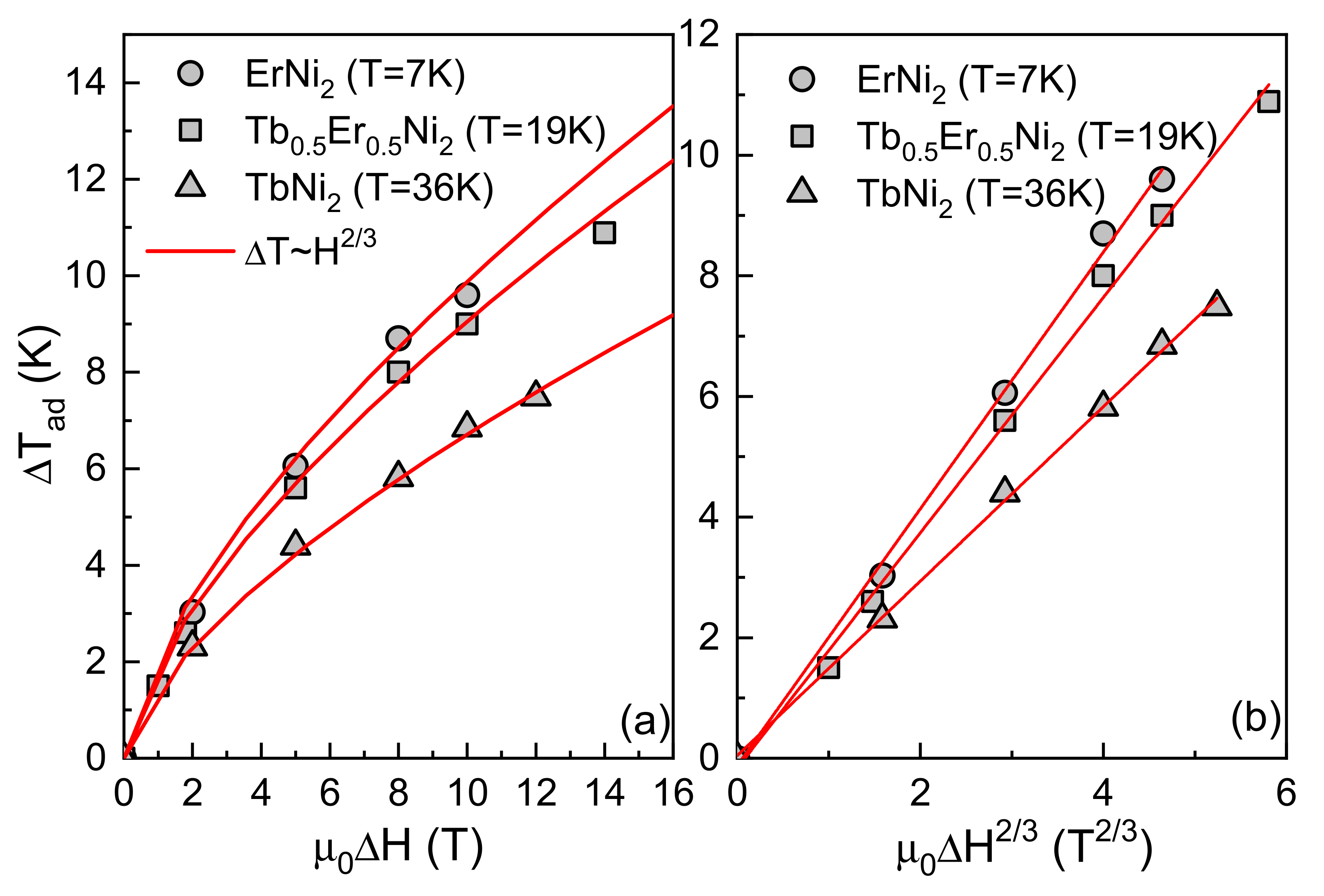
3.2. Direct Measurements of the Adiabatic Temperature Change
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tishin, A.M.; Spichkin, Y.I. The Magnetocaloric Effect and its Applications; IOP Publishing: Bristol, UK, 2003. [Google Scholar]
- De Oliveira, N.A.; von Ranke, P.J. Theoretical aspects of the magnetocaloric effect. Phys. Rep. 2010, 489, 89. [Google Scholar] [CrossRef]
- Kitanovski, A.; Tušek, J.; Tomc, U.; Plaznik, U.; Ožbolt, M.; Poredoš, A. Magnetocaloric Energy Conversion, from Theory to Applications; Springer International Publishing: Cham, Switzerland, 2015. [Google Scholar]
- Bazine, W.; Tahiri, N.; el Bounagui, O.; Ez-Zahraouy, H.; Benyoussef, A. Structural, electronic, magnetic, and magnetocaloric properties in intermetallic compound TbCu2Si2. J. Magn. Magn. Mater. 2019, 481, 72–76. [Google Scholar] [CrossRef]
- el Boukili, A.; Tahiri, N.; Salmani, E.; Ez-Zahraouy, H.; Hamedoun, M.; Benyoussef, A.; Balli, M.; Mounkachi, O. Magnetocaloric and cooling properties of the intermetallic compound AlFe2B2 in an AMR cycle system. Intermetallics 2019, 104, 84–89. [Google Scholar] [CrossRef]
- Chu, F.; Chen, Z.; Fuller, C.J.; Lin, C.L.; Mihalisin, T. Superconductivity and structural transformation in HfV2 and Nb-doped HfV2. J. Appl. Phys. 1996, 79, 6405. [Google Scholar] [CrossRef]
- Aoki, K.; Li, X.; Masumoto, T. Factors controlling hydrogen-induced amorphization of C15 Laves compounds. Acta Metall. Mater. 1992, 40, 1717–1726. [Google Scholar] [CrossRef]
- Chzhan, V.B.; Tereshina, I.S.; Karpenkov, A.Y.; Tereshina-Chitrova, E.A. Persistent values of magnetocaloric effect in the multicomponent Laves phase compounds with varied composition. Acta Mater. 2018, 154, 303–310. [Google Scholar] [CrossRef]
- Kirchmayr, H.R.; Burzo, E. Landolt–Börnstein, New Series III/19d2; Wijn, H.P.J., Ed.; Springer: Berlin, Germany, 1990. [Google Scholar]
- Buschow, K.H.J. Ferromagnetic Materials, 1; Wohlfarth, E.P., Ed.; North-Holland: Amsterdam, The Netherlands, 1980. [Google Scholar]
- Ćwik, J.; Koshkid’ko, Y.; de Oliveira, N.A.; Nenkov, K.; Hackemer, A.; Dilmieva, E.; Kolchugina, N.; Nikitin, S.; Rogacki, K. Magnetocaloric effect in Laves-phase rare-earth compounds with the second-order magnetic phase transition: Estimation of the high-field properties. Acta Mater. 2017, 133, 230–239. [Google Scholar] [CrossRef]
- Ćwik, J.; Koshkid’ko, Y.; Nenkov, K.; Tereshina, E.A.; Rogacki, K. Structural, magnetic and magnetocaloric properties of HoNi2 and ErNi2 compounds ordered at low temperatures. J. Alloys Compd. 2018, 735, 1088–1095. [Google Scholar] [CrossRef]
- Gignoux, D.; Givord, F. Quadrupolar interactions in ErNi2. J. Magn. Magn. Mater. 1983, 31–34, 217–218. [Google Scholar] [CrossRef]
- Ćwik, J.; Koshkid’ko, Y.; Nenkov, K.; Tereshina-Chitrova, E.; Kolchugina, N. Correlation between the structure and thermomagnetic properties of pseudo-binary (Tb,Er)Ni2 solid solutions. J. Alloys Compd. 2021, 859, 157870. [Google Scholar] [CrossRef]
- Koshkid’ko, Y.; Ćwik, J.; Ivanova, T.I.; Nikitin, S.A.; Miller, M.; Rogacki, K. Magnetocaloric properties of Gd in fields up to 14 T. J. Magn. Magn. Mater. 2017, 433, 234–238. [Google Scholar] [CrossRef]
- Ćwik, J.; Koshkid’ko, Y.; Nenkov, K.; Mikhailova, A.; Małecka, M.; Romanova, T.; Kolchugina, N.; de Oliveira, N.A. Experimental and theoretical analysis of magnetocaloric behavior of Dy1−xErxNi2 intermetallics (x = 0.25, 0.5, 0.75) and their composites for low-temperature refrigerators performing an Ericsson cycle. Phys. Rev. B 2021, 103, 214429. [Google Scholar] [CrossRef]
- Ćwik, J.; Koshkid’ko, Y.; Nenkov, K.; Tereshina-Chitrova, E.; Małecka, M.; Weise, B.; Kowalska, K. Magnetocaloric performance of the three-component Ho1-xErxNi2 (x = 0.25, 0.5, 0.75) Laves phases as composite refrigerants. Available online: https://assets.researchsquare.com/files/rs-1561990/v1/55c57056-a071-4ce3-bbe2-843a20e0a7f2.pdf?c=1650484221 (accessed on 7 June 2022).
- Ćwik, J.; Palewski, T.; Nenkov, K. Specific heat of the Tb1-xLaxNi2 solid solutions. Acta Phys. Pol. A 2008, 113, 339. [Google Scholar]
- Gschneidner, K.A.; Pecharsky, V.K.; Gailloux, M.J.; Takeya, H. Utilization of the magnetic entropy in active magnetic regenerator materials. In Advances in Cryogenic Engineering Materials; Summers, L.T., Ed.; Springer: Boston, MA, USA, 1996; Volume 42. [Google Scholar]
- Dörr, M.; Loewenhaupt, M.; Hahn, W.; Brück, E.; Hagmusa, I.H.; Klaasse, J.C.P.; Rotter, M. Specific heat of DyCu2 single crystals in high magnetic fields. Physica B 1999, 262, 340–347. [Google Scholar] [CrossRef]
- Tristan, N.V.; Palewski, T.; Nenkov, K.; Skokov, K.P.; Nikitin, S.A. Magnetic properties and specific heat of the Dy3Ni intermetallic compound. J. Phys. Condens. Matter 2003, 15, 5997–6004. [Google Scholar] [CrossRef]
- Hashimoto, T.; Kuzuhara, T.; Sahashi, M.; Inomata, K.; Tomokiyo, A.; Yayama, H. New application of complex magnetic materials to the magnetic refrigerant in an Ericsson magnetic refrigerator. J. Appl. Phys. 1987, 62, 3873. [Google Scholar] [CrossRef]
- Smaïli, A.; Chahine, R. Composite Magnetic Refrigerants for an Ericsson Cycle: New Method of Selection Using a Numerical Approach. In Advances in Cryogenic Engineering Materials; Springer: Boston, MA, USA, 1996; Volume 42, pp. 445–450. [Google Scholar]
- Ćwik, J.; Koshkid’ko, Y.; Małecka, M.; Weise, B.; Krautz, M.; Mikhailova, A.; Kolchugina, N. Magnetocaloric prospects of mutual substitutions of rare-earth elements in pseudobinary Tb1−xHoxNi2 compositions (x = 0.25–0.75). J. Alloys Compd. 2021, 886, 161295. [Google Scholar] [CrossRef]
- Samanta, T.; Das, I.; Banerjee, S. Magnetocaloric effect in Ho5Pd2: Evidence of large cooling power. Appl. Phys. Lett. 2007, 91, 152506. [Google Scholar] [CrossRef]
- Li, L.; Nishimura, K.; Huo, D.; Kadonaga, M.; Namiki, T.; Qian, Z. Large Magnetic Entropy Change in Dy1-xHoxNi2B2C (x = 0–1) Superconductors. Appl. Phys. Exp. 2011, 4, 093101. [Google Scholar] [CrossRef]
- Belov, K.P. Magnetic Transitions; Bureau, C., Ed.; Consultants Bureau: New York, NY, USA, 1961. [Google Scholar]
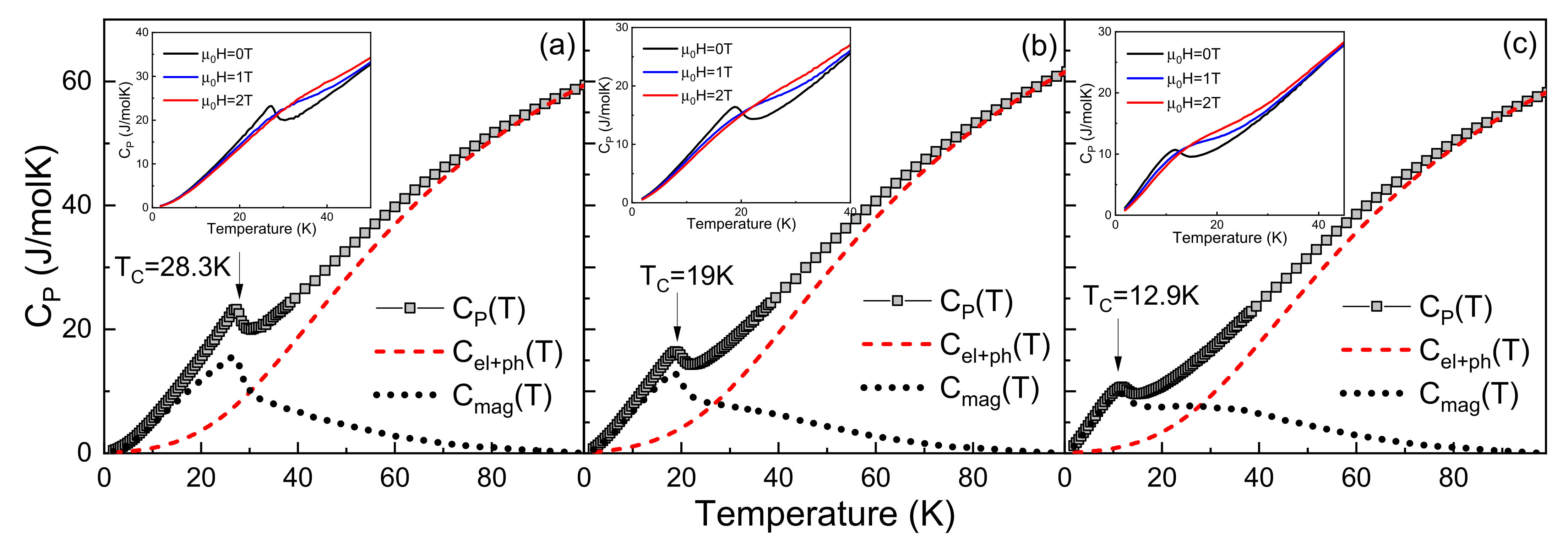
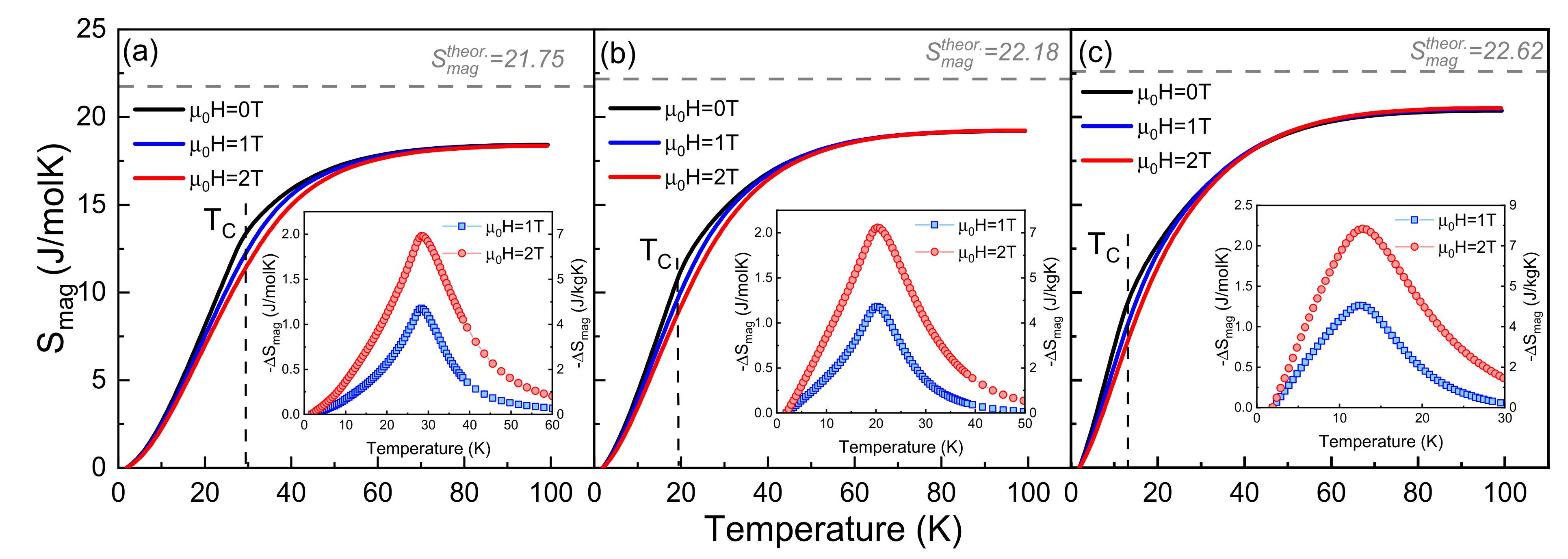

| Compound | TC, [K] | ƟD, [K] | γ, [mJ/molK2] | Smag, [J/molK] | ||
|---|---|---|---|---|---|---|
| Theor., Rln(2J + 1) | T = TC | T = 100 K | ||||
| TbNi2 | 37.1 [11] | 261 [18] | 17.5 [18] | - | - | - |
| Tb0.75Er0.25Ni2 | 28.3 | 262 | 54.5 | 21.75 | 12.9 | 18.4 |
| Tb0.5Er0.5Ni2 | 19.0 | 264 | 52.3 | 22.18 | 10.9 | 19.2 |
| Tb0.25Er0.75Ni2 | 12.9 | 268 | 50.1 | 22.62 | 9.6 | 20.5 |
| ErNi2 | 6.5 [12] | - | - | - | - | - |
| Compound | −ΔSmag (J/kgK) | ΔTad (K) | ||
|---|---|---|---|---|
| 0–1 T | 0–2 T | 0–1 T | 0–2 T | |
| TbNi2 [11] | 3.4 | 6.5 | 1.4 | 2.4 |
| Tb0.75Er0.25Ni2 | 4.2 | 7.1 | 1.6 | 2.6 |
| Tb0.5Er0.5Ni2 | 4.3 | 7.3 | 1.6 | 2.7 |
| Tb0.25Er0.75Ni2 | 4.6 | 7.8 | 1.6 | 2.8 |
| ErNi2 [12] | 8.6 | 13.0 | 2.2 | 3.8 |
| Composite 1 | 2.4 | - | - | - |
| Composite 2 | - | 4.7 | - | - |
| Compound | TC (K) | ΔTad (K) | A (K/T2/3) | |||||
|---|---|---|---|---|---|---|---|---|
| 0–1 T | 0–2 T | 0–5 T | 0–8 T | 0–10 T | 0–14 T | |||
| TbNi2 [11] | 37.1 | 1.5 ‡ | 2.4 | 4.6 | 6.2 | 6.9 | 8.4 ‡ | 1.45 |
| Tb0.5Er0.5Ni2 | 19.0 | 1.5 | 2.6 0–1.8 T | 5.6 | 8.0 | 9.0 | 11.0 | 1.95 |
| ErNi2 [12] | 6.2 | 2.1 ‡ | 3.5 | 6.2 | 8.8 | 9.8 | 12.2 ‡ | 2.13 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ćwik, J.; Koshkid’ko, Y.; Nenkov, K.; Tereshina-Chitrova, E.; Weise, B.; Kowalska, K. Low-Temperature Magnetothermodynamics Performance of Tb1-xErxNi2 Laves-Phases Compounds for Designing Composite Refrigerants. Crystals 2022, 12, 931. https://doi.org/10.3390/cryst12070931
Ćwik J, Koshkid’ko Y, Nenkov K, Tereshina-Chitrova E, Weise B, Kowalska K. Low-Temperature Magnetothermodynamics Performance of Tb1-xErxNi2 Laves-Phases Compounds for Designing Composite Refrigerants. Crystals. 2022; 12(7):931. https://doi.org/10.3390/cryst12070931
Chicago/Turabian StyleĆwik, Jacek, Yurii Koshkid’ko, Konstantin Nenkov, Evgenia Tereshina-Chitrova, Bruno Weise, and Karolina Kowalska. 2022. "Low-Temperature Magnetothermodynamics Performance of Tb1-xErxNi2 Laves-Phases Compounds for Designing Composite Refrigerants" Crystals 12, no. 7: 931. https://doi.org/10.3390/cryst12070931







