Ultrasound-Assisted Hydrothermal Synthesis of SrSnO3/g-C3N4 Heterojunction with Enhanced Photocatalytic Performance for Ciprofloxacin under Visible Light
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of g-C3N4 Photocatalyst
2.3. Preparation of SrSnO3 Photocatalyst
2.4. Preparation of SrSnO3/g-C3N4 Heterojunction Photocatalysts
2.5. Characterization
2.6. Photocatalytic Activity and Stability Evaluation
3. Results and Discussion
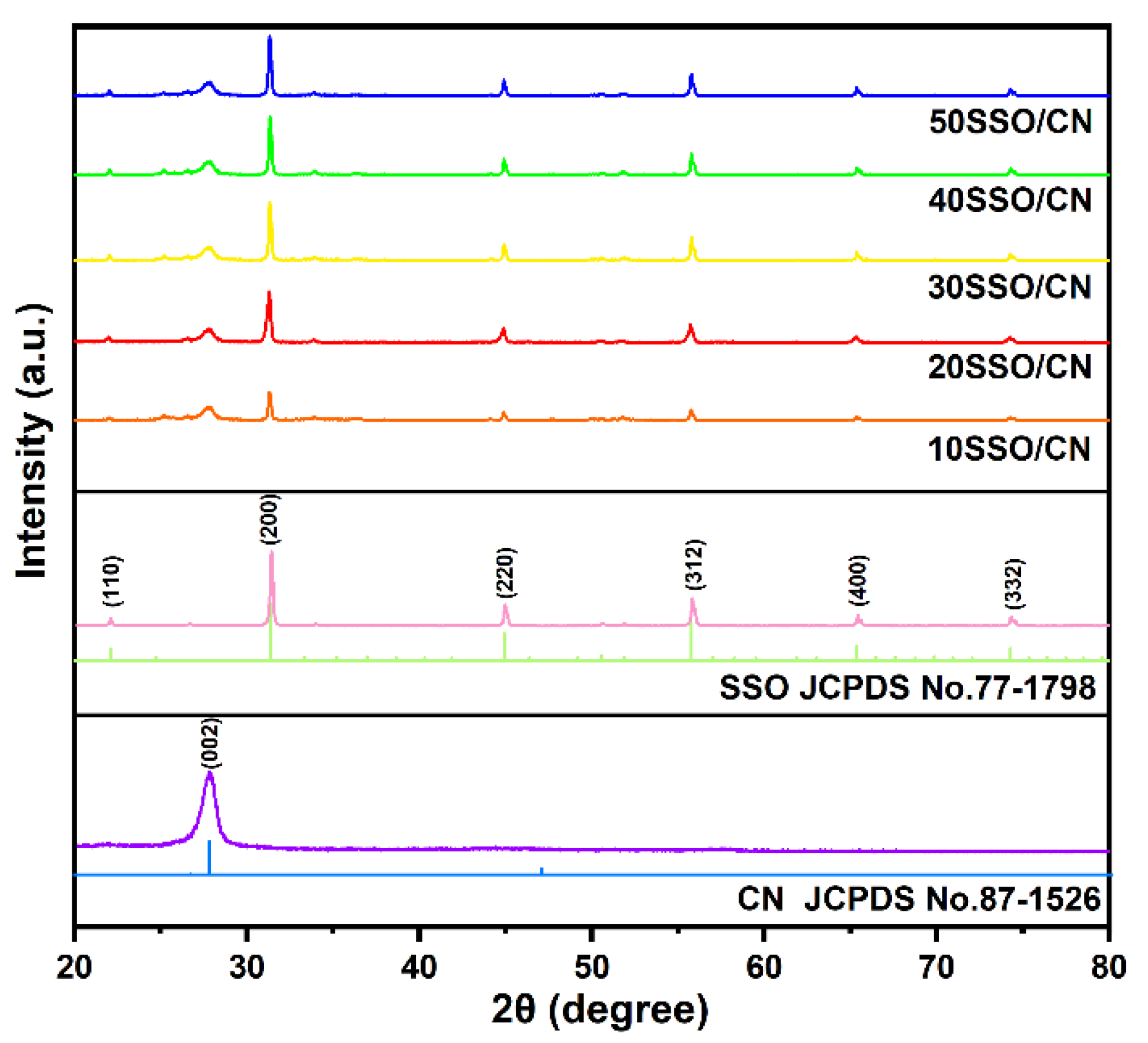
3.1. XRD Analysis
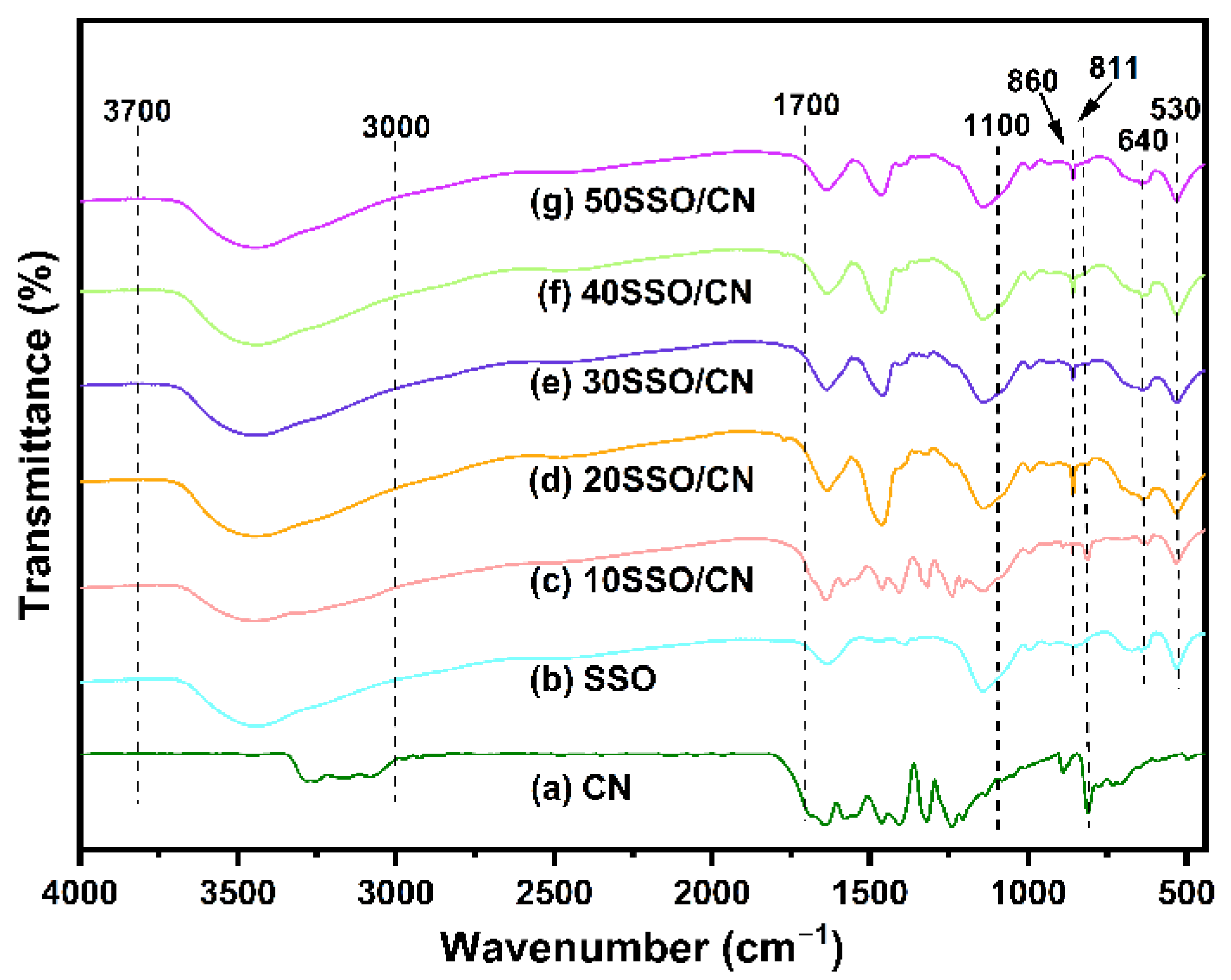
3.2. FTIR Analysis
3.3. XPS Analysis
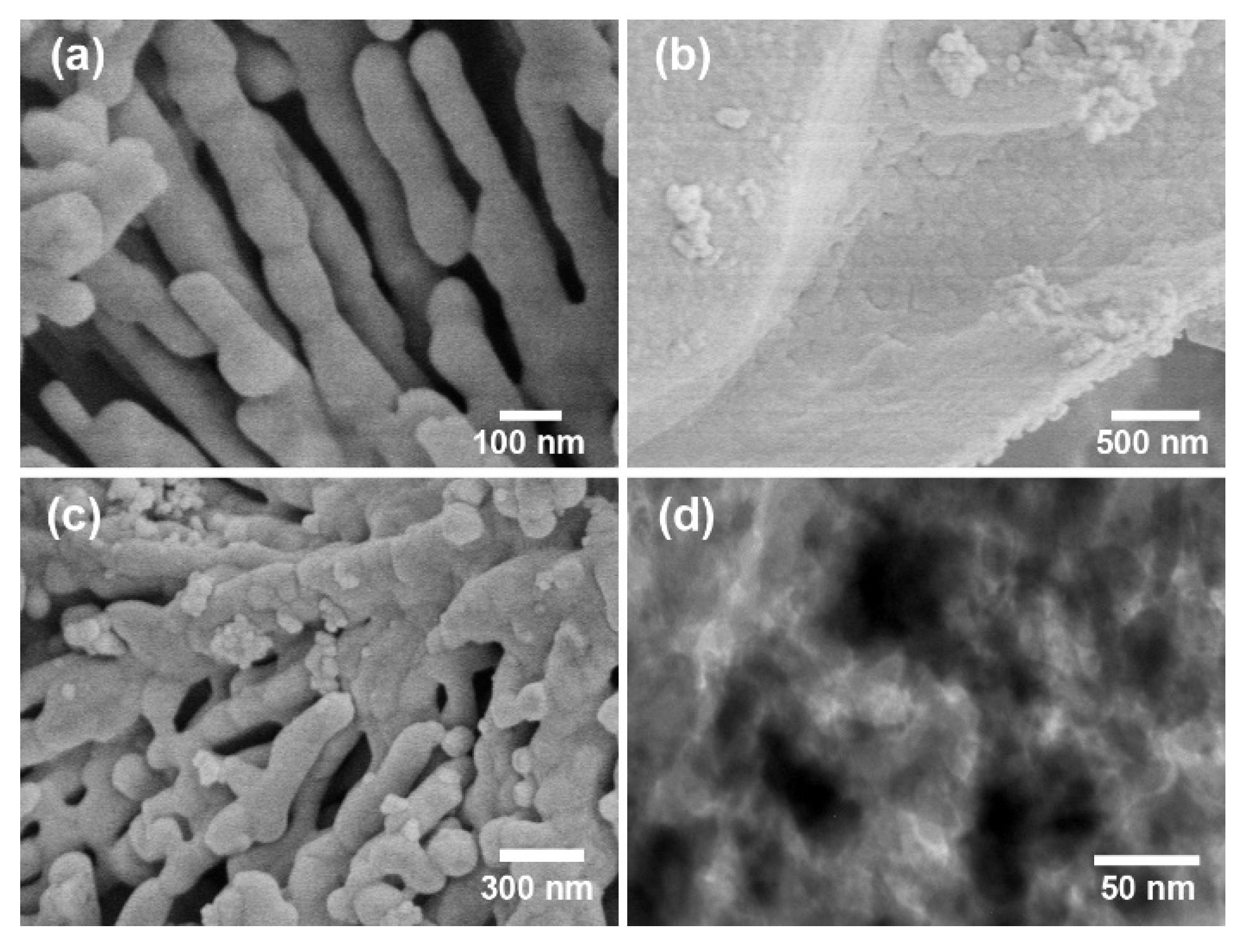
3.4. SEM and TEM Analysis
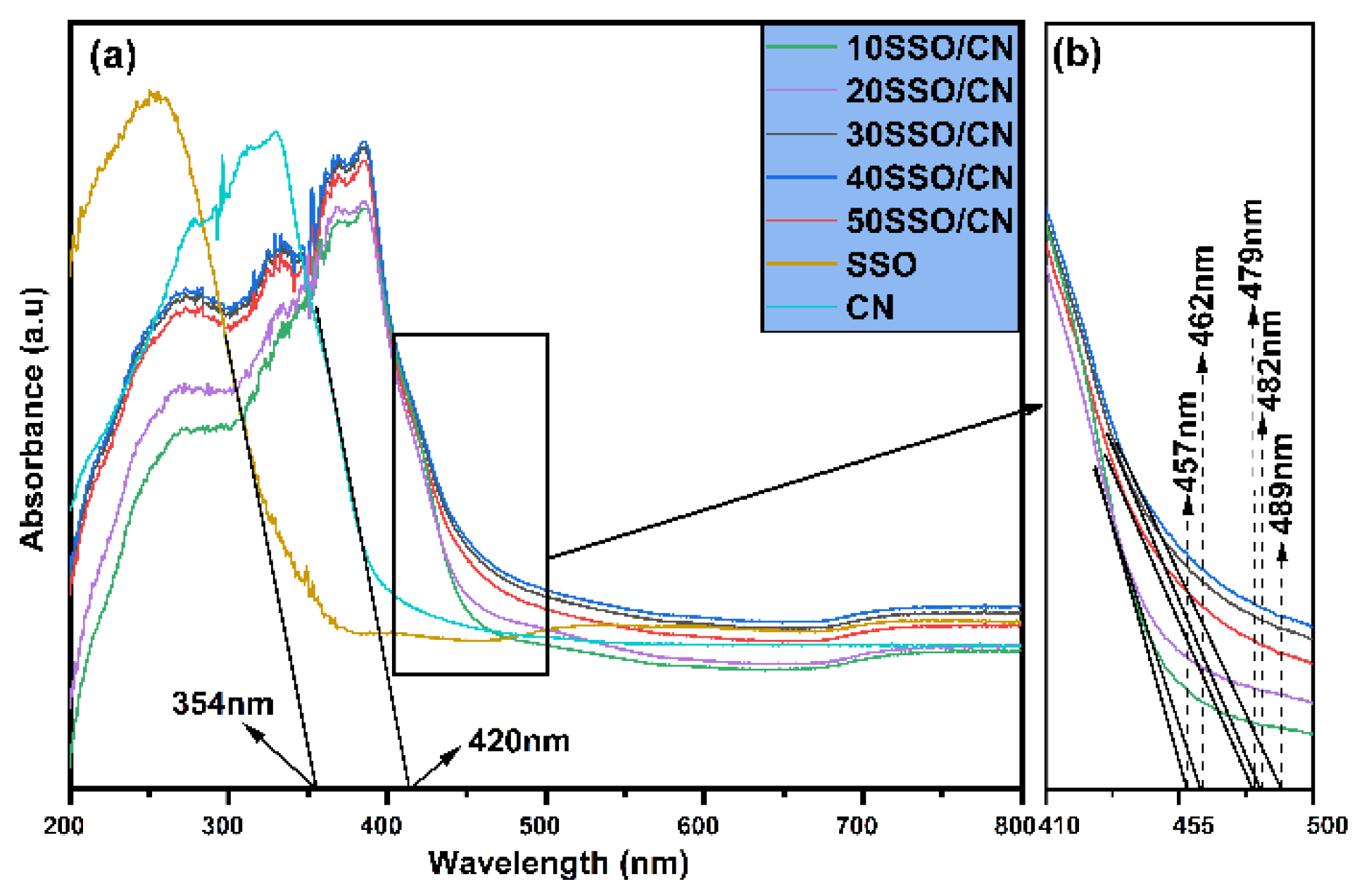
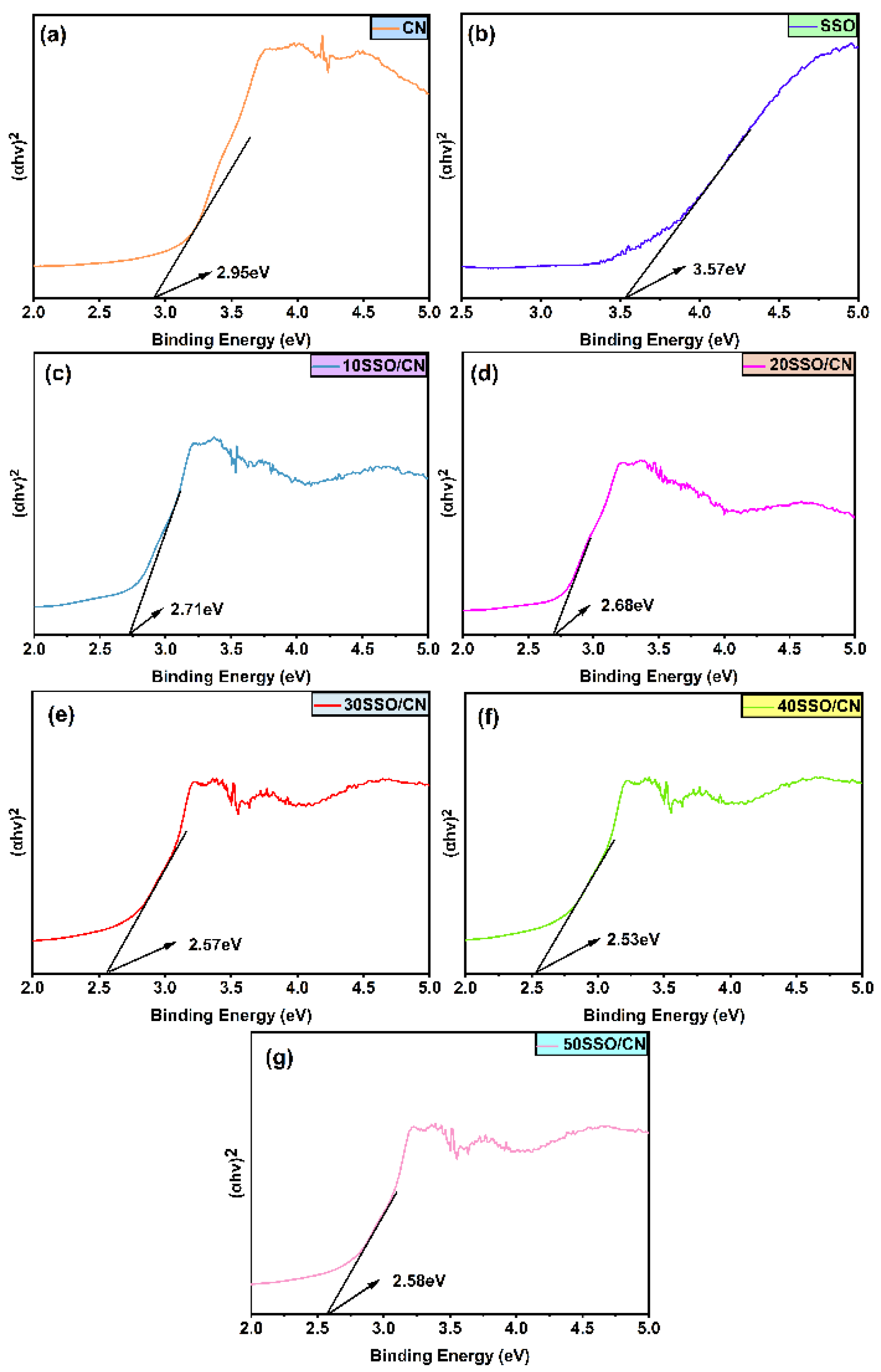
3.5. UV-Vis DRS Analysis

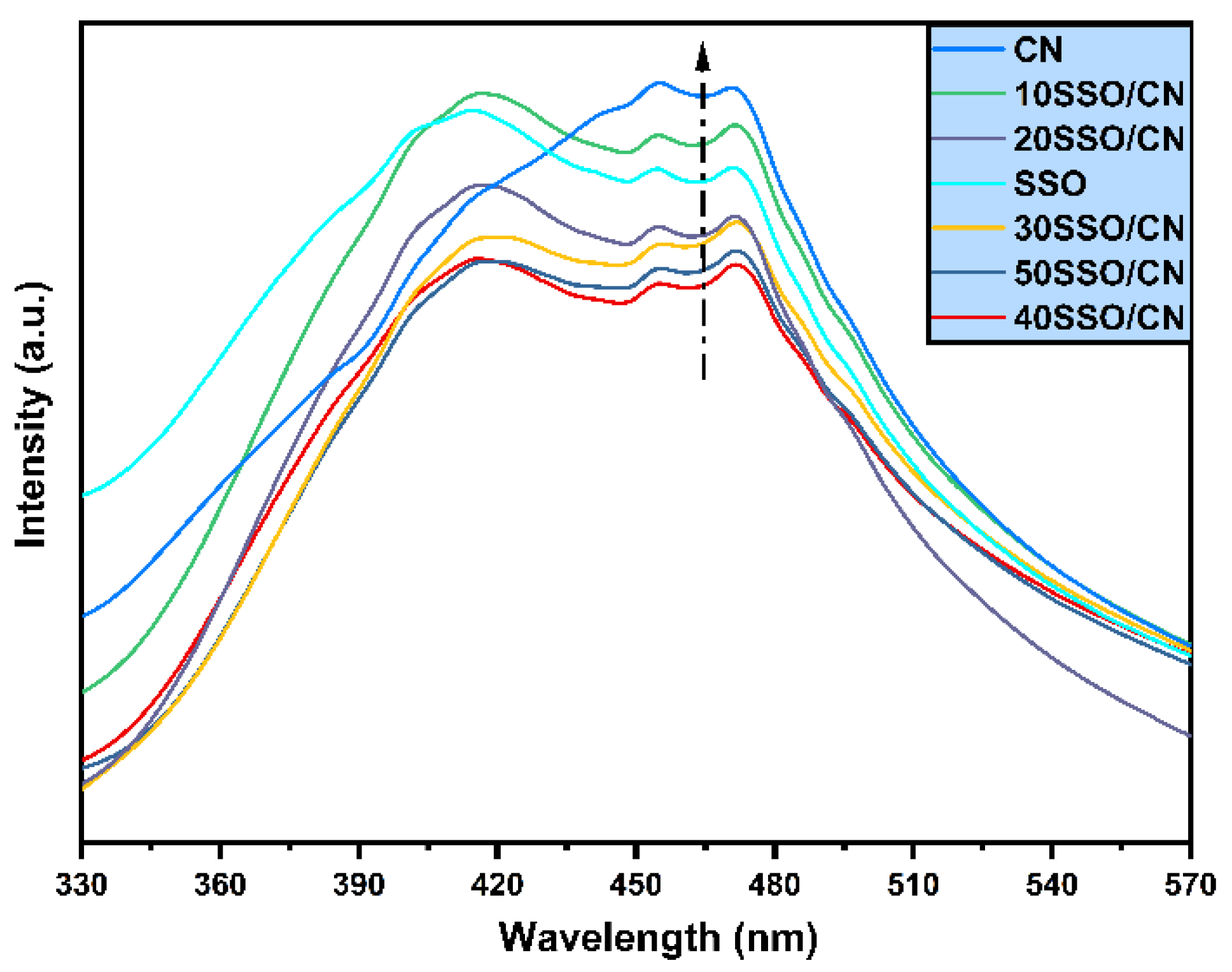
3.6. PL Analysis
3.7. Photocatalyst Activity
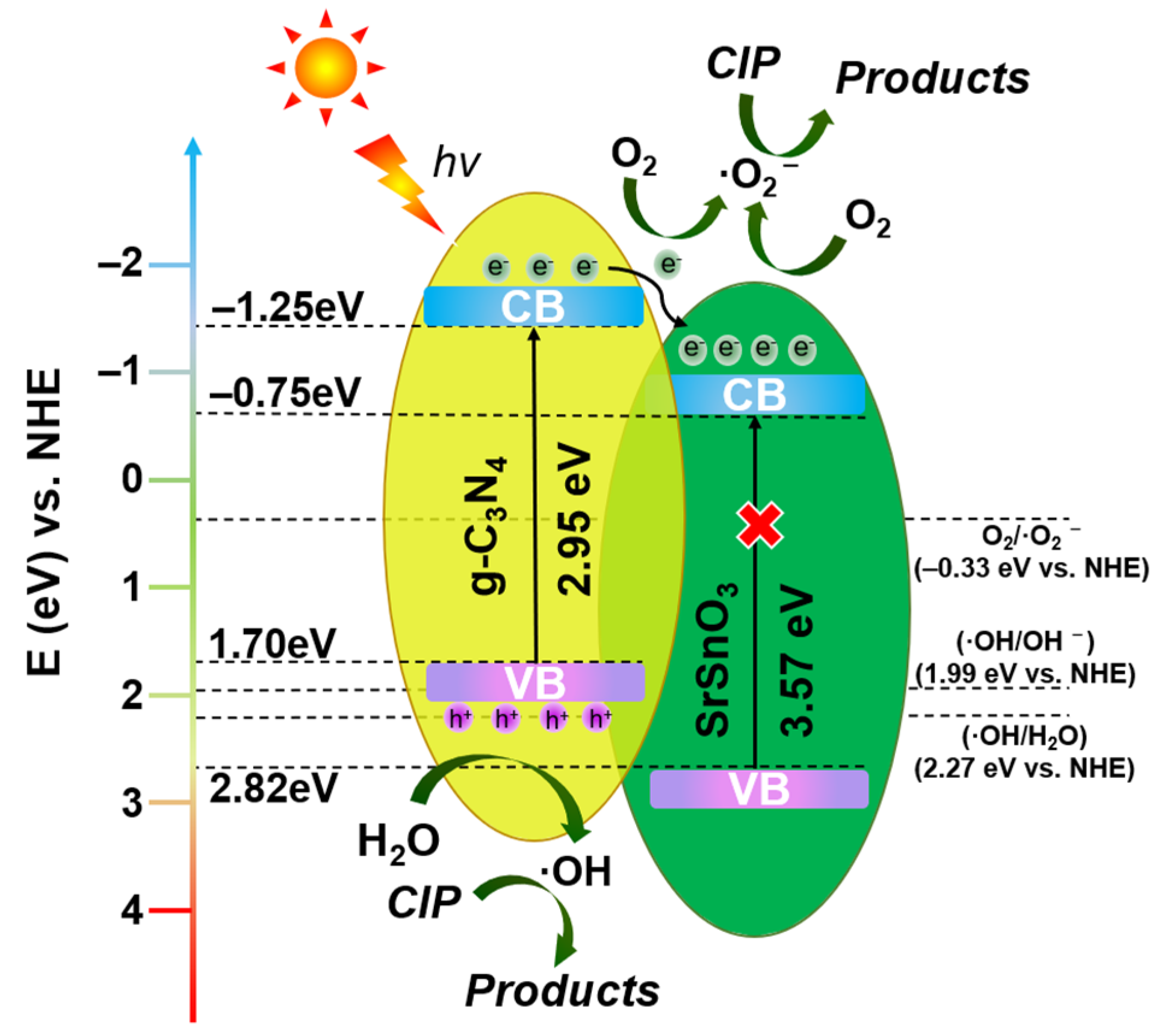
3.8. Photocatalytic Reaction Mechanism
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chen, B.; Zhou, L.; Tian, Y.; Yu, J.; Lei, J.; Wang, L.; Liu, Y.; Zhang, J. Z-Scheme Inverse Opal CN/BiOBr Photocatalysts for Highly Efficient Degradation of Antibiotics. Phys. Chem. Chem. Phys. 2019, 21, 12818–12825. [Google Scholar] [CrossRef]
- Xue, J.; Ma, S.; Zhou, Y.; Zhang, Z.; He, M. Facile Photochemical Synthesis of Au/Pt/g-C3N4 with Plasmon-Enhanced Photocatalytic Activity for Antibiotic Degradation. ACS Appl. Mater. Interfaces 2015, 7, 9630–9637. [Google Scholar] [CrossRef] [PubMed]
- Sun, R.; Chen, J.; Pan, C.; Sun, Y.; Mai, B.; Li, Q.X. Antibiotics and Food Safety in Aquaculture. J. Agric. Food Chem. 2020, 68, 11908–11919. [Google Scholar] [CrossRef]
- Sabri, N.A.; van Holst, S.; Schmitt, H.; van der Zaan, B.M.; Gerritsen, H.W.; Rijnaarts, H.H.M.; Langenhoff, A.A.M. Fate of Antibiotics and Antibiotic Resistance Genes during Conventional and Additional Treatment Technologies in Wastewater Treatment Plants. Sci. Total Environ. 2020, 741, 140199. [Google Scholar] [CrossRef]
- Baaloudj, O.; Assadi, A.A.; Azizi, M.; Kenfoud, H.; Trari, M.; Amrane, A.; Assadi, A.A.; Nasrallah, N. Synthesis and Characterization of ZnBi2O4 Nanoparticles: Photocatalytic Performance for Antibiotic Removal under Different Light Sources. Appl. Sci. 2021, 11, 3975. [Google Scholar] [CrossRef]
- Wen, J.; Xie, J.; Chen, X.; Li, X. A Review on g-C3N4 -Based Photocatalysts. Appl. Surf. Sci. 2017, 391, 72–123. [Google Scholar] [CrossRef]
- Dong, S.; Cui, L.; Zhang, W.; Xia, L.; Zhou, S.; Russell, C.K.; Fan, M.; Feng, J.; Sun, J. Double-Shelled ZnSnO3 Hollow Cubes for Efficient Photocatalytic Degradation of Antibiotic Wastewater. Chem. Eng. J. 2020, 384, 123279. [Google Scholar] [CrossRef]
- Safaralizadeh, E.; Mahjoub, A.R.; Fazlali, F.; Bagheri, H. Facile Construction of C3N4-TE@TiO2/UiO-66 with Double Z-Scheme Structure as High Performance Photocatalyst for Degradation of Tetracycline. Ceram. Int. 2021, 47, 2374–2387. [Google Scholar] [CrossRef]
- Kurniawan, T.A.; Mengting, Z.; Fu, D.; Yeap, S.K.; Othman, M.H.D.; Avtar, R.; Ouyang, T. Functionalizing TiO2 with Graphene Oxide for Enhancing Photocatalytic Degradation of Methylene Blue (MB) in Contaminated Wastewater. J. Environ. Manag. 2020, 270, 110871. [Google Scholar] [CrossRef] [PubMed]
- Yulizar, Y.; Apriandanu, D.O.B.; Ashna, R.I. La2CuO4-Decorated ZnO Nanoparticles with Improved Photocatalytic Activity for Malachite Green Degradation. Chem. Phys. Lett. 2020, 755, 137749. [Google Scholar] [CrossRef]
- Wang, D.; Xu, Y.; Sun, F.; Zhang, Q.; Wang, P.; Wang, X. Enhanced Photocatalytic Activity of TiO2 under Sunlight by MoS2 Nanodots Modification. Appl. Surf. Sci. 2016, 377, 221–227. [Google Scholar] [CrossRef]
- Askari, N.; Beheshti, M.; Mowla, D.; Farhadian, M. Facile Construction of Novel Z-Scheme MnWO4/Bi2S3 Heterojunction with Enhanced Photocatalytic Degradation of Antibiotics. Mater. Sci. Semicond. Process. 2021, 127, 105723. [Google Scholar] [CrossRef]
- Liang, Q.; Jin, J.; Liu, C.; Xu, S.; Li, Z. Constructing a Novel P-n Heterojunction Photocatalyst LaFeO3/g-C3N4 with Enhanced Visible-Light-Driven Photocatalytic Activity. J. Alloy. Compd. 2017, 709, 542–548. [Google Scholar] [CrossRef]
- Wang, Y.; Sun, X.; Xian, T.; Liu, G.; Yang, H. Photocatalytic Purification of Simulated Dye Wastewater in Different PH Environments by Using BaTiO3/Bi2WO6 Heterojunction Photocatalysts. Opt. Mater. 2021, 113, 110853. [Google Scholar] [CrossRef]
- Lu, G.; Zou, X.; Wang, F.; Wang, H.; Li, W. Facile Fabrication of CeVO4 Microspheres with Efficient Visible-Light Photocatalytic Activity. Mater. Lett. 2017, 195, 168–171. [Google Scholar] [CrossRef]
- Maeda, K.; Wang, X.; Nishihara, Y.; Lu, D.; Antonietti, M.; Domen, K. Photocatalytic Activities of Graphitic Carbon Nitride Powder for Water Reduction and Oxidation under Visible Light. J. Phys. Chem. C 2009, 113, 4940–4947. [Google Scholar] [CrossRef]
- Liu, X.; Ma, R.; Zhuang, L.; Hu, B.; Chen, J.; Liu, X.; Wang, X. Recent Developments of Doped g-C3N4 Photocatalysts for the Degradation of Organic Pollutants. Crit. Rev. Environ. Sci. Technol. 2021, 51, 751–790. [Google Scholar] [CrossRef]
- Minh Tri, N.L.; Kim, J.; Giang, B.L.; al Tahtamouni, T.M.; Huong, P.T.; Lee, C.; Viet, N.M.; Quang Trung, D. Ag-Doped Graphitic Carbon Nitride Photocatalyst with Remarkably Enhanced Photocatalytic Activity towards Antibiotic in Hospital Wastewater under Solar Light. J. Ind. Eng. Chem. 2019, 80, 597–605. [Google Scholar] [CrossRef]
- Zhang, S.; Gu, P.; Ma, R.; Luo, C.; Wen, T.; Zhao, G.; Cheng, W.; Wang, X. Recent Developments in Fabrication and Structure Regulation of Visible-Light-Driven g-C3N4-Based Photocatalysts towards Water Purification: A Critical Review. Catal. Today 2019, 335, 65–77. [Google Scholar] [CrossRef]
- Renukadevi, S.; Jeyakumari, A.P. Microwave Induced Inverse Spinel NiFe2O4 Decorated g-C3N4 Nanosheet for Enhanced Visible Light Photocatalytic Activity. J. Clust. Sci. 2021. [Google Scholar] [CrossRef]
- Ghorai, K.; Panda, A.; Hossain, A.; Bhattacharjee, M.; Chakraborty, M.; Bhattacharya, S.K.; Show, B.; Sarkar, A.; Bera, P.; Kim, H.; et al. LaNiO3/g-C3N4 Nanocomposite: An Efficient Z-Scheme Photocatalyst for Wastewater Treatment Using Direct Sunlight. J. Rare Earths 2022, 40, 725–736. [Google Scholar] [CrossRef]
- Zhang, B.; Hu, X.; Liu, E.; Fan, J. Novel S-Scheme 2D/2D BiOBr/g-C3N4 Heterojunctions with Enhanced Photocatalytic Activity. Chin. J. Catal. 2021, 42, 1519–1529. [Google Scholar] [CrossRef]
- Firooz, A.A.; Mahjoub, A.R.; Khodadadi, A.A.; Movahedi, M. High Photocatalytic Activity of Zn2SnO4 among Various Nanostructures of Zn2xSn1-XO2 Prepared by a Hydrothermal Method. Chem. Eng. J. 2010, 165, 735–739. [Google Scholar] [CrossRef]
- Huang, S.; Zhang, J.; Qin, Y.; Song, F.; Du, C.; Su, Y. Direct Z-Scheme SnO2/Bi2Sn2O7 Photocatalyst for Antibiotics Removal: Insight on the Enhanced Photocatalytic Performance and Promoted Charge Separation Mechanism. J. Photochem. Photobiol. A Chem. 2021, 404, 112947. [Google Scholar] [CrossRef]
- Gnanamoorthy, G.; Yadav, V.K.; Latha, D.; Karthikeyan, V.; Narayanan, V. Enhanced Photocatalytic Performance of ZnSnO3/rGO Nanocomposite. Chem. Phys. Lett. 2020, 739, 137050. [Google Scholar] [CrossRef]
- Jia, T.; Liu, M.; Yu, D.; Long, F.; Mo, S.; Deng, Z.; Wang, W. A Facile Approach for the Synthesis of Zn2SnO4/BiOBr Hybrid Nanocomposites with Improved Visible-Light Photocatalytic Performance. Nanomaterials 2018, 8, 313. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Xu, C.; Wan, F.; Zhou, D.; Yang, L.; Gu, H.; Xiong, J. Synthesis of Flower-like Bi2Sn2O7/Bi2WO6 Hierarchical Composites with Enhanced Visible Light Photocatalytic Performance. J. Alloy. Compd. 2019, 788, 1154–1161. [Google Scholar] [CrossRef]
- Moshtaghi, S.; Gholamrezaei, S.; Salavati Niasari, M.; Mehdizadeh, P. New Controllable Procedure for Preparation of SrSnO3 Nanostructures: Photo-Degradation of Azo Dyes and Photovoltaic Measurement. J. Mater. Sci. Mater. Electron. 2016, 27, 414–424. [Google Scholar] [CrossRef]
- de Sousa Filho, I.A.; Weber, I.T. SrSnO3/g-C3N4 Dry Phase Sunlight Photocatalysis. J. Photochem. Photobiol. A Chem. 2021, 412, 113255. [Google Scholar] [CrossRef]
- De Sousa Filho, I.A.; Arana, L.R.; Doungmo, G.; Grisólia, C.K.; Terrashke, H.; Weber, I.T. SrSnO3/g-C3N4 and Sunlight: Photocatalytic Activity and Toxicity of Degradation Byproducts. J. Environ. Chem. Eng. 2020, 8, 103633. [Google Scholar] [CrossRef]
- De Sousa Filho, I.A.; Freire, D.O.; Weber, I.T. Organic Load Removal and Microbial Disinfection of Raw Domestic Sewage Using SrSnO3/g-C3N4 with Sunlight. Environ. Sci. Pollut. Res. 2021, 28, 45009–45018. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Xia, H.; Wu, R.; Cao, Y.; Li, H. Fabrication of La2O3/g-C3N4 Heterojunction with Enhanced Photocatalytic Performance of Tetracycline Hydrochloride. Crystals 2021, 11, 1349. [Google Scholar] [CrossRef]
- Wang, H.; Yuan, X.; Wang, H.; Chen, X.; Wu, Z.; Jiang, L.; Xiong, W.; Zeng, G. Facile Synthesis of Sb2S3/Ultrathin g-C3N4 Sheets Heterostructures Embedded with g-C3N4 Quantum Dots with Enhanced NIR-Light Photocatalytic Performance. Appl. Catal. B Environ. 2016, 193, 36–46. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, A.; Cao, Y.; Xie, J.; Jia, W.; Jia, D. Interstitial N-Doped SrSnO3 Perovskite: Structural Design, Modification and Photocatalytic Degradation of Dyes. New J. Chem. 2019, 43, 10965–10972. [Google Scholar] [CrossRef]
- Li, C.; Zhu, Y.; Fang, S.; Wang, H.; Gui, Y.; Bi, L.; Chen, R. Preparation and Characterization of SrSnO3 Nanorods. J. Phys. Chem. Solids 2011, 72, 869–874. [Google Scholar] [CrossRef]
- Heidari, S.; Haghighi, M.; Shabani, M. Ultrasound Assisted Dispersion of Bi2Sn2O7-C3N4 Nanophotocatalyst over Various Amount of Zeolite Y for Enhanced Solar-Light Photocatalytic Degradation of Tetracycline in Aqueous Solution. Ultrason. Sonochem. 2018, 43, 61–72. [Google Scholar] [CrossRef] [PubMed]
- Lai, Y.; Meng, M.; Yu, Y.; Wang, X.; Ding, T. Photoluminescence and Photocatalysis of the Flower-like Nano-ZnO Photocatalysts Prepared by a Facile Hydrothermal Method with or without Ultrasonic Assistance. Appl. Catal. B Environ. 2011, 105, 335–345. [Google Scholar] [CrossRef]
- Long, Z.; Xian, G.; Zhang, G.; Zhang, T.; Li, X. BiOCl-Bi12O17Cl2 Nanocomposite with High Visible-Light Photocatalytic Activity Prepared by an Ultrasonic Hydrothermal Method for Removing Dye and Pharmaceutical. Chin. J. Catal. 2020, 41, 464–473. [Google Scholar] [CrossRef]
- Jia, J.; Wang, Y.; Xu, M.; Qi, M.L.; Wu, Y.; Zhao, G. MOF-Derived the Direct Z-Scheme g-C3N4/TiO2 with Enhanced Visible Photocatalytic Activity. J. Sol-Gel Sci. Technol. 2020, 93, 123–130. [Google Scholar] [CrossRef]
- Kappadan, S.; Thomas, S.; Kalarikkal, N. Enhanced Photocatalytic Performance of BaTiO3/g-C3N4 Heterojunction for the Degradation of Organic Pollutants. Chem. Phys. Lett. 2021, 771, 138513. [Google Scholar] [CrossRef]
- Tian, X.; Zhou, F.; Liu, X.; Zhong, H.; Wen, J.; Lian, S.; Ji, C.; Huang, Z.; Chen, Z.; Peng, H.; et al. Enhanced Photoluminescence and High Temperature Sensitivity from a Novel Pr3+ Doped SrSnO3/SnO2 Composite Phosphor. J. Solid State Chem. 2019, 280, 120997. [Google Scholar] [CrossRef]
- Saravanakumar, K.; Park, C.M. Rational Design of a Novel LaFeO3/g-C3N4/BiFeO3 Double Z-Scheme Structure: Photocatalytic Performance for Antibiotic Degradation and Mechanistic Insight. Chem. Eng. J. 2021, 423, 130076. [Google Scholar] [CrossRef]
- Xu, Y.; He, X.; Zhong, H.; Singh, D.J.; Zhang, L.; Wang, R. Solid Salt Confinement Effect: An Effective Strategy to Fabricate High Crystalline Polymer Carbon Nitride for Enhanced Photocatalytic Hydrogen Evolution. Appl. Catal. B Environ. 2019, 246, 349–355. [Google Scholar] [CrossRef]
- Saravanakumar, K.; Maheskumar, V.; Yea, Y.; Yoon, Y.; Muthuraj, V.; Park, C.M. 2D/2D Nitrogen-Rich Graphitic Carbon Nitride Coupled Bi2WO6 S-Scheme Heterojunction for Boosting Photodegradation of Tetracycline: Influencing Factors, Intermediates, and Insights into the Mechanism. Compos. Part B Eng. 2022, 234, 109726. [Google Scholar] [CrossRef]
- Faisal, M.; Rashed, M.A.; Ahmed, J.; Alsaiari, M.; Jalalah, M.; Alsareii, S.A.; Harraz, F.A. Ag Nanoparticle-Decorated Chitosan/SrSnO3 Nanocomposite for Ultrafast Elimination of Antibiotic Linezolid and Methylene Blue. Environ. Sci. Pollut. Res. 2022. [Google Scholar] [CrossRef] [PubMed]
- Venkatesh, G.; Geerthana, M.; Prabhu, S.; Ramesh, R.; Prabu, K.M. Enhanced Photocatalytic Activity of Reduced Graphene Oxide/SrSnO3 Nanocomposite for Aqueous Organic Pollutant Degradation. Optik 2020, 206, 164055. [Google Scholar] [CrossRef]
- Junploy, P.; Thongtem, S.; Thongtem, T. Photoabsorption and Photocatalysis of SrSnO3 Produced by a Cyclic Microwave Radiation. Superlattices Microstruct. 2013, 57, 1–10. [Google Scholar] [CrossRef]
- Hu, C.; Lei, E.; Hu, K.; Lai, L.; Zhao, D.; Zhao, W.; Rong, H. Simple Synthesis of 3D Flower-like g-C3N4/TiO2 Composite Microspheres for Enhanced Visible-Light Photocatalytic Activity. J. Mater. Sci. 2020, 55, 151–162. [Google Scholar] [CrossRef]
- Di, T.; Zhu, B.; Cheng, B.; Yu, J.; Xu, J. A Direct Z-Scheme g-C3N4/SnS2 Photocatalyst with Superior Visible-Light CO2 Reduction Performance. J. Catal. 2017, 352, 532–541. [Google Scholar] [CrossRef]
- Yin, R.; Luo, Q.; Wang, D.; Sun, H.; Li, Y.; Li, X.; An, J. SnO2/g-C3N4 Photocatalyst with Enhanced Visible-Light Photocatalytic Activity. J. Mater. Sci. 2014, 49, 6067–6073. [Google Scholar] [CrossRef]
- Beyhaqi, A.; Zeng, Q.; Chang, S.; Wang, M.; Taghi Azimi, S.M.; Hu, C. Construction of g-C3N4/WO3/MoS2 Ternary Nanocomposite with Enhanced Charge Separation and Collection for Efficient Wastewater Treatment under Visible Light. Chemosphere 2020, 247, 125784. [Google Scholar] [CrossRef]
- Junploy, P.; Thongtem, T.; Thongtem, S.; Phuruangrat, A. Decolorization of Methylene Blue by Ag/SrSnO3 Composites under Ultraviolet Radiation. J. Nanomater. 2014, 2014, 67. [Google Scholar] [CrossRef]
- Faisal, M.; Harraz, F.A.; Ismail, A.A.; Alsaiari, M.A.; Al-Sayari, S.A.; Al-Assiri, M.S. Novel Synthesis of Polyaniline/SrSnO3 Nanocomposites with Enhanced Photocatalytic Activity. Ceram. Int. 2019, 45, 20484–20492. [Google Scholar] [CrossRef]
- Deng, Y.; Tang, L.; Zeng, G.; Wang, J.; Zhou, Y.; Wang, J.; Tang, J.; Liu, Y.; Peng, B.; Chen, F. Facile Fabrication of a Direct Z-Scheme Ag2CrO4/g-C3N4 Photocatalyst with Enhanced Visible Light Photocatalytic Activity. J. Mol. Catal. A Chem. 2016, 421, 209–221. [Google Scholar] [CrossRef]
- Yu, M.; Liang, H.; Zhan, R.; Xu, L.; Niu, J. Sm-Doped g-C3N4/Ti3C2 MXene Heterojunction for Visible-Light Photocatalytic Degradation of Ciprofloxacin. Chin. Chem. Lett. 2021, 32, 2155–2158. [Google Scholar] [CrossRef]
- Balakumar, V.; Ramalingam, M.; Sekar, K.; Chuaicham, C.; Sasaki, K. Fabrication and Characterization of Carbon Quantum Dots Decorated Hollow Porous Graphitic Carbon Nitride through Polyaniline for Photocatalysis. Chem. Eng. J. 2021, 426, 131739. [Google Scholar] [CrossRef]
- Zheng, Y.; Liu, Y.; Guo, X.; Zhang, W.; Wang, Y.X.; Zhang, M.; Li, R.; Peng, Z.; Xie, H.; Huang, Y. In-Situ Construction of Morphology-Controllable 0D/1D g-C3N4 Homojunction with Enhanced Photocatalytic Activity. Appl. Surf. Sci. 2021, 563, 150317. [Google Scholar] [CrossRef]
- Zhang, B.; Li, C.; Zhang, Y.; Yuan, M.; Wang, J.; Zhu, J.; Ji, J.; Ma, Y. Improved Photocatalyst: Elimination of Triazine Herbicides by Novel Phosphorus and Boron Co-Doping Graphite Carbon Nitride. Sci. Total Environ. 2021, 757, 143810. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.; Zhao, Q.; Du, C.; Liu, Z. Enhanced Visible Light Photocatalytic Activity in BiOCl/SnO2: Heterojunction of Two Wide Band-Gap Semiconductors. RSC Adv. 2015, 5, 22740–22752. [Google Scholar] [CrossRef]
- Yang, X.C.; Yang, Y.L.; Zhang, S.L.; Liu, Y.F.; Fu, S.J.; Zhu, M.; Hu, J.F.; Zhang, Z.J.; Zhao, J.T. Facile Synthesis of Porous Nitrogen Doped Carbon Dots (NCDs)@g-C3N4 for Highly Efficient Photocatalytic and Anti-Counterfeiting Applications. Appl. Surf. Sci. 2019, 490, 592–597. [Google Scholar] [CrossRef]
- Hu, K.; Li, R.; Ye, C.; Wang, A.; Wei, W.; Hu, D.; Qiu, R.; Yan, K. Facile Synthesis of Z-Scheme Composite of TiO2 Nanorod/g-C3N4 Nanosheet Efficient for Photocatalytic Degradation of Ciprofloxacin. J. Clean. Prod. 2020, 253, 120055. [Google Scholar] [CrossRef]
- Leeladevi, K.; Arunpandian, M.; Kumar, J.V.; Chellapandi, T.; Thiruppathi, M.; Madhumitha, G.; Lee, J.W.; Nagarajan, E.R. Fabrication of 3D Pebble-like CeVO4/g-C3N4 Nanocomposite: A Visible Light-Driven Photocatalyst for Mitigation of Organic Pollutants. Diam. Relat. Mater. 2021, 116, 108424. [Google Scholar] [CrossRef]
- Navarro-Aguilar, A.I.; Obregón, S.; Sanchez-Martinez, D.; Hernández-Uresti, D.B. An Efficient and Stable WO3/g-C3N4 Photocatalyst for Ciprofloxacin and Orange G Degradation. J. Photochem. Photobiol. A Chem. 2019, 384, 112010. [Google Scholar] [CrossRef]
- Kadi, M.W.; Mohamed, R.M.; Bahnemann, D.W. Controlled Synthesis of Ag2O/g-C3N4 Heterostructures Using Soft and Hard Templates for Efficient and Enhanced Visible-Light Degradation of Ciprofloxacin. Ceram. Int. 2021, 47, 31073–31083. [Google Scholar] [CrossRef]
- Hernández-Uresti, D.B.; Sanchez-Martinez, D.; Torres-Martinez, L.M. Novel Visible Light-Driven PbMoO4/g-C3N4 Hybrid Composite with Enhanced Photocatalytic Performance. J. Photochem. Photobiol. A Chem. 2017, 345, 21–26. [Google Scholar] [CrossRef]
- Du, J.; Xu, Z.; Li, H.; Yang, H.; Xu, S.; Wang, J.; Jia, Y.; Ma, S.; Zhan, S. Ag3PO4/g-C3N4 Z-Scheme Composites with Enhanced Visible-Light-Driven Disinfection and Organic Pollutants Degradation: Uncovering the Mechanism. Appl. Surf. Sci. 2021, 541, 148487. [Google Scholar] [CrossRef]
- Zhu, H.; Yang, B.; Yang, J.; Yuan, Y.; Zhang, J. Persulfate-Enhanced Degradation of Ciprofloxacin with SiC/g-C3N4 Photocatalyst under Visible Light Irradiation. Chemosphere 2021, 276, 130217. [Google Scholar] [CrossRef]
- Sharma, S.; Kumar, K.; Thakur, N.; Chauhan, S.; Chauhan, M.S. Eco-Friendly Ocimum Tenuiflorum Green Route Synthesis of CuO Nanoparticles: Characterizations on Photocatalytic and Antibacterial Activities. J. Environ. Chem. Eng. 2021, 9, 105395. [Google Scholar] [CrossRef]
- Ma, Y.; Zhang, J.; Wang, Y.; Chen, Q.; Feng, Z.; Sun, T. Concerted Catalytic and Photocatalytic Degradation of Organic Pollutants over CuS/g-C3N4 Catalysts under Light and Dark Conditions. J. Adv. Res. 2019, 16, 135–143. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Tao, L.; Zhao, J.; Yue, X.; Deng, W.; Li, Y.; Wang, C. TiO2/g-C3N4 Nanosheets Hybrid Photocatalyst with Enhanced Photocatalytic Activity under Visible Light Irradiation. Res. Chem. Intermed. 2016, 42, 3609–3624. [Google Scholar] [CrossRef]
- Alammar, T.; Slowing, I.I.; Anderegg, J.; Mudring, A.V. Ionic-Liquid-Assisted Microwave Synthesis of Solid Solutions of Sr1−xBaxSnO3 Perovskite for Photocatalytic Applications. ChemSusChem 2017, 10, 3387–3401. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, C.; Yang, J.; Zhu, Y.; Xu, M.; Cui, Y.; Liu, L.; Ren, W.; Zhao, H.; Liang, B. Synthesis of 0D SnO2 Nanoparticles/2D g-C3N4 Nanosheets Heterojunction: Improved Charge Transfer and Separation for Visible-Light Photocatalytic Performance. J. Alloy. Compd. 2021, 871, 159561. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, X.; Nong, Q.; Lin, H.; Teng, B.; Zhang, Y.; Zhao, L.; Wu, T.; He, Y. Enhanced Visible-Light Photoactivity of g-C3N4 via Zn2SnO4 Modification. Appl. Surf. Sci. 2015, 329, 143–149. [Google Scholar] [CrossRef]
- Jiang, D.; Zhu, Y.; Chen, M.; Huang, B.; Zeng, G.; Huang, D.; Song, B.; Qin, L.; Wang, H.; Wei, W. Modified Crystal Structure and Improved Photocatalytic Activity of MIL-53 via Inorganic Acid Modulator. Appl. Catal. B Environ. 2019, 255, 117746. [Google Scholar] [CrossRef]
- Mao, X.; Xie, F.; Li, M. Facile Hydrolysis Synthesis of Novel Bi4O5Br2 Photocatalyst with Enhanced Visible Light Photocatalytic Activity for the Degradation of Resorcinol. Mater. Lett. 2016, 166, 296–299. [Google Scholar] [CrossRef]
- Dai, K.; Lu, L.; Liang, C.; Liu, Q.; Zhu, G. Heterojunction of Facet Coupled g-C3N4/Surface-Fluorinated TiO2 Nanosheets for Organic Pollutants Degradation under Visible LED Light Irradiation. Appl. Catal. B Environ. 2014, 156–157, 331–340. [Google Scholar] [CrossRef]
- Raja, A.; Rajasekaran, P.; Selvakumar, K.; Arunpandian, M.; Kaviyarasu, K.; Asath Bahadur, S.; Swaminathan, M. Visible Active Reduced Graphene Oxide-BiVO4-ZnO Ternary Photocatalyst for Efficient Removal of Ciprofloxacin. Sep. Purif. Technol. 2020, 233, 115996. [Google Scholar] [CrossRef]
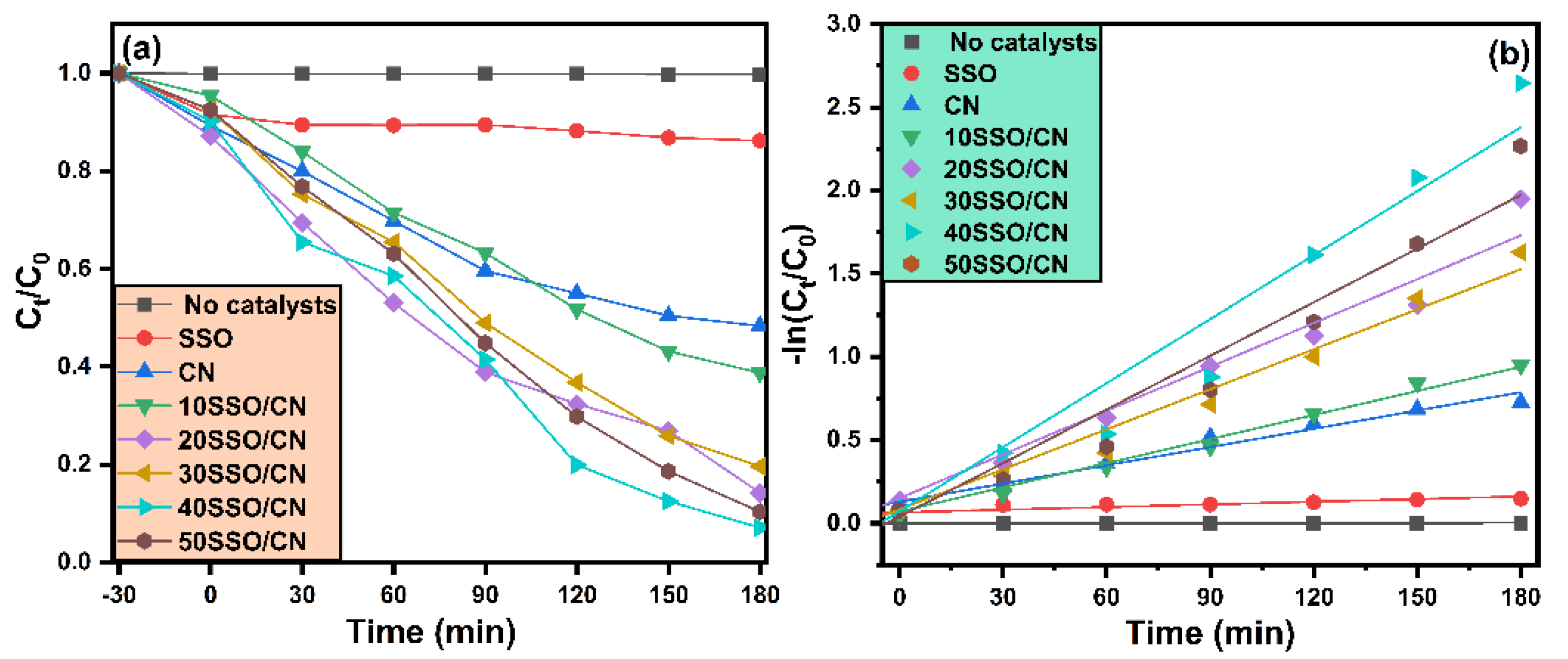

| Sample Name | Degradation (%) | K (Min−1) | R2 |
|---|---|---|---|
| SSO | 14% | 0.00053 | 0.71435 |
| CN | 52% | 0.00366 | 0.98234 |
| 10SSO/CN | 62% | 0.00483 | 0.98576 |
| 20SSO/CN | 86% | 0.00877 | 0.96844 |
| 30SSO/CN | 81% | 0.00801 | 0.97152 |
| 40SSO/CN | 93% | 0.01281 | 0.94085 |
| 50SSO/CN | 90% | 0.01074 | 0.93876 |
| Sample Name | Degradation (%) | Time (Min) | Light Source | Reference |
|---|---|---|---|---|
| TiO2/g-C3N4 | 93.4% | 60 min | Visible light | [61] |
| CeVO4/g-C3N4 | 92% | 70 min | Visible light | [62] |
| WO3/g-C3N4 | Almost 100% | 240 min | Visible light | [63] |
| Ag2O/g-C3N4 | 100% | 120 min | Visible light | [64] |
| PbMoO4/g-C3N4 | Almost 100% | 120 min | Visible light | [65] |
| Ag3PO4/g-C3N4 | 85.3% | 180 min | Visible light | [66] |
| SiC/g-C3N4 | 95% | 30 min | Visible light | [67] |
| SrSnO3/g-C3N4 | 93% | 180 min | Visible light | This work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, Z.; Xia, H.; Jiang, J.; Han, S.; Li, H. Ultrasound-Assisted Hydrothermal Synthesis of SrSnO3/g-C3N4 Heterojunction with Enhanced Photocatalytic Performance for Ciprofloxacin under Visible Light. Crystals 2022, 12, 1062. https://doi.org/10.3390/cryst12081062
Zhu Z, Xia H, Jiang J, Han S, Li H. Ultrasound-Assisted Hydrothermal Synthesis of SrSnO3/g-C3N4 Heterojunction with Enhanced Photocatalytic Performance for Ciprofloxacin under Visible Light. Crystals. 2022; 12(8):1062. https://doi.org/10.3390/cryst12081062
Chicago/Turabian StyleZhu, Zhengru, Haiwen Xia, Junchao Jiang, Songlin Han, and Hong Li. 2022. "Ultrasound-Assisted Hydrothermal Synthesis of SrSnO3/g-C3N4 Heterojunction with Enhanced Photocatalytic Performance for Ciprofloxacin under Visible Light" Crystals 12, no. 8: 1062. https://doi.org/10.3390/cryst12081062






