Structural and Antimicrobial Characterization of Co-Crystal [Ni(bpy)(acr)2(H2O)]·MA
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Physical Measurements
2.2. Synthesis of Complex
3. Results and Discussion
3.1. Complex Synthesis
3.2. Complex Characterization
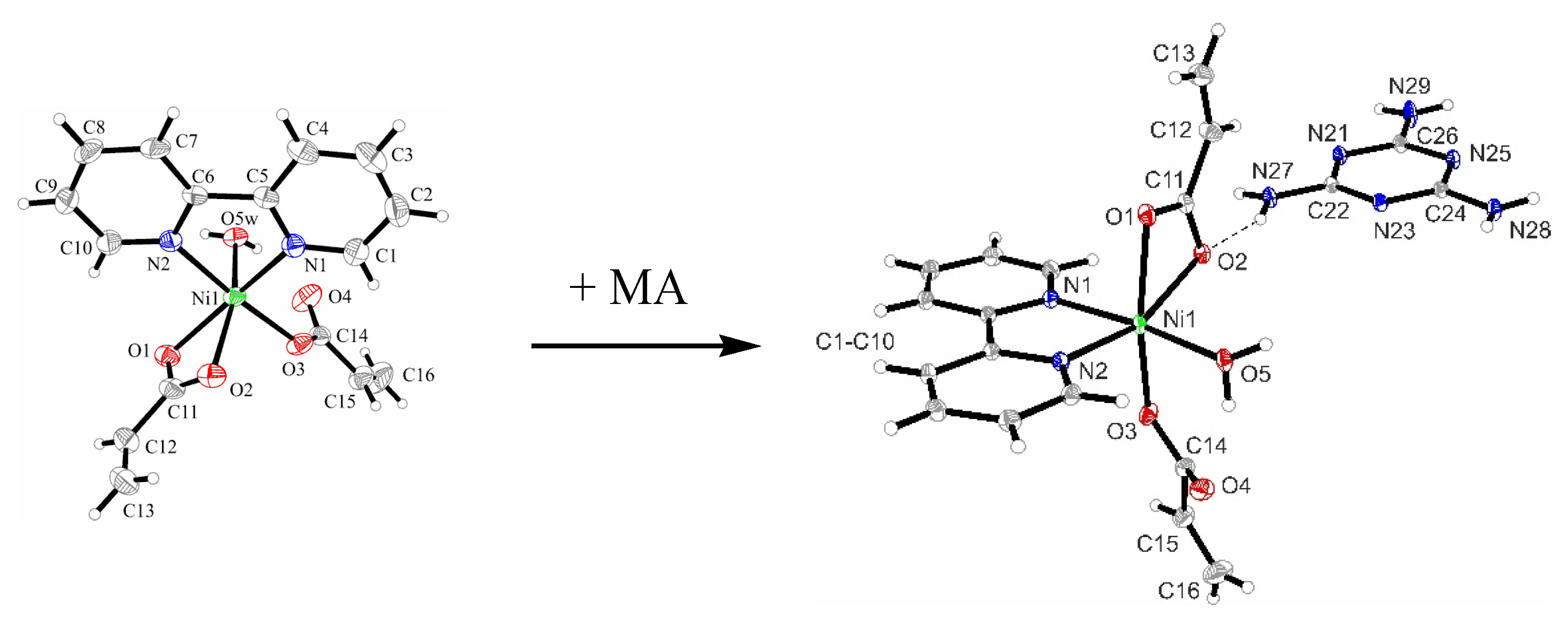
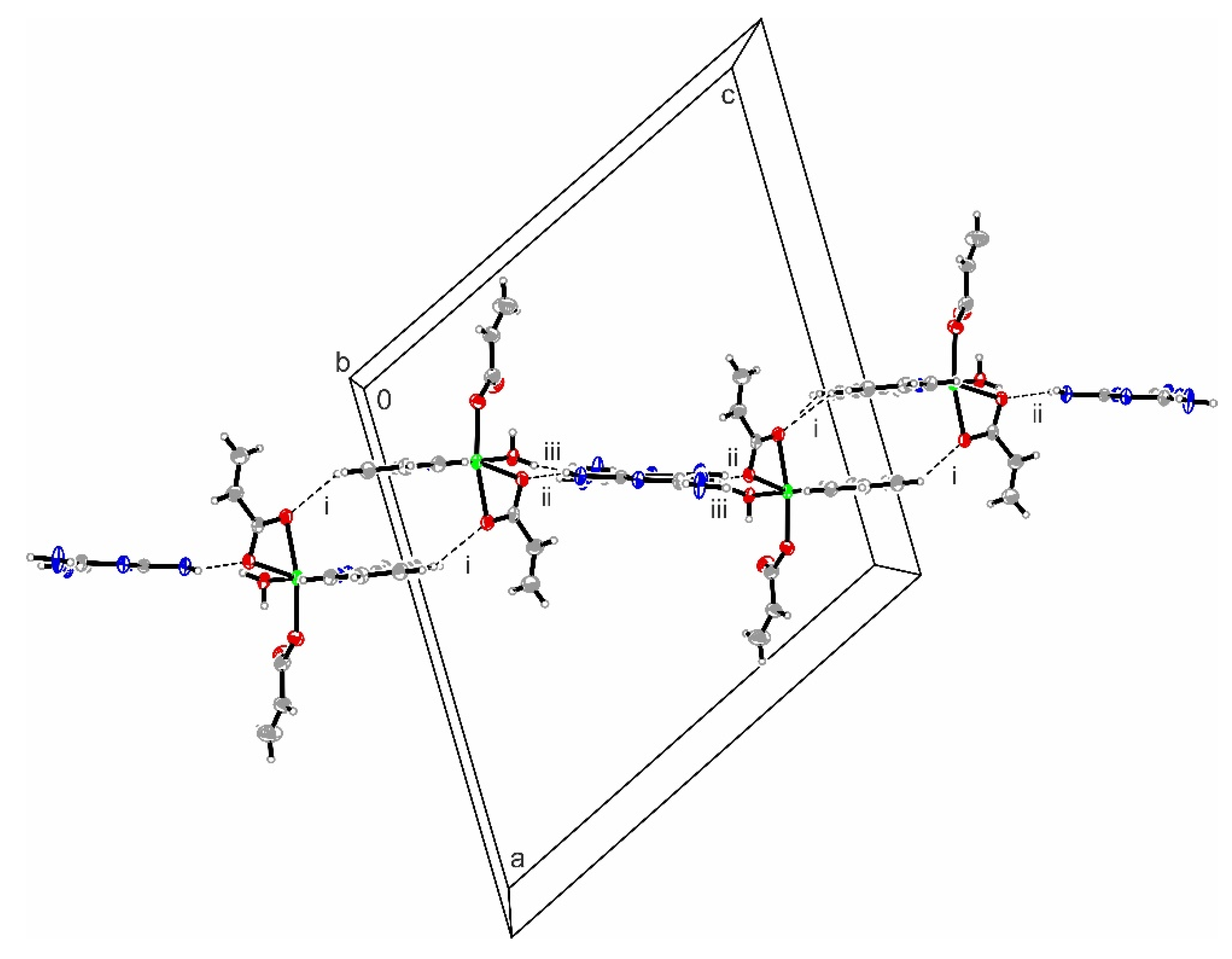
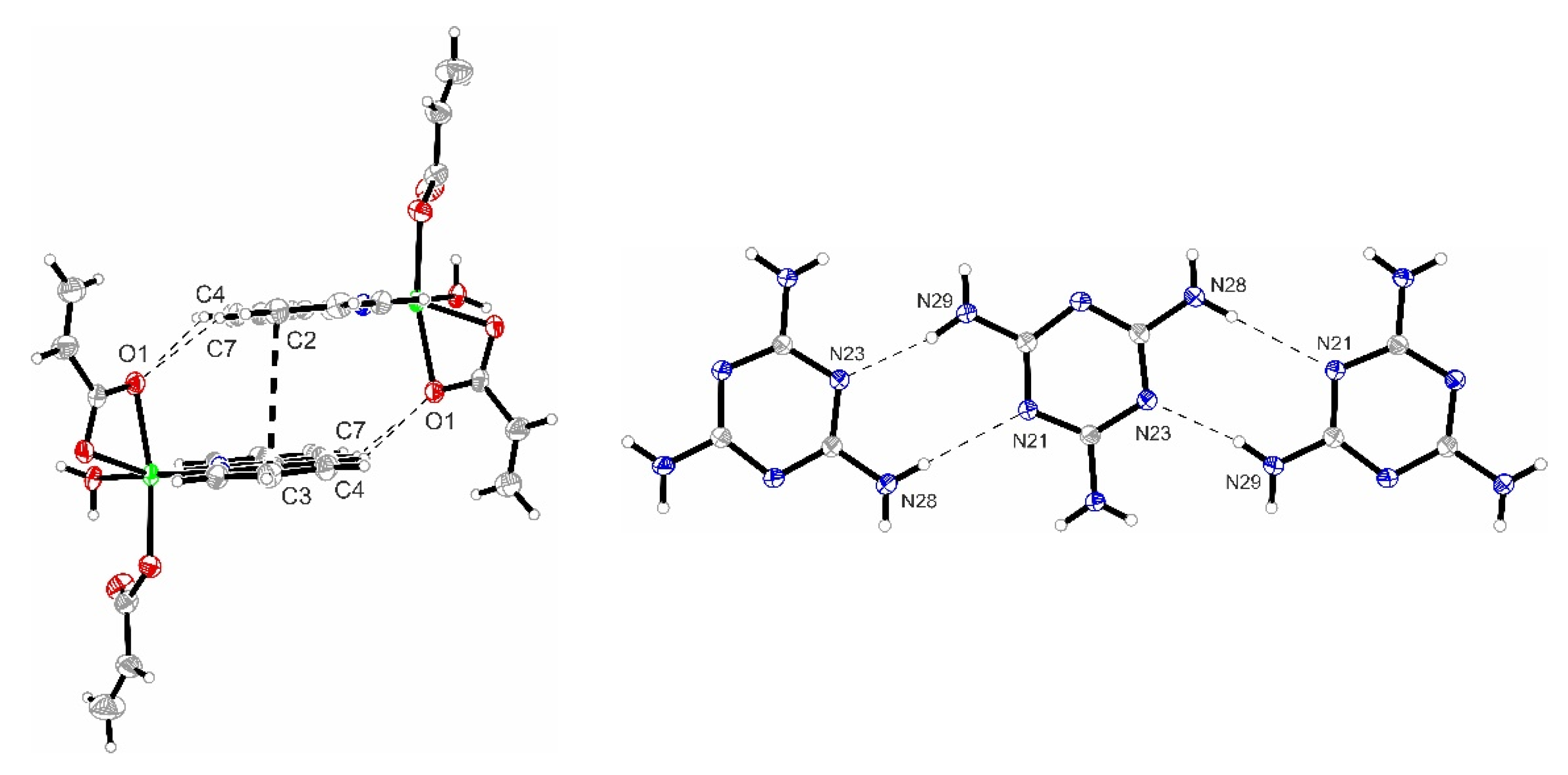
3.2.1. Description of the X-ray Crystal Structure of the Complex
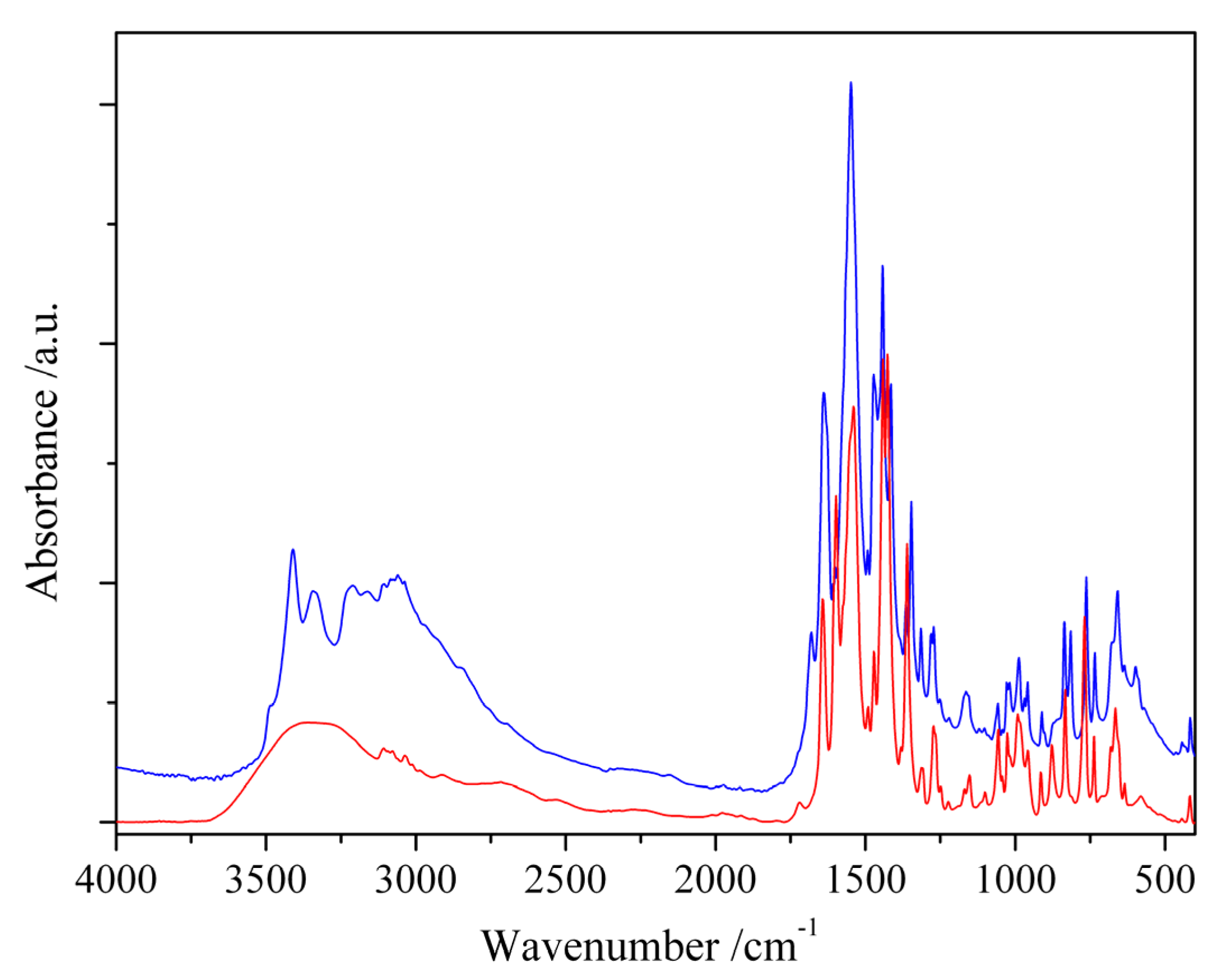
3.2.2. Infrared Spectra
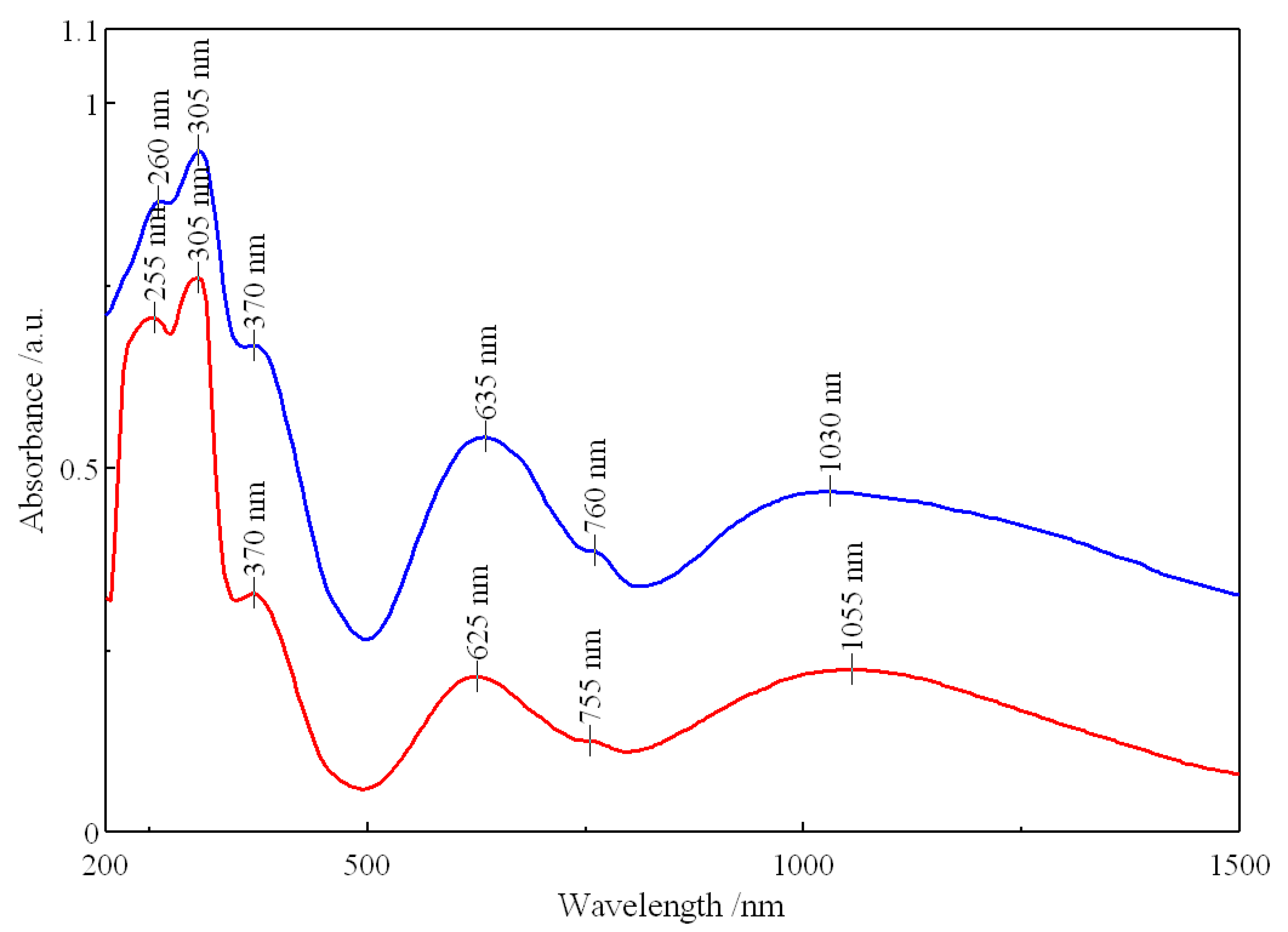
3.2.3. UV-Vis-NIR Spectra
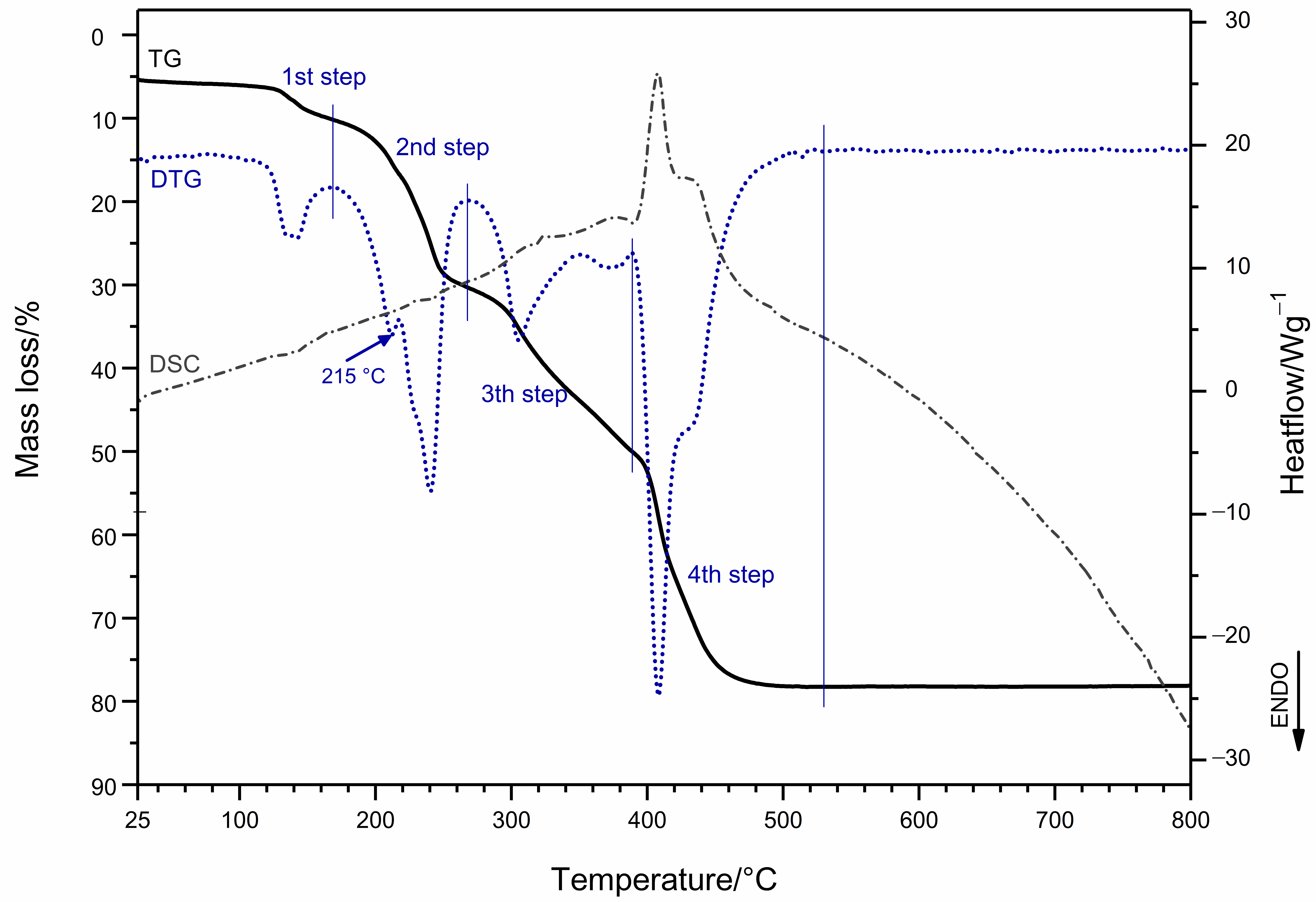
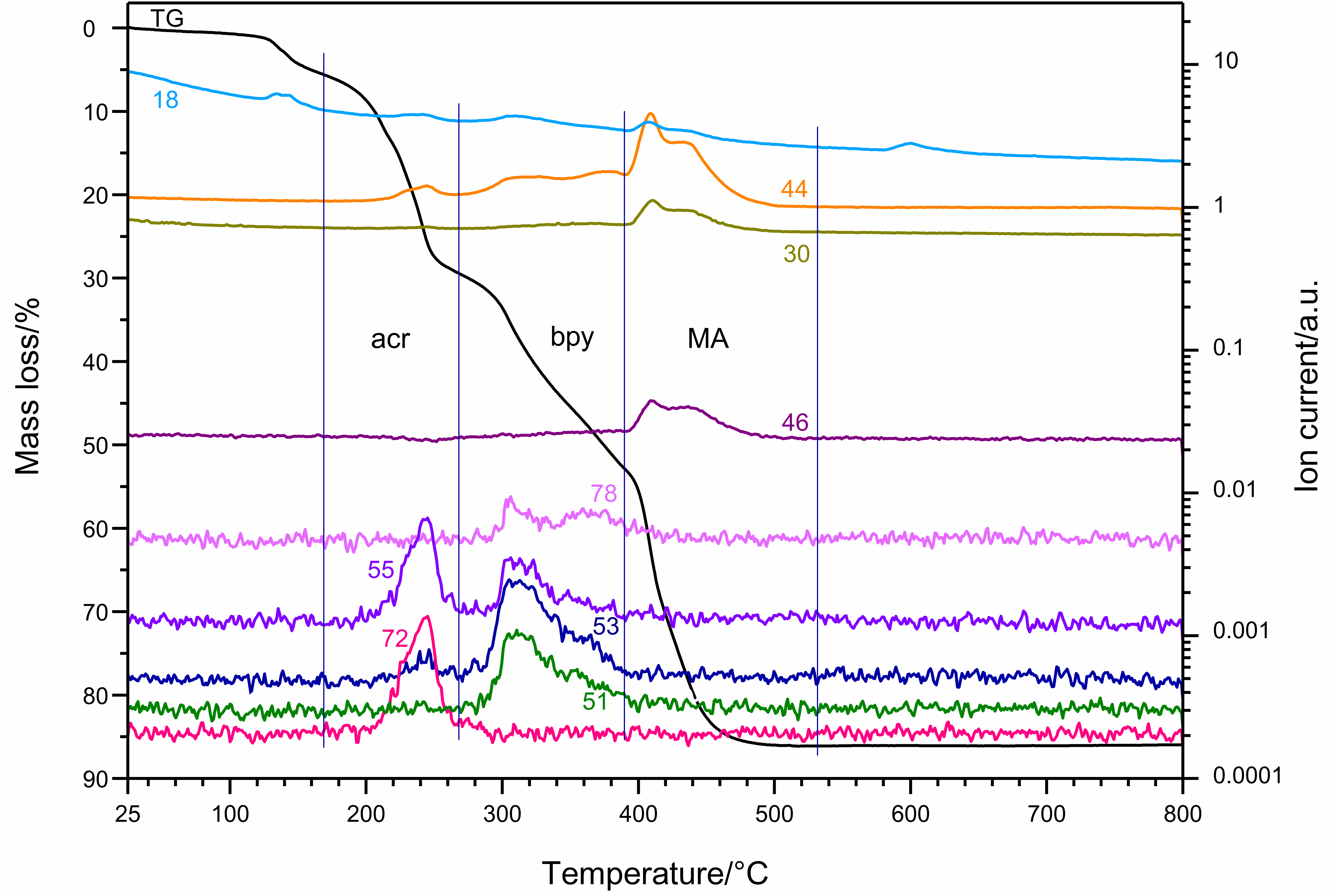
3.2.4. Thermal Behavior
3.2.5. Antimicrobial Assay
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Refat, M.; Adam, A.M.; El-Sayed, M. Biomarkers charge-transfer complexes of melamine with quinol and picric acid: Synthesis, spectroscopic, thermal, kinetic and biological studies. Arab. J. Chem. 2017, 10, S3482–S3492. [Google Scholar] [CrossRef] [Green Version]
- Goodgame, D.; Hussain, I.; White, A.; Williams, D. Synthesis and structure of a copper(II) melamine complex [Cu(MA)(µ-OCH3)(ONO2)(CH3OH)]2, with direct Cu-melamine coordination. J. Chem. Soc. Dalton Trans. 1999, 17, 2899–2900. [Google Scholar] [CrossRef]
- Yu, Y.-Q.; Lu, C.-Z.; He, X.; Chen, S.-M.; Zhang, Q.-Z.; Chen, L.-J.; Yang, W.-B. Synthesis, structure and fluorescent property of a novel inorganic-organic zinc compound. J. Chem. Cryst. 2004, 34, 905–909. [Google Scholar] [CrossRef]
- Chen, C.; Yeh, C.W.; Chen, J.D. Syntheses, structures and thermal properties of two new copper(II) melamine complexes. Polyhedron 2006, 25, 1307–1312. [Google Scholar] [CrossRef]
- Zhang, L.; Li, W.; Zhang, J.; Li, Z.-J.; Qin, Y.-Y.; Cheng, J.-K.; Yao, Y.-G. Antiferromagnetic interactions in melamine-bridged trinuclear cobalt complex. Inorg. Chem. Commun. 2008, 11, 279–282. [Google Scholar] [CrossRef]
- Li, Y.-H.; Sun, D.; Luo, G.-G.; Liu, F.-J.; Hao, H.-J.; Wen, Y.-M.; Zhao, Y.; Huang, R.-B.; Zheng, L.-S. Two Ag(I) coordination polymers derived from melamine and dicarboxylates: Syntheses, crystal structures and thermal stabilities. J. Mol. Struct. 2011, 1000, 85–91. [Google Scholar] [CrossRef]
- Sivashankar, K.; Ranganathan, A.; Pedireddin, V.R.; Rao, C.N.R. Novel supramolecular organizations in melamine complexes with 4,4′-bipyridyl and silver nitrate. J. Mol. Struct. 2001, 559, 41–48. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, J.; Li, J.-L.; Cheng, J.-K.; Yin, P.-X.; Yao, Y.-G. New coordination motifs of melamine directed by N-H···X (X=Cl or Br) hydrogen bonds. Inorg. Chem. 2007, 46, 5838–5840. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.-Y.; Bai, F.-Y.; Xing, Y.-H.; Li, Z.-P.; Cao, Y.-Z.; Zeng, X.-Q.; Ge, M.-F. A melamine–adipate-bridged binuclear copper complex with supramolecular architecture: Synthesis, structures, and properties of [Cu2(MA)(ad)2]·H2O and (MA)·(H2ad)·H2O. J. Coord. Chem. 2010, 63, 435–447. [Google Scholar] [CrossRef]
- Rana, A.; Bera, M.; Choudhuri, D.S.; Hazari, D.; Jana, S.K.; Zangrando, E.; Dalai, S. 3D coordination network of Ag(I) ions with µ3-bridging melamine ligands. J. Inorg. Organomet. Polym. 2012, 22, 360–368. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, K.; Fan, H.-T. Synthesis, crystal structure and catalytic property of a new Cu(I) coordination polymer constructed from melamine and azide ligands. Inorg. Chem. Commun. 2013, 38, 47–49. [Google Scholar] [CrossRef]
- Ranganathan, A.; El-Ghayoury, A.; Zorina, L.; Batail, P. The layered topology of a five components melaminium-melamine hybrid salt of a functional gold (II) dithiolene complex. Cryst. Eng. Comm. 2010, 12, 4268–4274. [Google Scholar] [CrossRef] [Green Version]
- Iliopoulos, K.; El-Ghayoury, A.; Derkowska, B.; Ranganathan, A.; Batail, P.; Gindre, D.; Sahraoui, B. Effect of the counter cation on the third order nonlinearity in anionic Au dithiolene complexes. Appl. Phys. Lett. 2012, 101, 261105. [Google Scholar] [CrossRef] [Green Version]
- Mirzaei, M.; Eshtiagh-Hosseini, H.; Bazargan, M. Syntheses and X-ray crystal structure studies of four new coordination complexes and salts based on proton-transferred pyridine-2,6-dicarboxylic acid N-oxide. Res. Chem. Intermed. 2015, 41, 9785–9803. [Google Scholar] [CrossRef]
- Mitra, M.; Hossain, A.; Manna, P.; Choudhury, S.R.; Kaenket, S.; Helliwell, M.; Bauzá, A.; Frontera, A.; Mukhopadhyay, S. Melamine-mediated self-assembly of a Cu(II)–methylmalonate complex assisted by π+–π+ and anti-electrostatic H-bonding interactions. J. Coord. Chem. 2017, 70, 463–474. [Google Scholar] [CrossRef]
- Krichen, F.; Walha, S.; Lhoste, J.; Bulou, A.; Hemon-Ribaud, A.; Goutenoire, F.; Kabadou, A. Supramolecular and heterometallic architectures based on [Fe(CN)6]3− metallotectons and diverse organic cations: Crystal structure, Hirshfeld surface analysis, spectroscopic and thermal properties. Inorg. Chim. Acta 2019, 486, 36–47. [Google Scholar] [CrossRef]
- Olar, R.; Scăețeanu Vasile, G.; Dănilă, M.; Daniliuc, C.-G.; Cerc Korošec, R.; Celan Korošin, N.; Badea, M.J. Synthesis and characterization of cobalt acrylate-melamine co-crystals. J. Therm. Anal. Calorim. 2019, 135, 2257–2264. [Google Scholar] [CrossRef]
- Liu, H.; Zhi, W.-W.; Liu, X.-Z. Two New Zn(II)/Cd(II) Complexes: Luminescent Properties and Treatment Activity on Cardiovascular Disease. J. Fluoresc. 2022, in press. [CrossRef]
- Vírseda, I.B.; Siddiqui, S.A.; Prado-Roller, A.; Eisterer, M.; Shiozawa, H. Crystal engineering with copper and melamine. RSC Adv. 2021, 11, 23943. [Google Scholar] [CrossRef]
- Chen, L.-D.; Zhou, L.-X.; Zheng, Y.-Q.; Zhu, H.-L. Two new Ag(I) supramolecular complexes based on melamine: Synthesis, structures and photocatalytic activity under visible light irradiation. Polyhedron 2017, 18, 150–158. [Google Scholar] [CrossRef]
- Baraka, A.; Hatem, H.; El-Geundi, M.S.; Tantawy, H.; Karaghiosoff, K.; Gobara, M.; Elbeih, A.; Shoaib, M.; Elsayed, M.A.; Kotb, M.M. A new cationic silver(I)/melamine coordination polymer, [Ag2(melamine)]2+: Synthesis, characterization and potential use for aqueous contaminant anion exchange. J. Solid State Chem. 2019, 274, 168–175. [Google Scholar] [CrossRef]
- Mastalir, M.; Kirchner, K. A triazine-based Ni(II) PNP pincer complex as catalyst for Kumada-Corriu and Negishi cross-coupling reactions. Monatsch. Chem. 2017, 148, 105–109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elsharkawy, R.G. Incorporation of copper/melamine complexes in silica surface and their sorption activity of organic dye. Iran. J. Chem. Chem. Eng. 2017, 36, 99–114. [Google Scholar]
- Zorainy, M.; Boffito, D.; Gobara, M.; Baraka, A.; Naeem, I.; Tantawy, H. Synthesis of a novel Ce(III)/melamine coordination polymer and its application for corrosion protection of AA2024 in NaCl solution. RSC Adv. 2021, 11, 6330–6345. [Google Scholar] [CrossRef] [PubMed]
- Olar, R.; Badea, M.; Marinescu, D.; Mardale, R. Thermal behaviour of new Cu(II) complexes with Schiff bases functionalised with 1,3,5-triazine moieties as potential antibacterial agents. J. Therm. Anal. Calorim. 2011, 105, 553–557. [Google Scholar] [CrossRef]
- Zhu, H.; Zhang, S.; Li, M.; Shao, Y.; Zhu, Z. Electrochemical sensor for melamine based on its complex. Chem. Commun. 2010, 46, 2259–2261. [Google Scholar] [CrossRef]
- Scăețeanu Vasile, G.; Chifiriuc, M.C.; Bleotu, C.; Karmezan, C.; Măruțescu, L.; Daniliuc, C.-G.; Maxim, C.; Calu, L.; Badea, M. Synthesis, Structural Characterization, Antimicrobial Activity, and In Vitro Biocompatibility of New Unsaturated Carboxylate Complexes with 2,2′-Bipyridine. Molecules 2018, 23, 157. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hooft, R.W.W.; Nonius, B.V. COLLECT, Program for Collecting Data on CCD Area Detectors; Bruker AXS: Delft, The Netherlands, 1998. [Google Scholar]
- Otwinowski, Z.; Minor, W. Processing of X-ray diffraction data collected in oscillation mode. Meth. Enzymol. 1997, 276, 307–326. [Google Scholar]
- Otwinowski, Z.; Borek, D.; Majewski, W.; Minor, W. Multiparametric scaling of diffraction intensities. Acta Crystallogr. A 2003, 59, 228–234. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. SHELXT—Integrated space-group and crystal-structure determination. Acta Crystallogr. 2015, A71, 3–8. [Google Scholar] [CrossRef] [Green Version]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. 2015, C71, 3–8. [Google Scholar]
- Bruker AXS Inc. XP—Interactive Molecular Graphics, Version 5.1; Bruker AXS Inc.: Madison, WI, USA, 1998.
- Chifiriuc, M.C.; Grumezescu, A.M.; Andronescu, E.; Ficai, A.; Cotar, A.I.; Grumezescu, V.; Bezirtzoglou, E.; Lazar, V.; Radulescu, R. Water dispersible magnetite nanoparticles influence the efficacy of antibiotics against planktonic and biofilm embedded Enterococcus faecalis cells. Anaerobe 2013, 22, 14–19. [Google Scholar] [CrossRef]
- Gingasu, D.; Mindru, I.; Mocioiu, O.C.; Preda, S.; Stanica, N.; Patron, L.; Ianculescu, A.; Oprea, O.; Nita, S.; Paraschiv, I.; et al. Synthesis of nanocrystalline cobalt ferrite through soft chemistry methods: A green chemistry approach using sesame seed extract. Mater. Chem. Phys. 2016, 182, 219–230. [Google Scholar] [CrossRef]
- Ye, B.-H.; Chen, X.-M.; Xue, G.-Q.; Li, L.N. Mononuclear nickel complexes assembled into two-dimensional networks via hydrogen bonds and π-π stacking interactions. J. Chem. Soc. Dalton Trans. 1998, 17, 2827–2832. [Google Scholar] [CrossRef]
- Begum, R.; Rehman, M.; Shahid, K.; Iqbal, M.; Tahir, M.N.; Ali, S. Synthesis, structural elucidation, DNA-binding and biological activity of nickel (II) mixed ligand carboxylate complexes. J. Mol. Struct. 2021, 1242, 130801. [Google Scholar] [CrossRef]
- Krešáková, L.; Miňo, A.; Holum, M.; Kuchár, J.; Werner, A.; Tomás, M.; Čižmár, E.; Falvello, L.; Černák, J. Heteroleptic complexes of Ni(II) with 2,2′-bipyridine and benzoato ligands. Magnetic properties of [Ni(bpy)(Bz)2]. Inorg. Chim. Acta 2021, 527, 120588. [Google Scholar] [CrossRef]
- Gunasekaran, S.; Srinivasan, K.; Xavier Jesu Raja, S. Fourier transform infrared and laser Raman scattering studies on the structure of melamine. Proc. Indian Natl. Sci. Acad. 1993, 59, 347–352. [Google Scholar]
- Mircescu, N.E.; Oltean, V.; Chis, V.; Leopold, N. FTIR, FT-Raman, SERS and DFT study on melamine. Vib. Spectrosc. 2012, 62, 165–171. [Google Scholar] [CrossRef]
- Lever, A.B.P. Inorganic Electronic Spectroscopy, 2nd ed.; Elsevier: Amsterdam, The Netherlands; London, UK; New York, NY, USA, 1984; pp. 507–544. [Google Scholar]
- 1,3,5-Triazine-2,4,6-triamine. Available online: https://webbook.nist.gov/cgi/cbook.cgi?ID=C108781&Units=SI&Mask=200#Mass-Spec (accessed on 19 June 2022).
- 2,2′-Bipyridine. Available online: https://webbook.nist.gov/cgi/cbook.cgi?ID=C366187&Units=SI&Mask=200#Mass-Spec (accessed on 19 June 2022).
- Melamine —Flame Retardants. Available online: http://fr.polymerinsights.com/fr-types/nitrogen-based/melamine (accessed on 19 June 2022).
- Haynes, W.M. (Ed.) CRC Handbook of Chemistry and Physics, 92nd ed.; CRC Press: Boca Raton, Fl, USA, 2011; p. 3516. [Google Scholar]
- Badea, M.; Olar, R.; Marinescu, D.; Vasile, G. Thermal behavior of some new complexes bearing ligands with polymerisable groups. J. Therm. Anal. Calorim. 2006, 85, 285–288. [Google Scholar] [CrossRef]
- Sheikhshoaie, I.; Lotfi, N.; Sieler, J.; Krautscheid, H.; Khaleghi, M. Synthesis, structures and antimicrobial activities of nickel(II) and zinc(II) diaminomaleonitrile-based complexes. Trans. Met. Chem. 2018, 43, 555–562. [Google Scholar] [CrossRef] [Green Version]
- Podda, E.; Arca, M.; Atzeni, G.; Coles, S.J.; Ibba, A.; Isaia, F.; Lippolis, V.; Orrù, G.J.; Orton, B.; Pintus, A.; et al. Antibacterial Activity of Amidodithiophosphonato Nickel(II) Complexes: An Experimental and Theoretical Approach. Molecules 2020, 25, 2052. [Google Scholar] [CrossRef] [PubMed]
- Jones, S. Permeability rules for antibiotic design. Nat. Biotechnol. 2017, 35, 639. [Google Scholar] [CrossRef] [PubMed]
| Complex | Ni-N | Ni-O (Chelate Carboxylate) | Ni-O (Unidentate Carboxylate) | Ni-O (Water) | Reference |
|---|---|---|---|---|---|
| [Ni(bpy)(acr)2(H2O)]·MA | 2.059(1) 2.049(2) | 2.131(1) 2.148(2) | 2.069(1) | 2.061(1) | this paper |
| [Ni(bpy)(acr)2(H2O)] (precursor complex) | 2.075(3) 2.064(3) | 2.126(2) 2.147(3) | 2.034(2) | 2.060(3) | [27] |
| [Ni(bpy)(acetate)2(H2O)2] | 2.069(2) | - | 2.079(2) | 2.082(2) | [36] |
| [Ni(dmbpy)(acetate)2(H2O)2] | 2.067(4) | - | 2.077(3) | 2.077(3) | [36] |
| [Ni(bpy)(pmc)2(H2O)2] | 2.0787 | - | 2.0903 | 2.0869 | [37] |
| [Ni(bpy)(Bz)2] | 2.049(3) 2.029(3) | 2.055(2) 2.077(2) 2.148(3) 2.156(3) | - | - | [38] |
| Step | Temperature Range/°C | Thermal Effect | Mass Loss (exp.)/% | Mass Loss (calc.)/% | Process |
|---|---|---|---|---|---|
| 1. | 108–168 | endothermic | 4.8 | 3.9 | water elimination |
| 2. | 168–268 | miscellaneous | 24.5 | 25.6 | acrylate ion decomposition |
| 3. | 268–385 | exothermic | 23.5 | 25.2 | bipyridine oxidative decomposition |
| 4. | 385–529 | exothermic | 34.1 | 31.2 | MA oxidative decomposition |
| 13.1 | 14.1 | Residue |
| Tested Compound/Tested Strain | S. aureus 25923 | E. faecalis 29212 | E. coli 25922 | P. aeruginosa 27853 |
|---|---|---|---|---|
| 1 [Ni(bpy)(acr)2(H2O)]·MA | 0.07/0.14 | 0.31/0.62 | 0.625/1.25 | 0.31/0.62 |
| [Ni(bpy)(acr)2(H2O)] | 0.07/0.19 | 0.15/0.40 | 0.625/1.67 | 0.31/0.83 |
| MA | 0.31/2.46 | 0.625/4.96 | 1.25/9.92 | 0.31/2.46 |
| Gentamycin | 0.001/0.002 | 0.016/0.034 | 0.001/0.002 | 0.002/0.004 |
| Tested Compound/Tested Strain | S. aureus 25923 | E. faecalis 29212 | E. coli 25922 | P. aeruginosa 27853 |
|---|---|---|---|---|
| 1 [Ni(bpy)(acr)2(H2O)]·MA | 0.15/0.30 | 0.31/0.62 | 0.625/1.25 | 1.25/2.50 |
| [Ni(bpy)(acr)2(H2O)] | 0.15/0.40 | 0.31/0.83 | 0.625/1.67 | 0.625/1.67 |
| MA | 1.25/9.92 | 0.625/4.96 | 1.25/9.92 | 0.625/4.96 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Olar, R.; Daniliuc, C.G.; Vasile Scăețeanu, G.; Cerc Korosec, R.; Čelan Korošin, N.; Chifiriuc, M.C.; Badea, M. Structural and Antimicrobial Characterization of Co-Crystal [Ni(bpy)(acr)2(H2O)]·MA. Crystals 2022, 12, 1078. https://doi.org/10.3390/cryst12081078
Olar R, Daniliuc CG, Vasile Scăețeanu G, Cerc Korosec R, Čelan Korošin N, Chifiriuc MC, Badea M. Structural and Antimicrobial Characterization of Co-Crystal [Ni(bpy)(acr)2(H2O)]·MA. Crystals. 2022; 12(8):1078. https://doi.org/10.3390/cryst12081078
Chicago/Turabian StyleOlar, Rodica, Constantin G. Daniliuc, Gina Vasile Scăețeanu, Romana Cerc Korosec, Nataša Čelan Korošin, Mariana Carmen Chifiriuc, and Mihaela Badea. 2022. "Structural and Antimicrobial Characterization of Co-Crystal [Ni(bpy)(acr)2(H2O)]·MA" Crystals 12, no. 8: 1078. https://doi.org/10.3390/cryst12081078
APA StyleOlar, R., Daniliuc, C. G., Vasile Scăețeanu, G., Cerc Korosec, R., Čelan Korošin, N., Chifiriuc, M. C., & Badea, M. (2022). Structural and Antimicrobial Characterization of Co-Crystal [Ni(bpy)(acr)2(H2O)]·MA. Crystals, 12(8), 1078. https://doi.org/10.3390/cryst12081078












