Abstract
N-methyl-D-aspartate (NMDA) receptor blockade can improve L-DOPA (l-3,4-dihydroxyphenylalanine)-induced dyskinesias in Parkinson’s disease (PD) patients. Amantadine is a well-tolerated and effective antiparkinsonian agent, recently found to possess NMDA antagonistic properties. Oxidative damage may contribute to dopaminergic (DAergic) neurodegeneration in the substantia nigra of patients with PD. N,N-dimethylglycine (DMG) (also known as vitamin B15 or pangamic acid) acts as an antioxidant, extending the lifespan of animal cells through protection from oxidation. In this study, we synthesized and tested in vivo the newly obtained compound N,N-dimethylglycine-amantadine (DMG-Am) for antiparkinsonian activity. MPTP (1-methyl-4–phenyl-1, 2, 3, 6-tetrahydropyridine) is a widely used neurotoxin to induce an experimental model which mimics Parkinson disease-like symptoms. The neuroprotective capacity of the new amantadine derivative DMG-Am was evaluated by its potential to ameliorate the neuromuscular coordination and behavioral changes worsened by the toxin. Our experimental results showed that DMG-Am applied for 12 consecutive days, 5 days simultaneously and 7 days after MPTP, restored motor and memory performance of the animals to the control level, indication of beneficial protective effect of this compound. In summary, our results reveal the potential of newly synthesized DMG-Am as promising antiparkinsonian agent.
1. Introduction
Levodopa (L-DOPA) is considered as standard treatment for Parkinson’s disease symptoms. However, the long-term use of Levodopa over the years may result in Levodopa-induced dyskinesias (LID), wearing-off effect and “on–off” effect all related to fluctuations in motor state. Nowadays, to evade the undesirable side effects, Levodopa is coadministered (adjuvant therapy) with dopamine agonists, monoamine oxidase type B (MAO-B) or Catechol-o-methyltransferase (COMT) inhibitors. Dopamine agonists mimic dopamine in the brain, MAO-B inhibitors block the enzyme that destroys dopamine in the brain e.g., longer effect while COMT inhibitors block the enzyme that breaks down levodopa. The adjuvant therapy usually lowers the risk of long-term complications than levodopa monotherapy and the adverse effects may be less severe. Unfortunately, approximately 40-60% of the patients using L-DOPA will develop both LID and motor fluctuations. For such patients the increase of dopaminergic dose will worsen LID and alternative approaches should be explored [1].
Amantadine has been initially used as an antiviral agent to treat the flu, but is currently used to relief motor symptoms in Parkinson’s disease. The mechanism of action of amantadine for treating Parkinson’s disease and alleviating LID is supposedly attributed to the blocking of NMDA receptors [2,3]. Amantadine use in Parkinson’s disease has been challenged as antidyskinetic efficacy wears off after a few months and the use of higher doses leads to side effects [4,5].
The occurrence of oxidative stress in Parkinson’s disease is well known. There is almost no doubt that oxidative stress leads to increased damages in the brain [6,7,8,9,10,11]. The degradation of dopamine by monoamine oxidase-B (MAO-B) to produce hydrogen peroxide (H2O2) further emphasizes how oxidative stress might arise in the substantia nigra (SN) of the brain. Oxidative stress may also be intimately linked to other processes associated with cell death, such as mitochondrial dysfunction, inflammation, excitotoxicity, and the toxic effects of nitric oxide [12].
The quaternary ammonium compounds, choline and betaine, and dimethylglycine (DMG) reside along a metabolic pathway linked to the synthesis of neurotransmitters and membrane phospholipids and to homocysteine remethylation. DMG and sarcosine are important intermediates in one-carbon metabolism. Abnormal levels of DMG are associated with a variety of diseases, and DMG has been proposed as therapeutic for specific pathologies [13,14,15]. DMG acting as a methyl donor, could improve body immunity, function as an antioxidant, prevent oxidative stress, and scavenge excess free radicals to avoid unwanted reactions in the body [16].
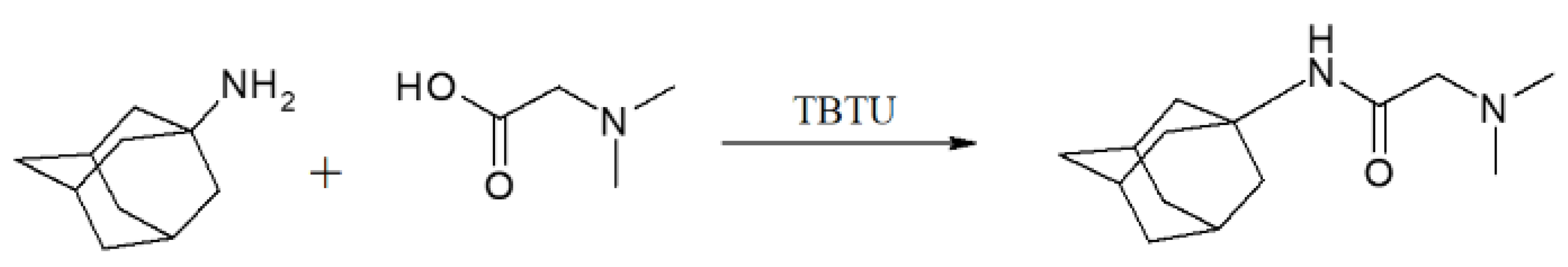
Nowadays, adamantane moiety is employed to either provide or improve the pharmacological activity of known drugs. The strategy that implies the addition of various substituents to the adamantane moiety has provided new alternatives for Alzheimer disease [17], Parkinson disease [18], or to anti-inflammatory drugs [19]. Following this rationale, we decided to combine amantadine and dimethylglycine, two known moieties with therapeutic activity, by employing a simple and cost effective synthetic procedure.
2. Materials and Methods
2.1. Materials
Amantadine, triethylamine (TEA), N,N-dimethylglycine, TBTU coupling reagent, and all necessary solvents were purchased from Sigma Aldrich. Aluminum TLC plates, silica gel coated with fluorescent indicator F254 are from Merck. All NMR spectra were recorded on Bruker AVANCE 500 MHz NMR spectrometer (Bruker, Ettlingen, Germany) in DMSO-d6. MS spectra were recorded on Bruker Esquire 3000 Plus Ion Trap Mass Spectrometer (Bruker Optics, Ettlingen, Germany) in ESI mode.
2.2. Synthesis of DMG-Am
Solution 1: Amantadine (1655 mg, 8.8 mmol) was dissolved in 5 mL DCM, TEA (1.23 mL, 8.8 mmol) was added to the solution. Solution 2: in separate flask N,N-dimethylglycine (1000 mg, 9.7 mmol) was dissolved in 10 mL DCM. Then TEA and (1.36 mL, 9.7 mmol) and TBTU (4360 mg, 13.6 mmol) were added. After 30 min, Solutions 1 and 2 were mixed together. The reaction mixture (nitrogen protected) was stirred at room temperature for 24 h. The reaction was monitored by TLC. After termination of the reaction, the mixture was washed with 5% NaHCO3 solution (twice), then dried with anhydrous Na2SO4. The obtained DMG-Am was purified by flash chromatography on silica gel column with elution system TCM:MeOH 4:1.
1H NMR (600 MHz, DMSO-d6) δ 10.01 (s, 1H), 8.27 (s, 1H), 3.82 (s, 2H), 2.78 (s, 6H), 2.03 (s, 3H), 1.95 (s, 6H), 1.63 (s, 6H). 13C NMR (151 MHz, DMSO-d6) δ 163.56, 58.25, 52.12, 43.37, 41.26, 36.34, 29.20. ESI-MS; m/z 237.2 [M+H]+.
2.3. Powder X-ray Diffraction (PXRD)
Powder XRD patterns were determined for the physical powder of the DMG-Am to establish crystalline properties, purity, and eventual presence of polymorphs. Powder samples of the synthesized quaternary ammonium compounds were analysed on Empyrean Powder X-ray diffractometer (Malvern Panalytical, Netherlands) in the range 2°–65° 2θ using Cu radiation (λ = 1.5406 Å) and PIXcel3D detector. The diffraction patterns of the precipitates were compared with those generated from the SCXRD experiment to confirm the presence or absence of additional phases.
2.4. Single Crystal X-ray Diffraction (SCXRD)
Single crystals of DMG-Am with suitable size and diffracting quality were mounted on nylon loops. The diffraction peak intensities and coordinates were collected on Bruker D8 Venture diffractometer (Bruker AXS, Karlsruhe, Germany) equipped with PhotonII CMOS detector using micro-focus MoKα radiation (λ = 0.71073 Å). Data were processed with CrysAlisPro software [20]. The structure was solved with intrinsic methods using ShelxT [21] and refined by the full-matrix least-squares method on F2 with ShelxL program [22]. All non-hydrogen atoms were located successfully from Fourier map and were refined anisotropically. Hydrogen atoms were placed on calculated positions (C–Hmethyl = 0.96 Å and C–Hmethylenic = 0.97 Å riding on the parent atom (Ueq = 1.2). Complete crystallographic data for the structure of compounds reported in this paper have been deposited in the CIF format with the Cambridge Crystallographic Data Center as 2179317. These data can be obtained free of charge via http://www.ccdc.cam.ac.uk/conts/retrieving.html, deposited on 15 June 2022, (or from the CCDC, 12 Union Road, Cambridge CB2 1EZ, UK; Fax: +441223336033; E-mail: deposit@ccdc.cam.ac.uk).
2.5. Thermal Analysis (DSC)
Differential scanning calorimetry was conducted to estimate studies of association and polymorphism, thermotropic properties, and thermal behaviors of DMG-Am. DSC analyses were performed on a Discovery DSC250 (TA instruments, New Castle DE, USA). Samples weighing between 1 and 5 mg were heated in aluminum pans from 20 to 350 °C (10 °C·min−1) in argon (flow rate 10 mL·min−1). Dehydration, melting point, and decomposition of the synthesized compound were determined from the DSC experiments.
2.6. Neurobehavioral Studies
Mice (C57BL/6, male, 8 weeks old) were obtained from Erboj (Animal Breeding Center, Slivniza, Sofia). The animals were housed two per cage under constant laboratory conditions (25 ± 3 °C, 12/12 h light/dark cycle) with food and water available ad libitum. Habituation period was for 5 days before starting the experiment. The experimental protocol was in accordance to requirements of European Communities Council Directive (86/609/EEC).
Before conducting neurobehavioral studies, we performed experiments on 20 male 8-week-old C57BL/6 mice in order to evaluate the effective dose of DMG-Am, which was calculated to be 72 mg/kg i.p.
Mice were divided into four experimental groups (n = 10 in each group) as follows: (1) control, treated with normal saline (i.p.) for 12 consecutive days; (2) MPTP (30 mg/kg/day, i.p., applied daily for 5 consecutive days [23]; (3) MPTP+DMG-Am (72 mg/kg/day, i.p.) applied for 12 consecutive days, 5 days simultaneously with MPTP and 7 days after MPTP; and (4) DMG-Am (72 mg/kg/day i.p.) applied daily for 12 consecutive days.
2.6.1. Rotarod Test
Mice from all groups were placed on a gyratory with a fixed speed of 7 rpm/min, and the time on rotarod was determined. The observed period was 5 min. All animals were pre-trained on the rotarod apparatus before treatment in order to reach stable performance. The training consisted of one session per day in 3 consecutive days. The test was made on the 13th day, where the average time per group was calculated after the experiment and repeated four times [24].
2.6.2. Passive Avoidance Test
Learning and memory performance in mice was evaluated using passive avoidance learning test [25]. During the acquisition phase, each animal was placed in the illuminated compartment. When the rodents innately entered into the dark compartment, they received a mild electrical foot shock (0.5 mA, 3 s). In this trial, the initial latency (IL, acquisition latency time) of entrance into the dark chamber was recorded, and mice with ILs > 60 s were excluded from the study. The test phase was on the 13th and 14th days. Each mouse was placed in the illuminated chamber, and the entry into the dark chamber was measured as step through latency (STL). The behavioural observations were carried out between 9 a.m. to 12 a.m.
2.6.3. Statistical Analysis
The results were expressed as means ± the standard error of the mean (SEM) or as percentage changes over the mean compared to the control. Statistical analyses of the data were performed by one-way analysis of variance (ANOVA) followed by Dunnett post-hoc comparison test. Differences were considered significant at p < 0.05.
3. Results
The newly synthesized compound DMG-Am is fully characterized with MS, 1H and 13C-NMR in solution and by single crystal analysis powder diffraction and DSC.
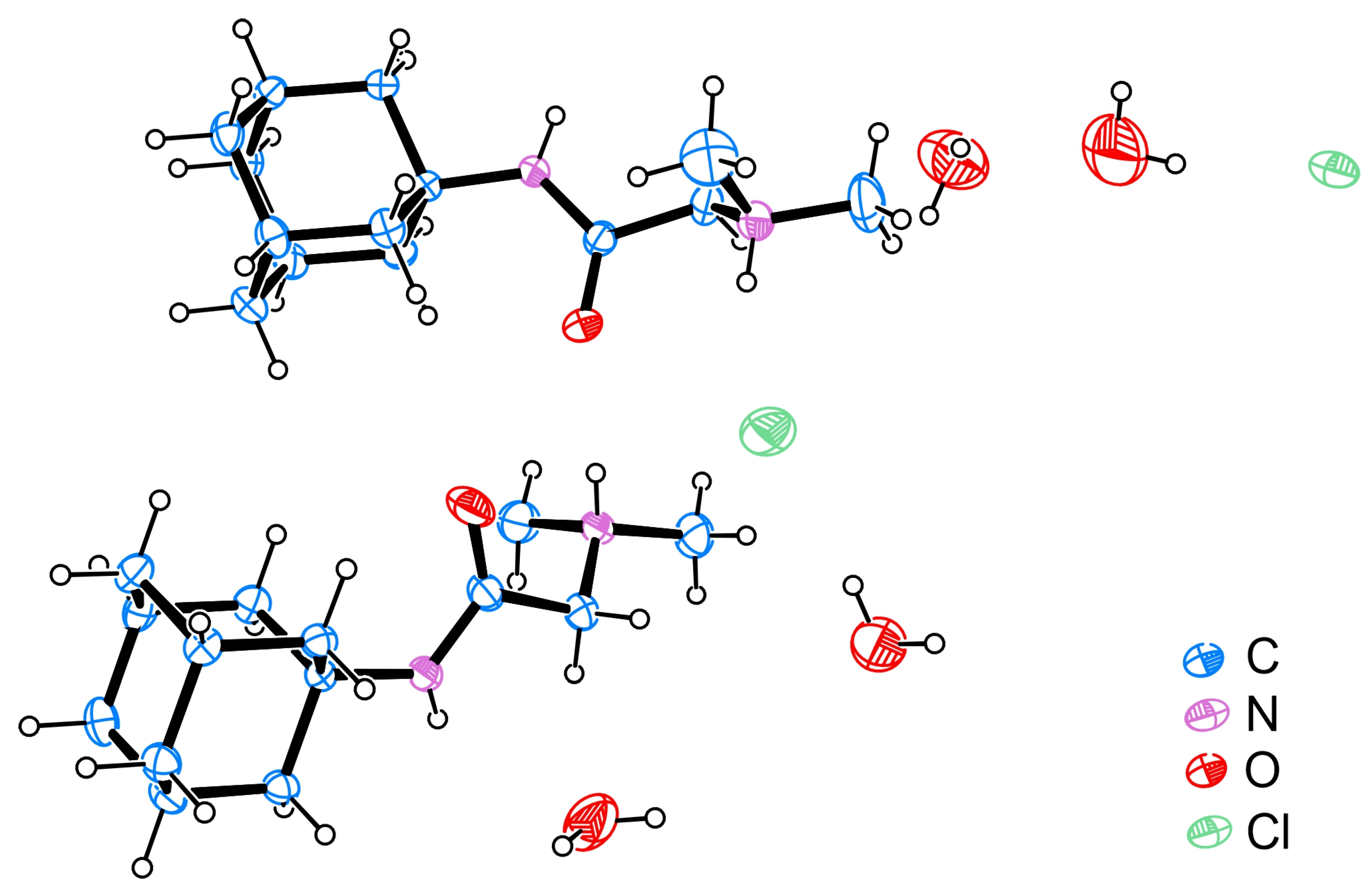
The DMG-Am crystallizes as dihydrate chlorine salt in the triclinic crystal system (Space group P-1), bearing two molecules in the asymmetric unit (ASU) (Table 1, Figure 1). The values for selected bond lengths and angles determined from the SCXRD experiment (Tables S1 and S2) are in agreement with those of other similar structures in the Cambridge structural database [26,27,28,29].

Table 1.
Most important data collection and crystallographic refinement parameters for DMG-Am, DMG, and DMG.hemihydrate and amantadine (Am).

Figure 1.
ORTEP [32] view of the molecules present in the asymmetric unit of DMG-Am; the thermal ellipsoids are drawn with 50% probability, hydrogen atoms are shown as small spheres with arbitrary radii.
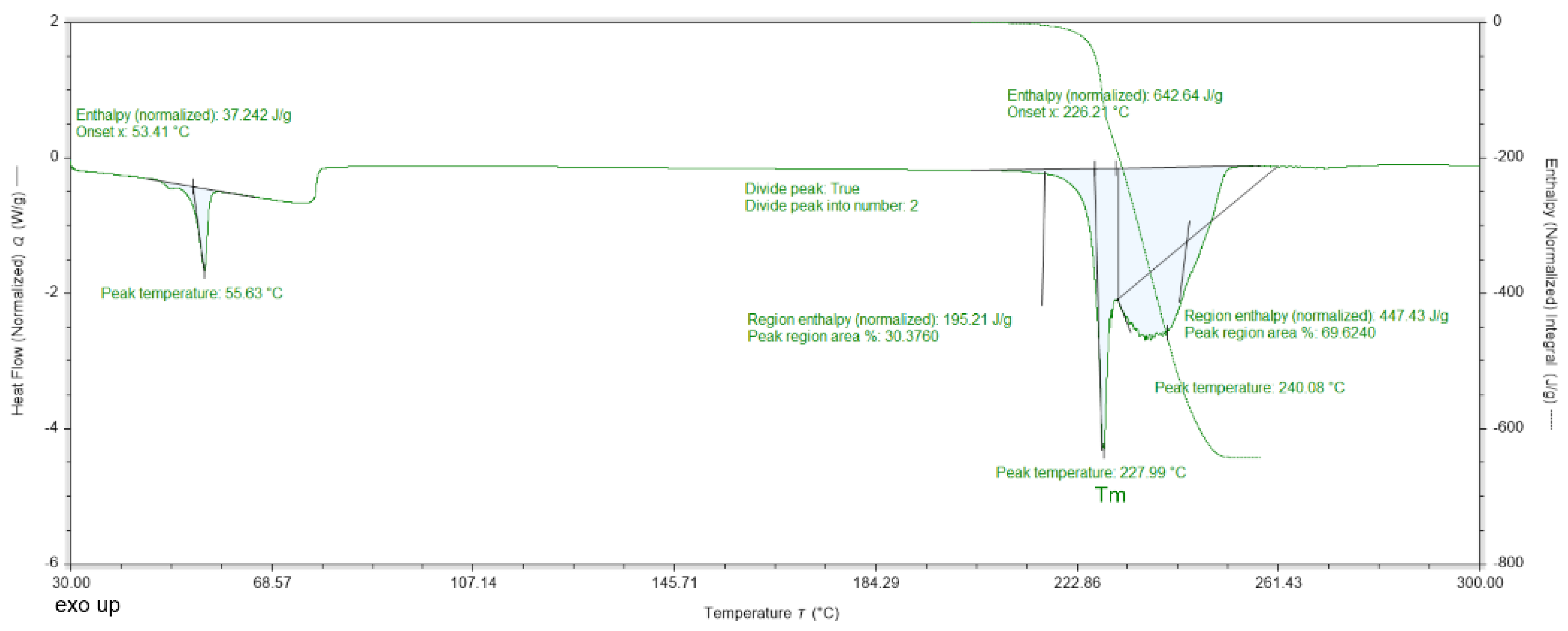
The DSC data for DMG-Am show three endothermic peaks (at 55, 228, and 240 °C, Figure 2). The first endothermic peak (55.63 °C) is associated with the release of water molecules. The second endothermic peak is associated with the melting of compound 1; the last peak is the decomposition. The second and third endothermic peaks with maxima at 228 °C and 240 °C, respectively, overlap, but the melting is observed as a sharp peak, while the decomposition is more prolonged. The DSC data for Am are shown in Figure 3.

Figure 2.
Graphical representation of the results from the DSC analyses for DMG-Am. The maximum of the endothermic peak, corresponding to the melting temperature (Tm) is denoted.

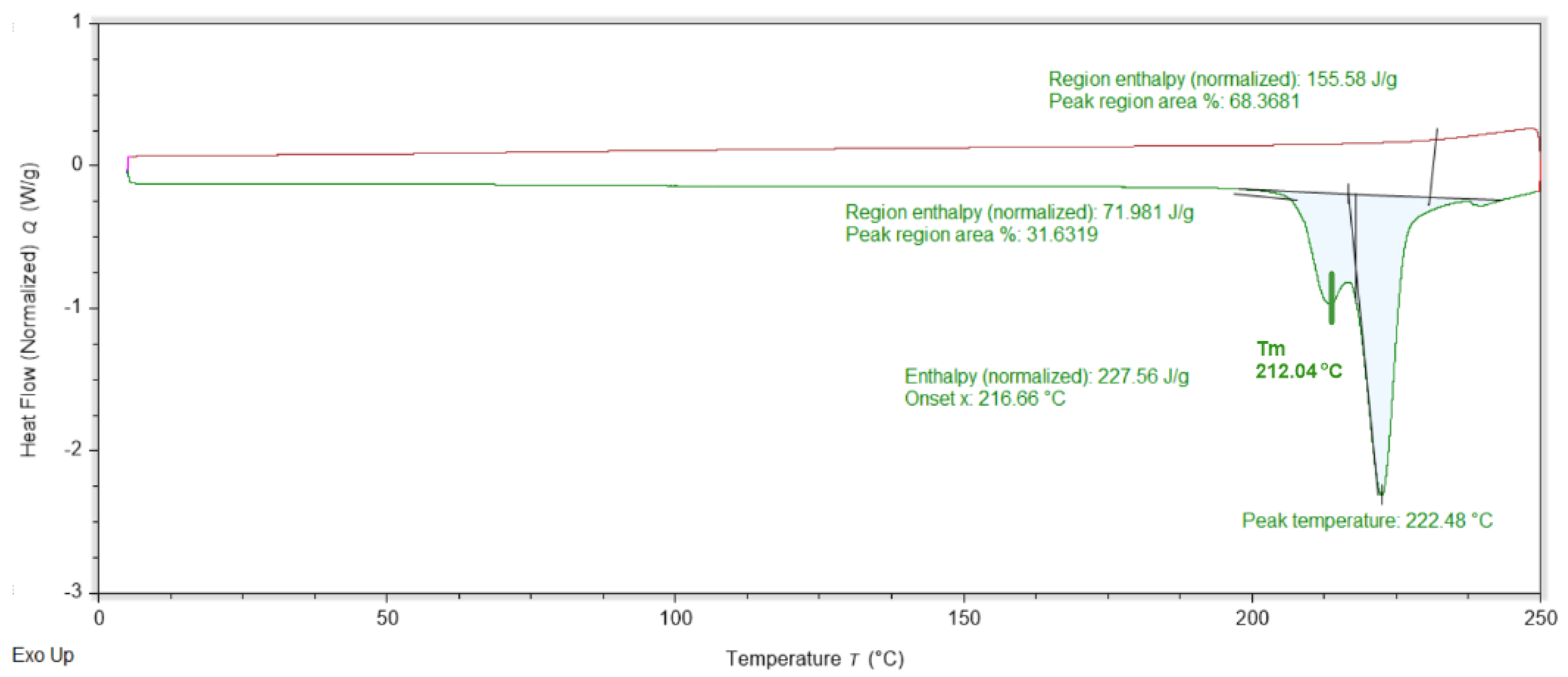
Figure 3.
Graphical representation of the results from the DSC analyses for amantadine.HCl. The maximum of the endothermic peak, corresponding to the melting temperature (Tm) is denoted followed by the decomposition peak. The red line corresponds to the cooling stage from 250 to 0 °C.
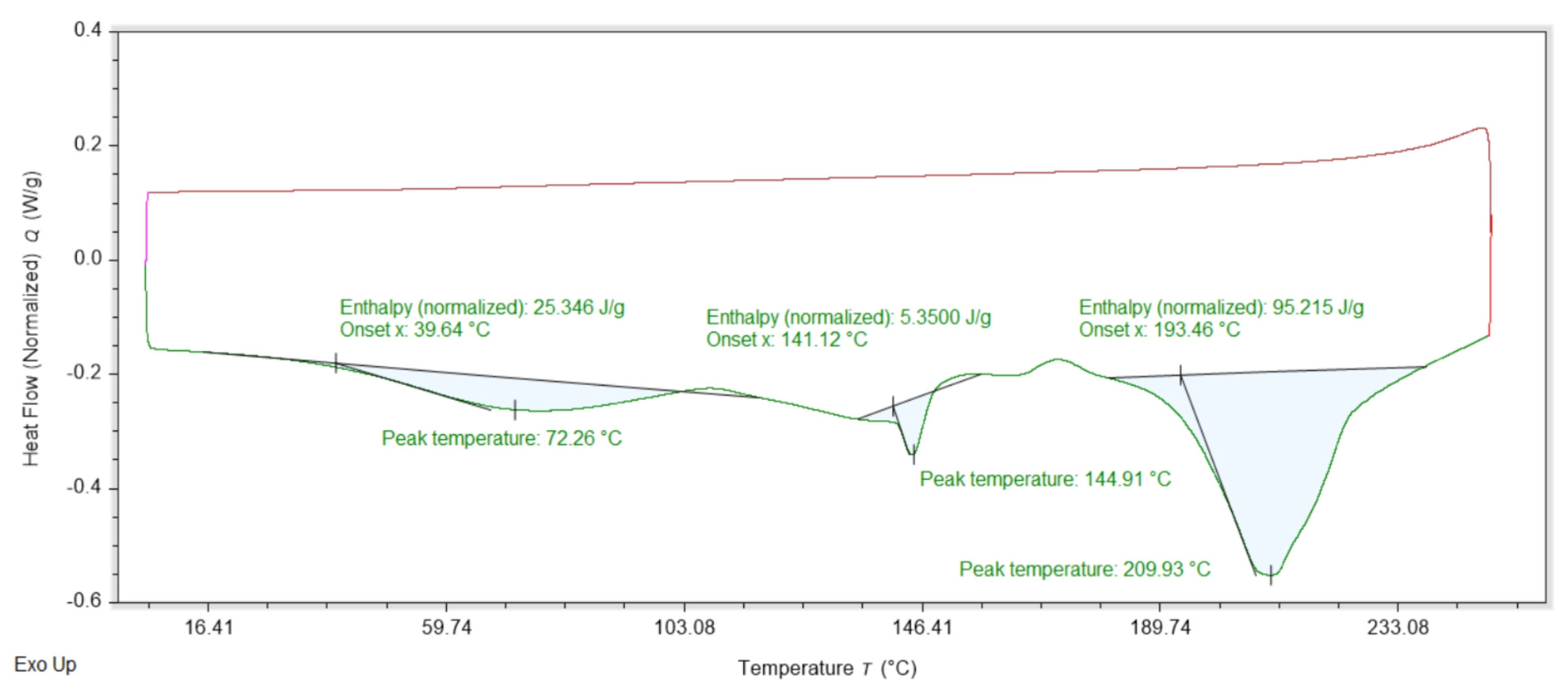
The DSC data for Am is shown on Figure 3. The heating stage discloses only two overlapping endothermic peaks at 212 and 222 °C. During the cooling back stage, no peaks were registered. The DSC data for DMG.0.5H2O is shown on Figure 4. Three endothermic peaks can be observed at 72, 144 and 196 °C. During the cooling stage no effects are registered.

Figure 4.
Graphical representation of the results from the DSC analyses for DMG.0.5H2O. The red line corresponds to the cooling stage from 250 to 0 °C.
In the present investigations we used subchronic PD regimen, developed by Tatton and Kish [33], which involves one injection of 30 mg/kg/free base MPTP (1-methyl-4–phenyl-1, 2, 3, 6-tetrahydropyridine) daily for five consecutive days. This regimen causes apoptosis and depletes striatal dopamine by 40–50% in adult C57/BL mice. The changes can be behaviourally evaluated. Rotarod and passive avoidance tests were employed. Passive avoidance tests were performed on the 13th and 14th day after the first MPTP treatment.
4. Discussion
The synthesis of DMG-Am features an amide bond formation realized according to method described by Knorr et al. [34] (Scheme 1). The reaction runs in very mild conditions at room temperature in DCM solution and inert environment for 24 h. Reaction progression is easily monitored by means of TLC in a system TCM:MeOH 4:1. The obtained chromatograms are visualized by Ninhydrin Reagent 2% Solution. At the end of the reaction time, the reaction mixture is filtered and washed with 5% NaHCO3 solution twice, then dried with anhydrous Na2SO4. DMG-Am was purified by flash chromatography on silica gel column with elution system TCM:MeOH 4:1 to obtain the final pure product with 88% yield.

Scheme 1.
Synthesis of DMG-amantadine.
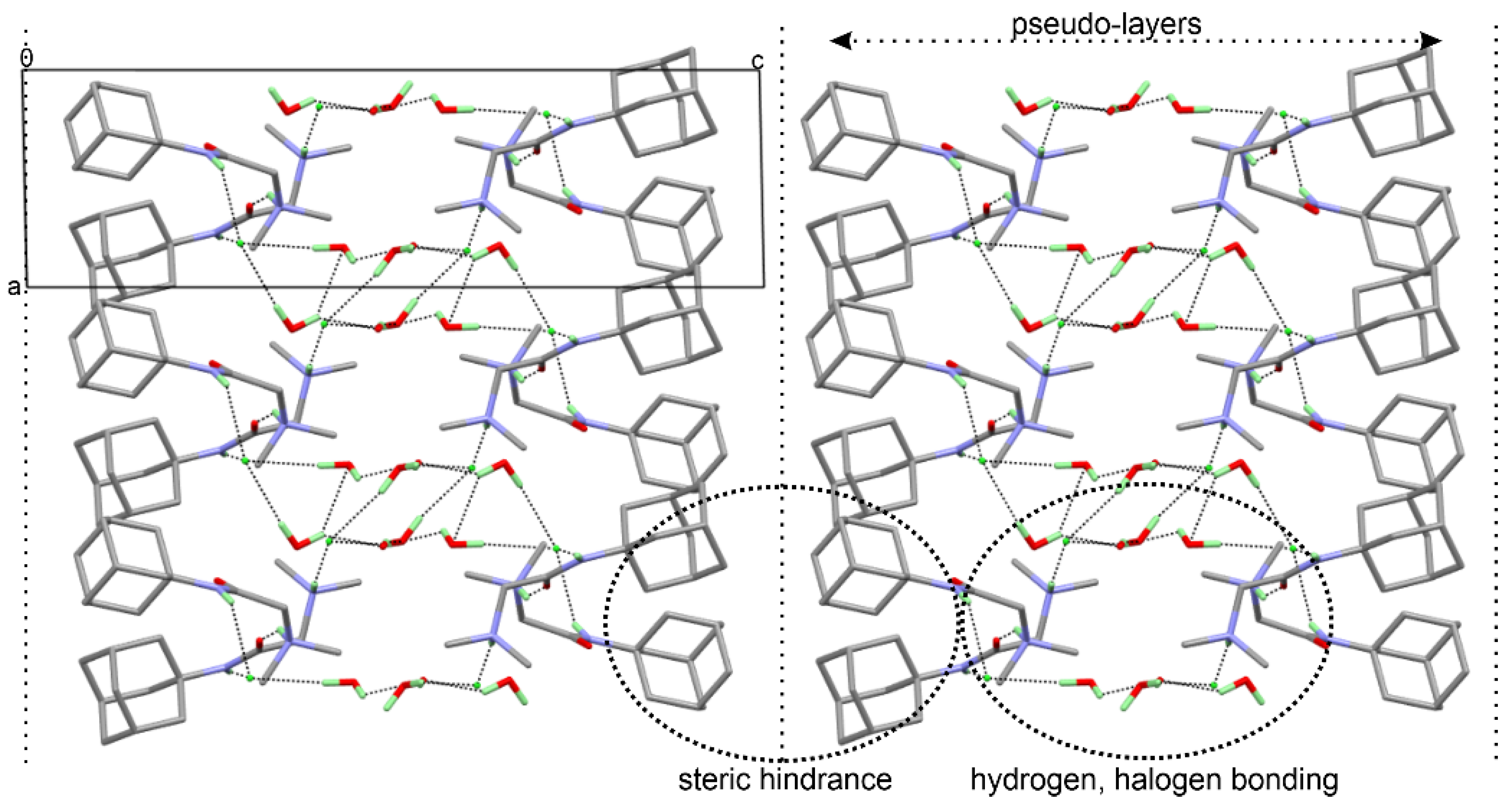
After the purification of DMG-Am, colourless single crystals of the title compound were obtained by a slow evaporation method from hot benzene. The crystal structure discloses that DMG-Am is actually a quaternary ammonium chlorine salt. Interestingly the N-H…Cl halogen interaction was different for the two molecules present in the ASU. In molecule 1, only the N71 nitrogen was involved in the halogen bonding (N71H71…Cl1, Table 2). In the second molecule, N72 interacted with Cl1 (N72H72…Cl1), but in addition, the second nitrogen N22 participated in the N22H22…Cl2 halogen bond. The analogical centre (N11) of DMG-Am molecule 1 was not involved in halogen bonding but as an alternative an N11-H11…O62 hydrogen bond was detected.

Table 2.
Detected hydrogen, halogen, and weak C-H…O bonding interaction in the crystal structure of DMG-Am.
The water molecules were involved in hydrogen bonding with Cl1 and Cl2 and produced some weak C-H…O interactions helping the stabilization of the crystal structure (Table 2). The three-dimensional arrangement of the of DMG-Am molecules shows the formation of pseudo-layers propagating along the c axis. The adamantly moieties are on the outside of the layers, while the inside of the layers features the water molecules, the chlorine, and the N,N-dimethylglycyl, e.g., the moieties involved in the halogen and hydrogen bonding interactions (Figure 5).

Figure 5.
View of the three-dimensional packing of the molecules along b direction of DMG-Am revealing the formation of pseudo-layers; hydrogen and halogen bonding interactions are shown as dotted lines.
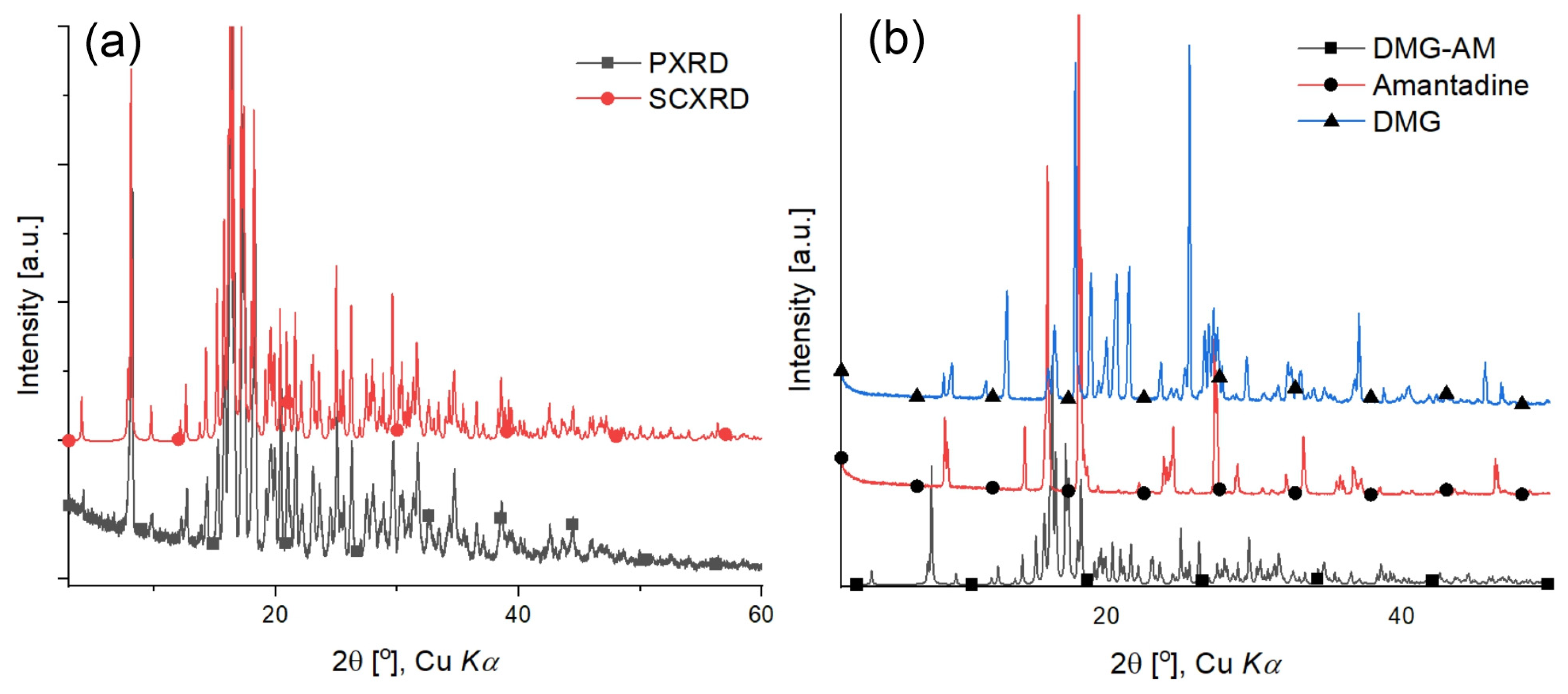
The powder diffraction pattern of the DMG-Am precipitate was compared with the generated from the SCXRD experiment (Figure 6a and Table S3). In both patterns, the position and intensities of the diffraction peaks correlate very well. No additional diffraction peaks were observed in the PXRD. The PXRD of the synthesized DMG-Am and starting compounds Am and DMG.0.5H2O are shown on Figure 6b. Based on the peak positions and intensities, it is clearly noticeable that the patterns differ. Thus, one can exclude the presence of additional phases, polymorphs, or impurities in the purified DMG-Am.

Figure 6.
Comparison of: (a) the Powder X-ray diffraction pattern (red) versus single crystal X-ray diffraction pattern (generated via Mercury software [35], blue) for DMG-Am; and (b) comparison of the PXRD of the starting compounds AM and DMG and DMG-Am.
The DSC data for DMG-Am show three endothermic peaks. The first endothermic peak starts around 30 °C, has a distinct maximum at 55.63 °C and finishes about 75 °C. It is associated with the release of the four water molecules present in the crystal structure. The second and third endothermic peaks with maxima at 228 °C and 240 °C respectively, are related to the concurrent melting and decomposition of DMG-Am. (for the employed heating rate of 10 °C·min−1).
The PXRD of the employed amantadine collected at room temperature is consistent with that of the monoclinic C2/c polymorph form (Figure S1, [31]). Accordingly, the DSC data for amantadine shown on Figure 3 will be interpreted as the monoclinic C2/c high temperature polymorph form of amantadine.HCl [36]. The DSC starting temperature was set at 0 °C (273 K), e.g., the sample is initially cooled down from room temperature to 0 °C in the DSC and kept at this temperature for 10 min (isothermal equilibration). Upon heating, the DSC data do not register a conversion/transition from one polymorph form to another, e.g., the monoclinic form is conserved. This is consistent with the SCXRD data collected at −130 °C (143 K) also disclosing C2/c space group. The endothermic peaks at 212 and 222 °C are the melting and decomposition of Am. This is supported by the cooling back from 250 to 0 °C DSC data as peaks are registered, e.g., the decomposition is irreversible.
The PXRD of the employed DMG shows a hemihydrate, not an anhydrous form (Figure S2). The DSC data for DMG.0.5H2O are shown on Figure 4 and reveal three endothermic peaks. The first endothermic peak is relatively broad with a maximum at 72 °C and is related to the release of the water molecule. The last endothermic peak is the decomposition of the DMG. The decomposition is also irreversible as peaks are not registered upon cooling of the sample from 250 to 0 °C. The endothermic peak at 144 °C may be linked to the melting of the DMG.
The characterization of the compound DMG-Am reveals high purity and stability, suggesting that it is suitable for biological use at ambient conditions.
MPTP is a widely used neurotoxin as an experimental model which mimics some Parkinson‘s disease-like symptoms. It is a potent, highly lipophilic agent with capability to cross the blood–brain barrier rapidly after systemic administration. MPTP with its active toxic compound (MPP+) inflicts selective degeneration of dopaminergic neurons projecting from substantia nigra pars compacta (SNpc) into striatum [37]. The selective loss of dopaminergic neurons in CNS appears to be the direct cause of neurodegeneration in patients with PD [38]. To date, very little is known why and how the neurodegenerative process begins and progresses, and there are few therapeutic options available for treatment of PD. However, the decrease in striatal DAergic innervation in PD is responsible for the motor disturbances characteristic of the disease, such as akinesia, muscular rigidity, and tremor. It is therefore essential to search for new neuroprotective strategies.
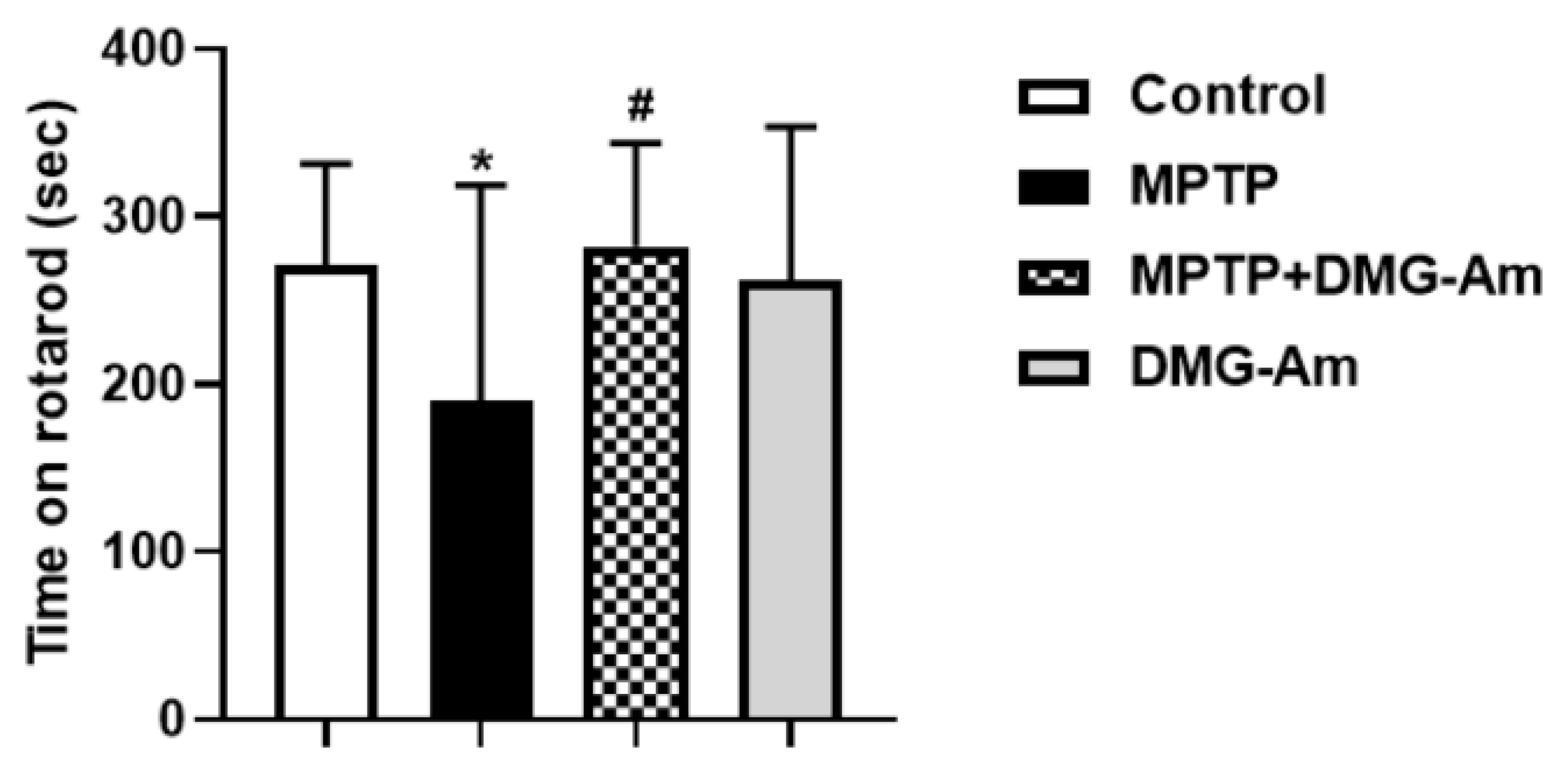
From the rotarod test, the average rotating beam time for the control group was 271.6 s, while for the MPTP group it was 189.6 s, which is 30% less than control values (p < 0.05) and means worsened neuromuscular coordination in the MPTP-treated group as compared to the control (Figure 7). In the MPTP group treated with DMG-Am (72 mg/kg, i.p.), mice spent significantly longer time (an increase of 49%) on the rotating beam compared to MPTP (p < 0.05). In DMG-Am-treated animals there were no significant differences with the control group of mice (Figure 7).

Figure 7.
Effect of DMG-Am on neuromuscular coordination. Asterisks above bars indicate significant differences in number of falls per minute for each experimental group versus control at * p < 0.05. Hashtags above bars indicate significant difference in number of falls per minute versus the MPTP-treated group at # p < 0.05. Statistical analysis was performed by one-way analysis of variance (ANOVA) followed by Dunnett’s post hoc comparison test.
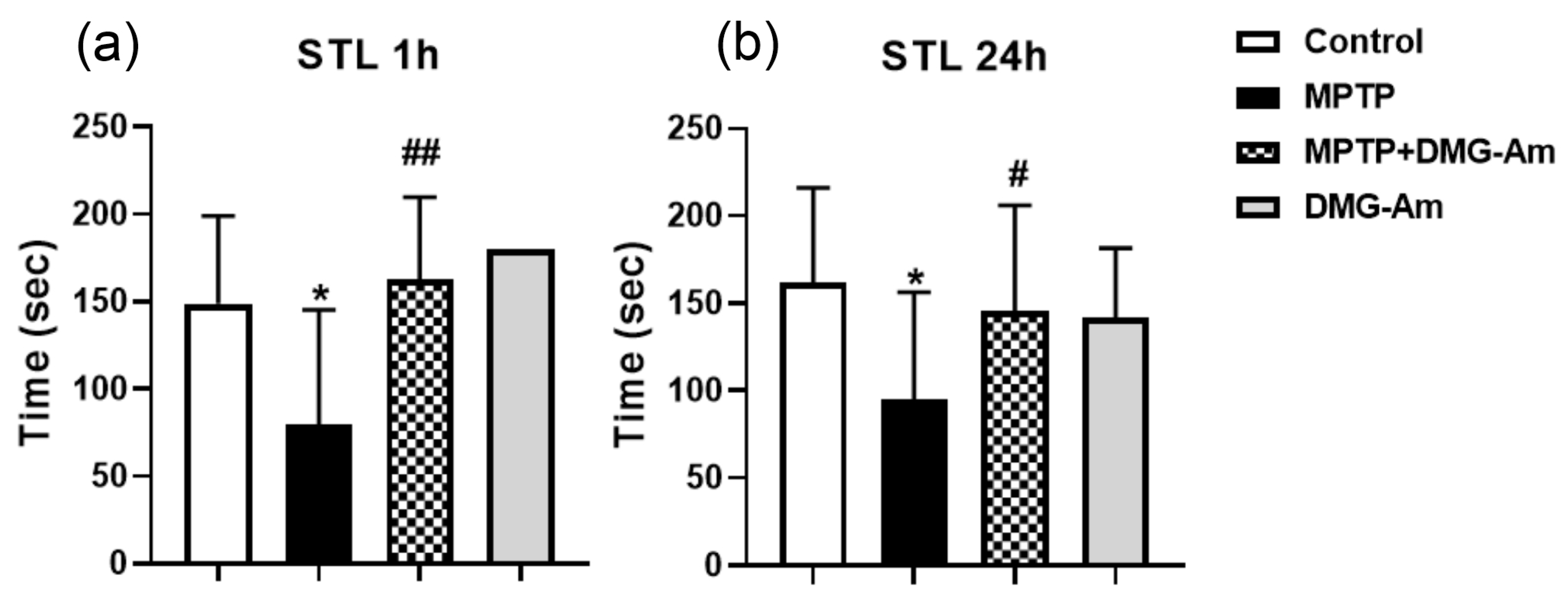
The passive avoidance test performed on the 13th and 14th day after the first MPTP treatment showed that step-through latency (STL) time in the MPTP group was significantly reduced compared to controls. One hour after the acquisition phase, the reduction was by 47% (p < 0.01), and by 41% (p < 0.01) 24 h after training (Figure 8). In the MPTP group treated with DMG-Am, SLT was restored to the control levels, both 1 h (p < 0.01) and 24 h (p < 0.01) after acquisition. In DMG-Am-treated animals there was no significant difference to the control group STL time (Figure 8).

Figure 8.
Effect of DMG-Am on step-through latency (STL) in the single-trial passive avoidance test in a mice model of PD: (a) STL time 1 h; and (b) STL time 24 h. Significance vs. control group: * p < 0.05; significance vs. MPTP-treated group # p < 0.05; ## p < 0.01. Statistical analysis was performed by one-way analysis of variance (ANOVA), followed by Dunnett’s post hoc comparison test.
Our results showed that the systemic administration of MPTP worsened the motor and memory performance of the animals in the behaviour test used. This manifests itself as less time spent on the gyratory in the rotarod test and reduction of STL 1 h and 24 h after the acquisition phase in the passive avoidance test, an indication for memory lost effect.
The neuroprotective capacity of the new amantadine derivative DMG-Am was evaluated by its potential to ameliorate the memory performance and neuromuscular coordination in an experimental model of Parkinson’s disease. Our experimental results showed that DMG-Am applied for 12 consecutive days, 5 days simultaneously and 7 days after MPTP, restored motor and memory performance of the animals to the control level, an indication of the beneficial protective effect of this compound. In the DMG-Am-treated group, the motor and memory behaviour of the animals were as in the control group.
5. Conclusions
A new amantadine derivative was synthesized and characterized as a potential antiparkinsonian drug. An experimental model of Parkinson’s disease was simulated in mice by treatment with MPTP. The studied biological activity in vivo showing that DMG-amantadine restored neuromuscular coordination and memory performance of the animals treated with MPTP to the level of the control group of animals could represent an attractive strategy for the treatment of this disease.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/cryst12091227/s1, Table S1: Observed Bond lengths for DMG-Am; Table S2: Observed Bond angles for DMG-Am. Table S3. Tabular form of the values from DMG-Am observed characteristic PXRD peaks; values are from derivation from pure Kα1 (odd) and Kα2 (even) using profile fitting.
Author Contributions
Formal analysis, I.S.; neurobehavioral investigations (rotarod test, passive avoidance test), R.K., L.T. and M.L.; synthesis (NMR), R.C.; crystal growth, H.S.-D.; powder XRD, B.S.; single crystal, H.S.-D., B.S., differential scanning calorimetry, and H.S.-D.; methodology, I.S., B.S. and R.C.; software, B.S.; writing—original draft, I.S., B.S., R.K. and H.S.-D. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Bulgarian National Science Fund (BNSF), grant number KP-06-Russia/7-2019.
Institutional Review Board Statement
The animal study protocol was approved by the Commission of Bioethics (CBE) at the Institute of Neurobiology, Bulgarian Academy of Sciences —BAS/CBE/018/2020.
Informed Consent Statement
Not applicable.
Data Availability Statement
Complete crystallographic data for the structure of DMG-Am reported in this paper have been deposited in the CIF format with the Cambridge Crystallographic Data Center as 2179317. These data can be obtained free of charge via http://www.ccdc.cam.ac.uk/conts/retrieving.html, deposited on 15 June 2022 (or from the CCDC, 12 Union Road, Cambridge CB2 1EZ, UK; Fax: +441223336033; E-mail: deposit@ccdc.cam.ac.uk).
Acknowledgments
We are grateful for the financial support from South-West University “Neofit Rilski”.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Hauser, R.A.; Walsh, R.R.; Pahwa, R.; Chernick, D.; Formella, A.E. Amantadine ER (Gocovri®) Significantly Increases on Time without Any Dyskinesia: Pooled Analyses from Pivotal Trials in Parkinson’s Disease. Front. Neurol. 2021, 12, 645706. [Google Scholar] [CrossRef] [PubMed]
- Kong, M.; Ba, M.; Ren, C.; Yu, L.; Dong, S.; Yu, G.; Liang, H. An updated meta-analysis of amantadine for treating dyskinesia in Parkinson’s disease. Oncotarget 2017, 8, 57316. [Google Scholar] [CrossRef] [PubMed]
- Poewe, W.; Mahlknecht, P. Pharmacologic treatment of motor symptoms associated with Parkinson disease. Neurol. Clin. 2020, 38, 255–267. [Google Scholar] [CrossRef] [PubMed]
- Oertel, W.; Eggert, K.; Pahwa, R.; Tanner, C.M.; Hauser, R.A.; Trenkwalder, C.; Ehret, R.; Azulay, J.P.; Isaacson, S.; Felt, L. Randomized, placebo-controlled trial of ADS-5102 (amantadine) extended-release capsules for levodopa-induced dyskinesia in Parkinson’s disease (EASE LID 3). Mov. Disord. 2017, 32, 1701–1709. [Google Scholar] [CrossRef]
- Pahwa, R.; Tanner, C.M.; Hauser, R.A.; Isaacson, S.H.; Nausieda, P.A.; Truong, D.D.; Agarwal, P.; Hull, K.L.; Lyons, K.E.; Johnson, R. ADS-5102 (amantadine) extended-release capsules for levodopa-induced dyskinesia in Parkinson disease (EASE LID study): A randomized clinical trial. JAMA Neurol. 2017, 74, 941–949. [Google Scholar] [CrossRef]
- Rezaei, M.; Alirezaei, M. Protective effects of Althaea officinalis L. extract in 6-hydroxydopamine-induced hemi-Parkinsonism model: Behavioral, biochemical and histochemical evidence. J. Physiol. Sci. 2014, 64, 171–176. [Google Scholar] [CrossRef]
- Fahn, S. Levodopa-induced neurotoxicity. CNS Drugs 1997, 8, 376–393. [Google Scholar] [CrossRef]
- Shulman, L.M. Levodopa toxicity in Parkinson disease: Reality or myth?: Reality—practice patterns should change. Arch. Neurol. 2000, 57, 406–408. [Google Scholar] [CrossRef]
- Weiner, W.J. Is levodopa toxic? Arch. Neurol. 2000, 57, 408–410. [Google Scholar] [CrossRef]
- Graham, D.G. Oxidative pathways for catecholamines in the genesis of neuromelanin and cytotoxic quinones. Mol. Pharmacol. 1978, 14, 633–643. [Google Scholar]
- Tse, D.C.; McCreery, R.L.; Adams, R.N. Potential oxidative pathways of brain catecholamines. J. Med. Chem. 1976, 19, 37–40. [Google Scholar] [CrossRef]
- Jenner, P. Oxidative stress in Parkinson’s disease. Ann. Neurol. 2003, 53, S26–S38. [Google Scholar] [CrossRef] [PubMed]
- Holm, P.I.; Ueland, P.M.; Kvalheim, G.; Lien, E.A. Determination of choline, betaine, and dimethylglycine in plasma by a high-throughput method based on normal-phase chromatography–tandem mass spectrometry. Clin. Chem. 2003, 49, 286–294. [Google Scholar] [CrossRef]
- Friesen, R.W.; Novak, E.M.; Hasman, D.; Innis, S.M. Relationship of dimethylglycine, choline, and betaine with oxoproline in plasma of pregnant women and their newborn infants. J. Nutr. 2007, 137, 2641–2646. [Google Scholar] [CrossRef]
- Luka, Z.; Pakhomova, S.; Loukachevitch, L.V.; Newcomer, M.E.; Wagner, C. Folate in demethylation: The crystal structure of the rat dimethylglycine dehydrogenase complexed with tetrahydrofolate. Biochem. Biophys. Res. Commun. 2014, 449, 392–398. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bai, K.; Xu, W.; Zhang, J.; Kou, T.; Niu, Y.; Wan, X.; Zhang, L.; Wang, C.; Wang, T. Assessment of free radical scavenging activity of dimethylglycine sodium salt and its role in providing protection against lipopolysaccharide-induced oxidative stress in mice. PLoS ONE 2016, 11, e0155393. [Google Scholar] [CrossRef] [PubMed]
- Palanimuthu, D.; Wu, Z.; Jansson, P.J.; Braidy, N.; Bernhardt, P.V.; Richardson, D.R.; Kalinowski, D.S. Novel chelators based on adamantane-derived semicarbazones and hydrazones that target multiple hallmarks of Alzheimer’s disease. Dalton Trans. 2018, 47, 7190–7205. [Google Scholar] [CrossRef] [PubMed]
- Lees, A. Alternatives to levodopa in the initial treatment of early Parkinson’s disease. Drugs Aging 2005, 22, 731–740. [Google Scholar] [CrossRef]
- Kouatly, O.; Geronikaki, A.; Kamoutsis, C.; Hadjipavlou-Litina, D.; Eleftheriou, P. Adamantane derivatives of thiazolyl-N-substituted amide, as possible non-steroidal anti-inflammatory agents. Eur. J. Med. Chem. 2009, 44, 1198–1204. [Google Scholar] [CrossRef]
- Rigaku Oxford Diffraction, CrysAlis pro. 2022. Available online: https://www.rigaku.com/products/crystallography/crysalis (accessed on 26 August 2022).
- Sheldrick, G.M. SHELXT–Integrated space-group and crystal-structure determination. Acta Crystallogr. Sect. A Found. Adv. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Sheldrick, G. SHELXL-97. Program for Crystal-Structure Refinement; ScienceOpen, Inc.: Burlington, MA, USA, 1997. [Google Scholar]
- Shin, K.S.; Zhao, T.T.; Choi, H.S.; Hwang, B.Y.; Lee, C.K.; Lee, M.K. Effects of gypenosides on anxiety disorders in MPTP-lesioned mouse model of Parkinson׳ s disease. Brain Res. 2014, 1567, 57–65. [Google Scholar] [CrossRef]
- Manna, S.; Bhattacharyya, D.; Mandal, T.; Dey, S. Neuropharmacological effects of deltamethrin in rats. J. Vet. Sci. 2006, 7, 133–136. [Google Scholar] [CrossRef] [PubMed]
- Jarvik, M.; Kopp, R. An improved one-trial passive avoidance learning situation. Psychol. Rep. 1967, 21, 221–224. [Google Scholar] [CrossRef] [PubMed]
- Basarić, N.; Molčanov, K.; Matković, M.; Kojić-Prodić, B.; Mlinarić-Majerski, K. Adamantane-retropeptides, new building blocks for molecular channels. Tetrahedron 2007, 63, 7985–7996. [Google Scholar] [CrossRef]
- Cabildo, P.; Claramunt, R.M.; Sanz, D.; Foces-Foces, M.C.; Cano, F.H.; Fayet, J.P.; Vertut, M.C.; Elguero, J. Adamantylazoles. 5. The molecular structure of 1-(1-adamantyl)pyrazoles. J. Heterocycl. Chem. 1986, 23, 1045–1050. [Google Scholar] [CrossRef]
- Villhauer, E.B.; Brinkman, J.A.; Naderi, G.B.; Burkey, B.F.; Dunning, B.E.; Prasad, K.; Mangold, B.L.; Russell, M.E.; Hughes, T.E. 1-[[(3-Hydroxy-1-adamantyl)amino]acetyl]-2-cyano-(S)-pyrrolidine: A Potent, Selective, and Orally Bioavailable Dipeptidyl Peptidase IV Inhibitor with Antihyperglycemic Properties. J. Med. Chem. 2003, 46, 2774–2789. [Google Scholar] [CrossRef]
- Kelly, R.P.; Falcone, M.; Lamsfus, C.A.; Scopelliti, R.; Maron, L.; Meyer, K.; Mazzanti, M. Metathesis of a UV imido complex: A route to a terminal UV sulfide. Chem. Sci. 2017, 8, 5319–5328. [Google Scholar] [CrossRef]
- Minkov, V.S.; Boldyreva, E.V. The effect of partial methylation of the glycine amino group on crystal structure in N, N-dimethylglycine and its hemihydrate. Acta Crystallogr. Sect. C Cryst. Struct. Commun. 2012, 68, o283–o287. [Google Scholar] [CrossRef]
- Bélanger-Gariépy, F.; Brisse, F.; Harvey, P.; Butler, I.; Gilson, D. Structure of adamantanamine hydrochloride at 143 K. Acta Crystallogr. Sect. C Cryst. Struct. Commun. 1987, 43, 756–759. [Google Scholar] [CrossRef]
- Farrugia, L.J. WinGX and ORTEP for Windows: An update. J. Appl. Crystallogr. 2012, 45, 849–854. [Google Scholar] [CrossRef]
- Tatton, N.; Kish, S. In situ detection of apoptotic nuclei in the substantia nigra compacta of 1-methyl-4-phenyl-1, 2, 3, 6-tetrahydropyridine-treated mice using terminal deoxynucleotidyl transferase labelling and acridine orange staining. Neuroscience 1997, 77, 1037–1048. [Google Scholar] [CrossRef]
- Knorr, R.; Trzeciak, A.; Bannwarth, W.; Gillessen, D. New coupling reagents in peptide chemistry. Tetrahedron Lett. 1989, 30, 1927–1930. [Google Scholar] [CrossRef]
- Macrae, C.F.; Sovago, I.; Cottrell, S.J.; Galek, P.T.; McCabe, P.; Pidcock, E.; Platings, M.; Shields, G.P.; Stevens, J.S.; Towler, M. Mercury 4.0: From visualization to analysis, design and prediction. J. Appl. Crystallogr. 2020, 53, 226–235. [Google Scholar] [CrossRef] [PubMed]
- Bazyleva, A.B.; Blokhin, A.V.; Kabo, A.G.; Kabo, G.J.; Emel’yanenko, V.N.; Verevkin, S.P. Thermodynamic properties of 1-aminoadamantane. J. Chem. Thermodyn. 2008, 40, 509–522. [Google Scholar] [CrossRef]
- Smeyne, R.J.; Jackson-Lewis, V. The MPTP model of Parkinson’s disease. Mol. Brain Res. 2005, 134, 57–66. [Google Scholar] [CrossRef]
- Schapira, A.H.; Jenner, P. Etiology and pathogenesis of Parkinson’s disease. Mov. Disord. 2011, 26, 1049–1055. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).