A Rare Structural Motif for a Luminescent Cu(I) Coordination Polymer with 3-(Pyridin-2-yl)triimidazotriazine
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis of [Cu2I2(TT-Py)]n (1)
2.2. Crystal Structure Analysis
2.3. Theoretical Methods
2.4. Photophysical Characterization
3. Results
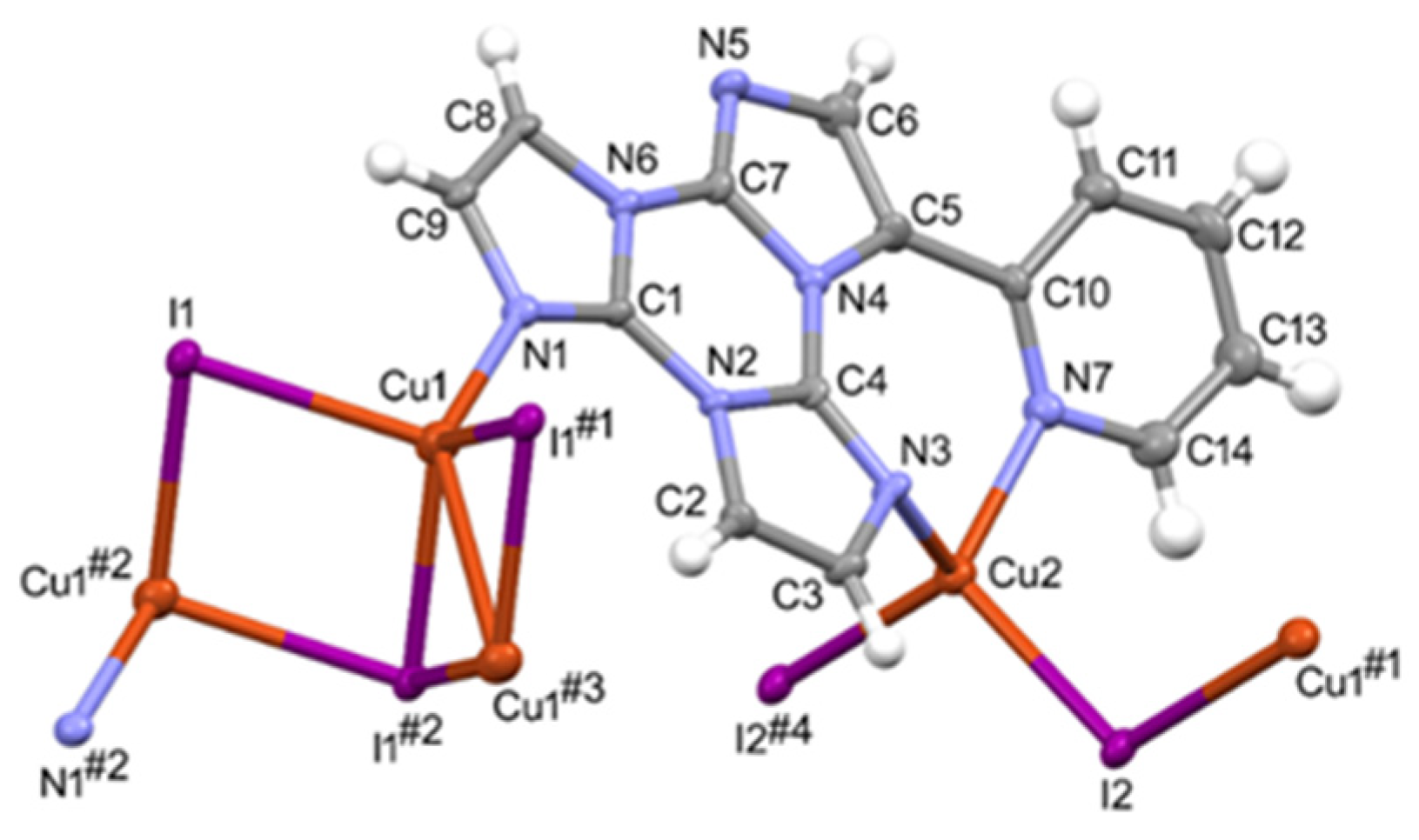
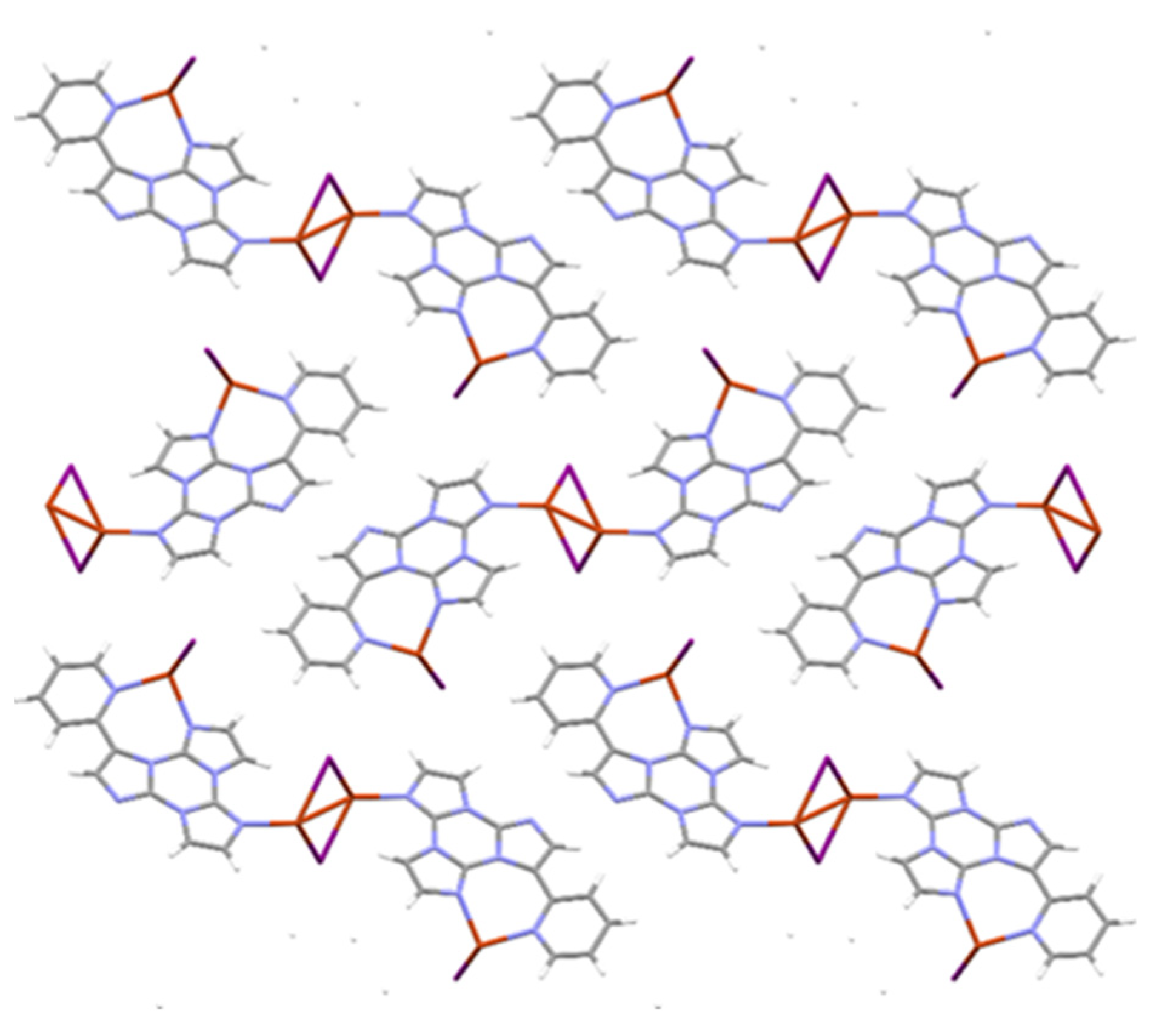
3.1. Crystal Structure Characterization
3.2. X-ray Powder Diffraction (XRPD) and Thermal Analysis
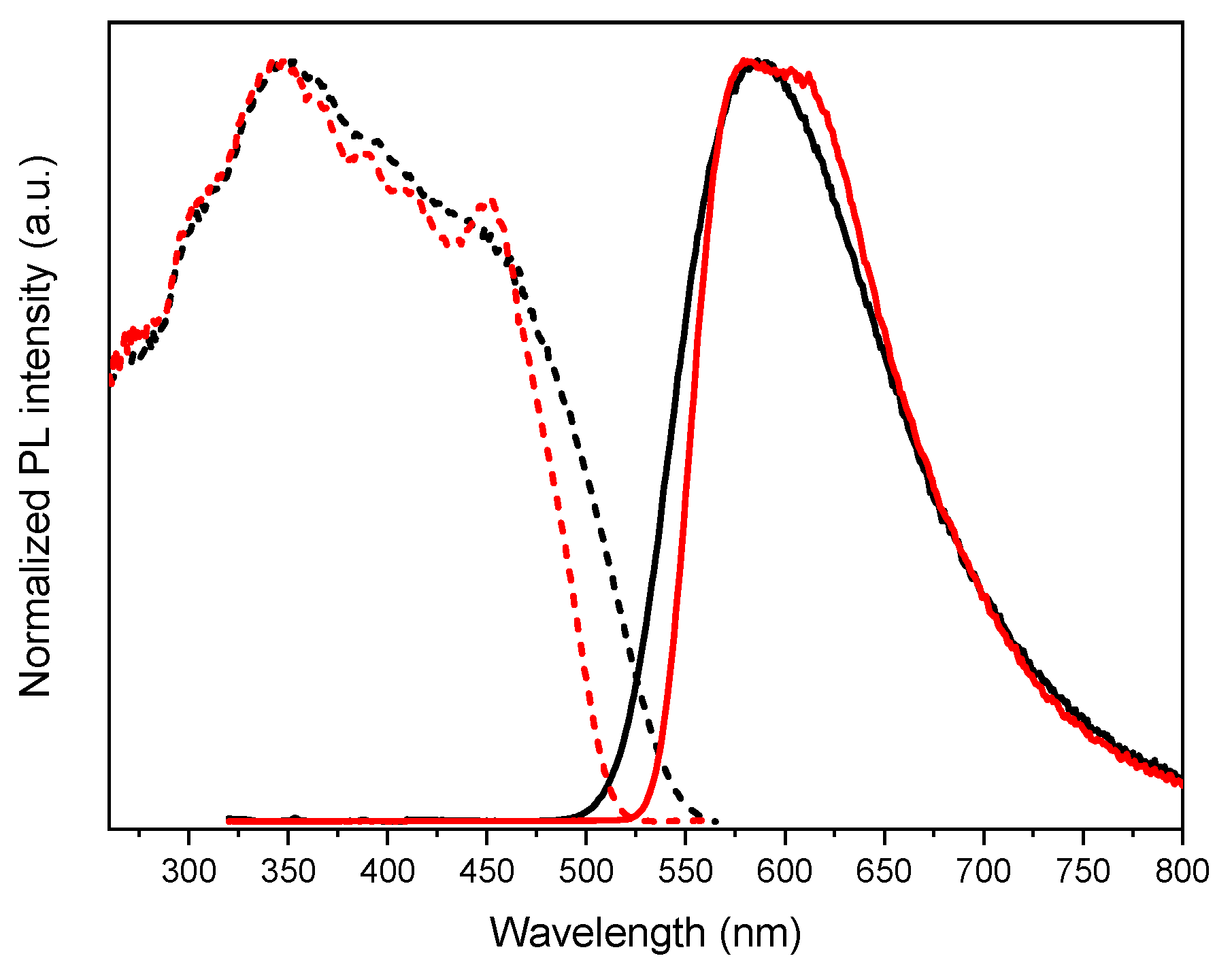
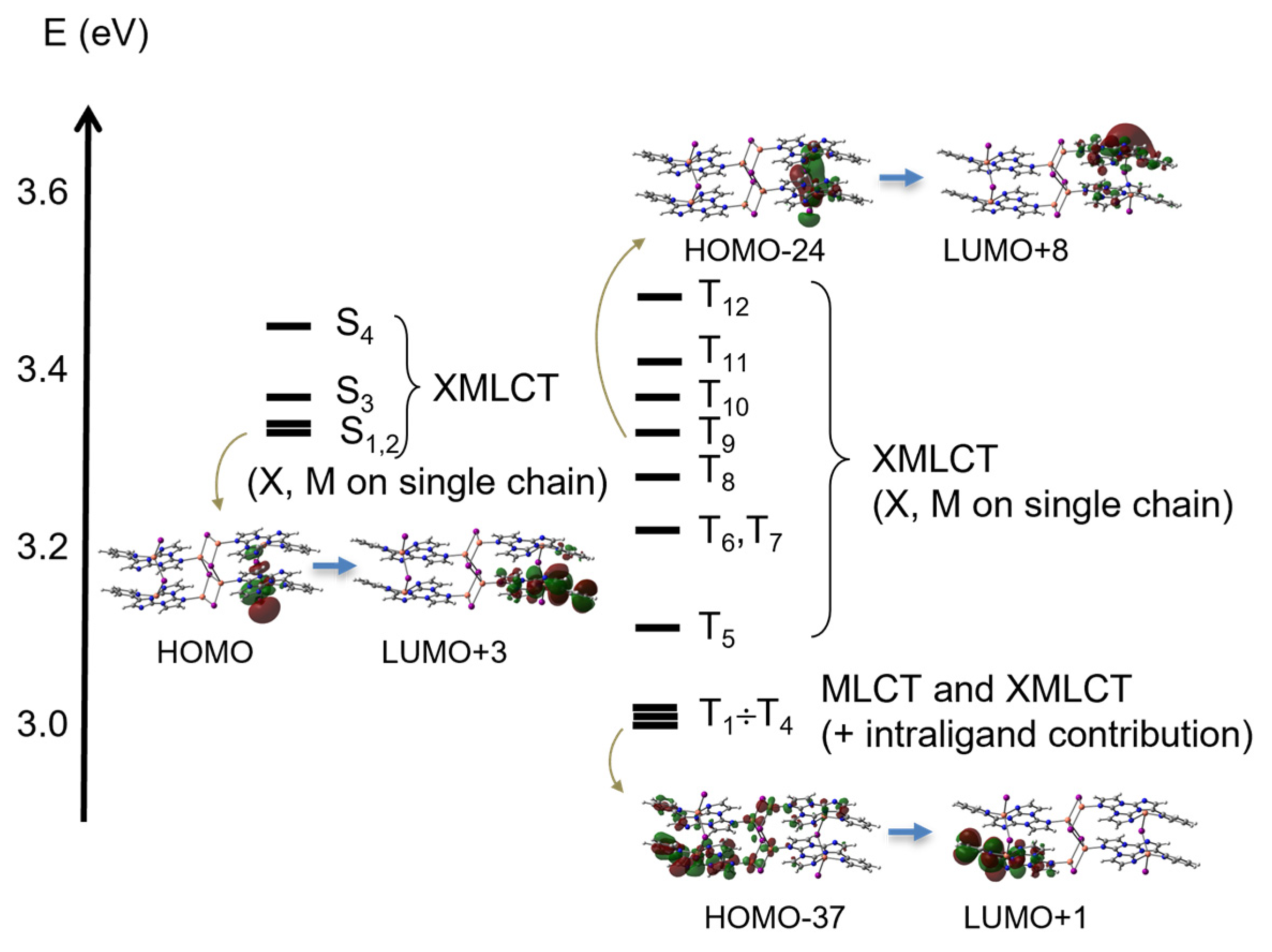
3.3. Photophysical Characterization and Theoretical Investigation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References and Note
- Kobayashi, A.; Kato, M. Stimuli-Responsive Luminescent Copper(I) Complexes for Intelligent Emissive Devices. Chem. Lett. 2016, 46, 154–162. [Google Scholar] [CrossRef]
- Czerwieniec, R.; Leitl, M.J.; Homeier, H.H.H.; Yersin, H. Cu(I) Complexes—Thermally Activated Delayed Fluorescence. Photophysical Approach and Material Design. Coord. Chem. Rev. 2016, 325, 2–28. [Google Scholar] [CrossRef]
- Cariati, E.; Lucenti, E.; Botta, C.; Giovanella, U.; Marinotto, D.; Righetto, S. Cu(I) Hybrid Inorganic–Organic Materials with Intriguing Stimuli Responsive and Optoelectronic Properties. Coord. Chem. Rev. 2016, 306, 566–614. [Google Scholar] [CrossRef]
- Tsuge, K.; Chishina, Y.; Hashiguchi, H.; Sasaki, Y.; Kato, M.; Ishizaka, S.; Kitamura, N. Luminescent Copper(I) Complexes with Halogenido-Bridged Dimeric Core. Coord. Chem. Rev. 2016, 306, 636–651. [Google Scholar] [CrossRef]
- Dumur, F. Recent Advances in Organic Light-Emitting Devices Comprising Copper Complexes: A Realistic Approach for Low-Cost and Highly Emissive Devices? Org. Electron. 2015, 21, 27–39. [Google Scholar] [CrossRef]
- Singha, S.; Kim, D.; Seo, H.; Cho, S.W.; Ahn, K.H. Fluorescence Sensing Systems for Gold and Silver Species. Chem. Soc. Rev. 2015, 44, 4367–4399. [Google Scholar] [CrossRef]
- Hamze, R.; Peltier, J.L.; Sylvinson, D.; Jung, M.; Cardenas, J.; Haiges, R.; Soleilhavoup, M.; Jazzar, R.; Djurovich, P.I.; Bertrand, G.; et al. Eliminating Nonradiative Decay in Cu(I) Emitters: >99% Quantum Efficiency and Microsecond Lifetime. Science 2019, 363, 601–606. [Google Scholar] [CrossRef]
- Yam, V.W.-W.; Au, V.K.-M.; Leung, S.Y.-L. Light-Emitting Self-Assembled Materials Based on d8 and d10 Transition Metal Complexes. Chem. Rev. 2015, 115, 7589–7728. [Google Scholar] [CrossRef]
- Xin, X.-L.; Chen, M.; Ai, Y.; Yang, F.; Li, X.-L.; Li, F. Aggregation-Induced Emissive Copper(I) Complexes for Living Cell Imaging. Inorg. Chem. 2014, 53, 2922–2931. [Google Scholar] [CrossRef]
- Wang, S.; Morgan, E.E.; Panuganti, S.; Mao, L.; Vishnoi, P.; Wu, G.; Liu, Q.; Kanatzidis, M.G.; Schaller, R.D.; Seshadri, R. Ligand Control of Structural Diversity in Luminescent Hybrid Copper(I) Iodides. Chem. Mater. 2022, 34, 3206–3216. [Google Scholar] [CrossRef]
- Ford, P.C.; Cariati, E.; Bourassa, J. Photoluminescence Properties of Multinuclear Copper(I) Compounds. Chem. Rev. 1999, 99, 3625–3647. [Google Scholar] [CrossRef]
- Conesa-Egea, J.; Zamora, F.; Amo-Ochoa, P. Perspectives of the Smart Cu-Iodine Coordination Polymers: A Portage to the World of New Nanomaterials and Composites. Coord. Chem. Rev. 2019, 381, 65–78. [Google Scholar] [CrossRef]
- Perruchas, S. Molecular Copper Iodide Clusters: A Distinguishing Family of Mechanochromic Luminescent Compounds. Dalt. Trans. 2021, 50, 12031–12044. [Google Scholar] [CrossRef]
- Schlachter, A.; Viau, L.; Fortin, D.; Knauer, L.; Strohmann, C.; Knorr, M.; Harvey, P.D. Control of Structures and Emission Properties of (CuI)n 2-Methyldithiane Coordination Polymers. Inorg. Chem. 2018, 57, 13564–13576. [Google Scholar] [CrossRef]
- Troyano, J.; Zapata, E.; Perles, J.; Amo-Ochoa, P.; Fernández-Moreira, V.; Martínez, J.I.; Zamora, F.; Delgado, S. Multifunctional Copper(I) Coordination Polymers with Aromatic Mono- and Ditopic Thioamides. Inorg. Chem. 2019, 58, 3290–3301. [Google Scholar] [CrossRef] [PubMed]
- Troyano, J.; Perles, J.; Amo-Ochoa, P.; Zamora, F.; Delgado, S. Strong Luminescent Copper(I) Halide Coordination Polymers and Dinuclear Complexes with Thioacetamide and N,N′-Donor Ligands. CrystEngComm 2016, 18, 1809–1817. [Google Scholar] [CrossRef]
- Artem’Ev, A.V.; Doronina, E.P.; Rakhmanova, M.I.; Tarasova, O.A.; Bagryanskaya, I.Y.; Nedolya, N.A. Chemoselective Mechanochemical Route toward a Bright TADF-Emitting CuI-Based Coordination Polymer. Inorg. Chem. Front. 2019, 6, 671–679. [Google Scholar] [CrossRef]
- Troyano, J.; Castillo, Ó.; Amo-Ochoa, P.; Martínez, J.I.; Zamora, F.; Delgado, S. Reversible Transformation between Cu(I)-Thiophenolate Coordination Polymers Displaying Luminescence and Electrical Properties. CrystEngComm 2019, 21, 3232–3239. [Google Scholar] [CrossRef]
- Artem’ev, A.V.; Baranov, A.Y.; Rakhmanova, M.I.; Malysheva, S.F.; Samsonenko, D.G. Copper(I) Halide Polymers Derived from Tris [2-(Pyridin-2-Yl)Ethyl]Phosphine: Halogen-Tunable Colorful Luminescence Spanning from Deep Blue to Green. New J. Chem. 2020, 44, 6916–6922. [Google Scholar] [CrossRef]
- Vinogradova, K.A.; Shekhovtsov, N.A.; Berezin, A.S.; Sukhikh, T.S.; Rogovoy, M.I.; Artem’ev, A.V.; Bushuev, M.B. Coordination-Induced Emission Enhancement in Copper(I) Iodide Coordination Polymers Supported by 2-(Alkylsulfanyl)Pyrimidines. Dalt. Trans. 2021, 50, 9317–9330. [Google Scholar] [CrossRef]
- Conesa-Egea, J.; Moreno-Vázquez, A.; Fernández-Moreira, V.; Ballesteros, Y.; Castellanos, M.; Zamora, F.; Amo-Ochoa, P. Micro and Nano Smart Composite Films Based on Copper-Iodine Coordination Polymer as Thermochromic Biocompatible Sensors. Polymers 2019, 11, 1047. [Google Scholar] [CrossRef] [PubMed]
- An, Z.; Zheng, C.; Tao, Y.; Chen, R.; Shi, H.; Chen, T.; Wang, Z.; Li, H.; Deng, R.; Liu, X.; et al. Stabilizing Triplet Excited States for Ultralong Organic Phosphorescence. Nat. Mater. 2015, 14, 685–690. [Google Scholar] [CrossRef] [PubMed]
- Lucenti, E.; Forni, A.; Botta, C.; Carlucci, L.; Giannini, C.; Marinotto, D.; Previtali, A.; Righetto, S.; Cariati, E. H-Aggregates Granting Crystallization-Induced Emissive Behavior and Ultralong Phosphorescence from a Pure Organic Molecule. J. Phys. Chem. Lett. 2017, 8, 1894–1898. [Google Scholar] [CrossRef] [PubMed]
- Cariati, E.; Forni, A.; Lucenti, E.; Marinotto, D.; Previtali, A.; Righetto, S.; Botta, C.; Bold, V.; Kravtsov, V.; Fonari, M.S. Extrinsic Heavy Metal Atom Effect on the Solid-State Room Temperature Phosphorescence of Cyclic Triimidazole. Chem. Asian J. 2019, 14, 853–858. [Google Scholar] [CrossRef]
- Fonari, M.S.; Kravtsov, V.C.; Bold, V.; Lucenti, E.; Cariati, E.; Marinotto, D.; Forni, A. Structural Landscape of Zn(II) and Cd(II) Coordination Compounds with Two Isomeric Triimidazole Luminophores: Impact of Crystal Packing Patterns on Emission Properties. Cryst. Growth Des. 2021, 21, 4184–4200. [Google Scholar] [CrossRef]
- Lucenti, E.; Cariati, E.; Previtali, A.; Marinotto, D.; Forni, A.; Bold, V.; Kravtsov, V.C.; Fonari, M.S.; Galli, S.; Carlucci, L. Versatility of Cyclic Triimidazole to Assemble 1D, 2D, and 3D Cu(I) Halide Coordination Networks. Cryst. Growth Des. 2019, 19, 1567–1575. [Google Scholar] [CrossRef]
- Malpicci, D.; Lucenti, E.; Forni, A.; Marinotto, D.; Previtali, A.; Carlucci, L.; Mercandelli, P.; Botta, C.; Righetto, S.; Cariati, E. Ag(I) and Cu(I) Cyclic-Triimidazole Coordination Polymers: Revealing Different Deactivation Channels for Multiple Room Temperature Phosphorescences. Inorg. Chem. Front. 2021, 8, 1312–1323. [Google Scholar] [CrossRef]
- Melnic, E.; Kravtsov, V.C.; Lucenti, E.; Cariati, E.; Forni, A.; Siminel, N.; Fonari, M.S. Regulation of π⋯π Stacking Interactions between Triimidazole Luminophores and Comprehensive Emission Quenching by Coordination to Cu(II). New J. Chem. 2021, 45, 9040–9052. [Google Scholar] [CrossRef]
- Forni, A.; Cariati, E.; Carlucci, L.; Lucenti, E.; Marinotto, D.; Pieraccini, S.; Sironi, M. Interpreting the Different Emissive Properties of Cyclic Triimidazole-Based CuI and AgI Coordination Polymers: A QTAIM and IQA Study. Acta Cryst. Sect. B Struct. Sci. Cryst. Eng. Mater. 2021, 77, 865–870. [Google Scholar] [CrossRef]
- Lucenti, E.; Forni, A.; Previtali, A.; Marinotto, D.; Malpicci, D.; Righetto, S.; Giannini, C.; Virgili, T.; Kabacinski, P.; Ganzer, L.; et al. Unravelling the Intricate Photophysical Behavior of 3-(Pyridin-2-Yl)Triimidazotriazine AIE and RTP Polymorphs. Chem. Sci. 2020, 11, 7599–7608. [Google Scholar] [CrossRef]
- SADABS 2012. Area Detector Absorption Correction; Bruker AXS Inc.: Madison, WI, USA, 2012.
- Burla, M.C.; Caliandro, R.; Carrozzini, B.; Cascarano, G.L.; Cuocci, C.; Giacovazzo, C.; Mallamo, M.; Mazzone, A.; Polidori, G. Crystal Structure Determination and Refinement Via SIR2014. J. Appl. Cryst. 2015, 48, 306–309. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal Structure Refinement with SHELXL. Acta Cryst. Sect. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16 Rev. A.03; Gaussian: Wallingford, CT, USA, 2016. [Google Scholar]
- Pritchard, B.P.; Altarawy, D.; Didier, B.; Gibson, T.D.; Windus, T.L. New Basis Set Exchange: An Open, Up-to-Date Resource for the Molecular Sciences Community. J. Chem. Inf. Model. 2019, 59, 4814–4820. [Google Scholar] [CrossRef]
- Feller, D. The Role of Databases in Support of Computational Chemistry Calculations. J. Comput. Chem. 1996, 17, 1571–1586. [Google Scholar] [CrossRef]
- Schuchardt, K.L.; Didier, B.T.; Elsethagen, T.; Sun, L.; Gurumoorthi, V.; Chase, J.; Li, J.; Windus, T.L. Basis Set Exchange: A Community Database for Computational Sciences. J. Chem. Inf. Model. 2007, 47, 1045–1052. [Google Scholar] [CrossRef] [PubMed]
- Glukhovtsev, M.N.; Pross, A.; McGrath, M.P.; Radom, L. Extension of Gaussian-2 (G2) Theory to Bromine- and Iodine-Containing Molecules: Use of Effective Core Potentials. J. Chem. Phys. 1995, 103, 1878–1885. [Google Scholar] [CrossRef]
- Chai, J.-D.; Head-Gordon, M. Systematic Optimization of Long-Range Corrected Hybrid Density Functionals. J. Chem. Phys. 2008, 128, 84106. [Google Scholar] [CrossRef]
- Malpicci, D.; Giannini, C.; Lucenti, E.; Forni, A.; Marinotto, D.; Cariati, E. Mono-, Di-, Tri-Pyrene Substituted Cyclic Triimidazole: A Family of Highly Emissive and RTP Chromophores. Photochem 2021, 1, 477–487. [Google Scholar] [CrossRef]
- Previtali, A.; He, W.; Forni, A.; Malpicci, D.; Lucenti, E.; Marinotto, D.; Carlucci, L.; Mercandelli, P.; Ortenzi, M.A.; Terraneo, G.; et al. Tunable Linear and Nonlinear Optical Properties from Room Temperature Phosphorescent Cyclic Triimidazole-Pyrene Bio-Probe. Chem. Eur. J. 2021, 27, 16690–16700. [Google Scholar] [CrossRef] [PubMed]
- Previtali, A.; Lucenti, E.; Forni, A.; Mauri, L.; Botta, C.; Giannini, C.; Malpicci, D.; Marinotto, D.; Righetto, S.; Cariati, E. Solid State Room Temperature Dual Phosphorescence from 3-(2-Fluoropyridin-4-Yl)Triimidazo[1,2-a:1′,2′-c:1″,2″-e][1,3,5]Triazine. Molecules 2019, 24, 2552. [Google Scholar] [CrossRef]
- Giannini, C.; Forni, A.; Malpicci, D.; Lucenti, E.; Marinotto, D.; Previtali, A.; Carlucci, L.; Cariati, E. Room Temperature Phosphorescence from Organic Materials: Unravelling the Emissive Behaviour of Chloro-Substituted Derivatives of Cyclic Triimidazole. Eur. J. Org. Chem. 2021, 2021, 2041–2049. [Google Scholar] [CrossRef]
- Lucenti, E.; Forni, A.; Botta, C.; Carlucci, L.; Colombo, A.; Giannini, C.; Marinotto, D.; Previtali, A.; Righetto, S.; Cariati, E. The Effect of Bromo Substituents on the Multifaceted Emissive and Crystal-Packing Features of Cyclic Triimidazole Derivatives. ChemPhotoChem 2018, 2, 801–805. [Google Scholar] [CrossRef]
- Lucenti, E.; Forni, A.; Botta, C.; Carlucci, L.; Giannini, C.; Marinotto, D.; Pavanello, A.; Previtali, A.; Righetto, S.; Cariati, E. Cyclic Triimidazole Derivatives: Intriguing Examples of Multiple Emissions and Ultralong Phosphorescence at Room Temperature. Angew. Chem. Int. Ed. 2017, 56, 16302–16307. [Google Scholar] [CrossRef]
- Lucenti, E.; Forni, A.; Botta, C.; Giannini, C.; Malpicci, D.; Marinotto, D.; Previtali, A.; Righetto, S.; Cariati, E. Intrinsic and Extrinsic Heavy-Atom Effects on the Multifaceted Emissive Behavior of Cyclic Triimidazole. Chem. Eur. J. 2019, 25, 2452–2456. [Google Scholar] [CrossRef] [PubMed]
- Conesa-Egea, J.; Redondo, C.D.; Martínez, J.I.; Gómez-García, C.J.; Castillo, Ó.; Zamora, F.; Amo-Ochoa, P. Supramolecular Interactions Modulating Electrical Conductivity and Nanoprocessing of Copper–Iodine Double-Chain Coordination Polymers. Inorg. Chem. 2018, 57, 7568–7577. [Google Scholar] [CrossRef]
- Hassanein, K.; Conesa-Egea, J.; Delgado, S.; Castillo, O.; Benmansour, S.; Martínez, J.I.; Abellán, G.; Gómez-García, C.J.; Zamora, F.; Amo-Ochoa, P. Electrical Conductivity and Strong Luminescence in Copper Iodide Double Chains with Isonicotinato Derivatives. Chem. Eur. J. 2015, 21, 17282–17292. [Google Scholar] [CrossRef]
- Shannon, R.D. Revised Effective Ionic Radii and Systematic Studies of Interatomic Distances in Halides and Chalcogenides. Acta Cryst. Sect. A 1976, 32, 751–767. [Google Scholar] [CrossRef]
- Li, J.-C.; Li, H.-X.; Li, H.-Y.; Gong, W.-J.; Lang, J.-P. Ligand Coordination Site-Directed Assembly of Copper(I) Iodide Complexes of ((Pyridyl)-1-Pyrazolyl)Pyridine. Cryst. Growth Des. 2016, 16, 1617–1625. [Google Scholar] [CrossRef]
- It should be noted that geometry optimization of discrete models implies a loss of the symmetry characterizing the polymeric structure owing to major boundary effects, giving rise to an artificial splitting of the electronic excitation levels.

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Malpicci, D.; Blasi, D.; Marinotto, D.; Forni, A.; Cariati, E.; Lucenti, E.; Carlucci, L. A Rare Structural Motif for a Luminescent Cu(I) Coordination Polymer with 3-(Pyridin-2-yl)triimidazotriazine. Crystals 2023, 13, 149. https://doi.org/10.3390/cryst13010149
Malpicci D, Blasi D, Marinotto D, Forni A, Cariati E, Lucenti E, Carlucci L. A Rare Structural Motif for a Luminescent Cu(I) Coordination Polymer with 3-(Pyridin-2-yl)triimidazotriazine. Crystals. 2023; 13(1):149. https://doi.org/10.3390/cryst13010149
Chicago/Turabian StyleMalpicci, Daniele, Delia Blasi, Daniele Marinotto, Alessandra Forni, Elena Cariati, Elena Lucenti, and Lucia Carlucci. 2023. "A Rare Structural Motif for a Luminescent Cu(I) Coordination Polymer with 3-(Pyridin-2-yl)triimidazotriazine" Crystals 13, no. 1: 149. https://doi.org/10.3390/cryst13010149
APA StyleMalpicci, D., Blasi, D., Marinotto, D., Forni, A., Cariati, E., Lucenti, E., & Carlucci, L. (2023). A Rare Structural Motif for a Luminescent Cu(I) Coordination Polymer with 3-(Pyridin-2-yl)triimidazotriazine. Crystals, 13(1), 149. https://doi.org/10.3390/cryst13010149










