Biomedical Applications of Titanium Alloys Modified with MOFs—Current Knowledge and Further Development Directions
Abstract
:1. Metal-Organic Frameworks (MOFs)
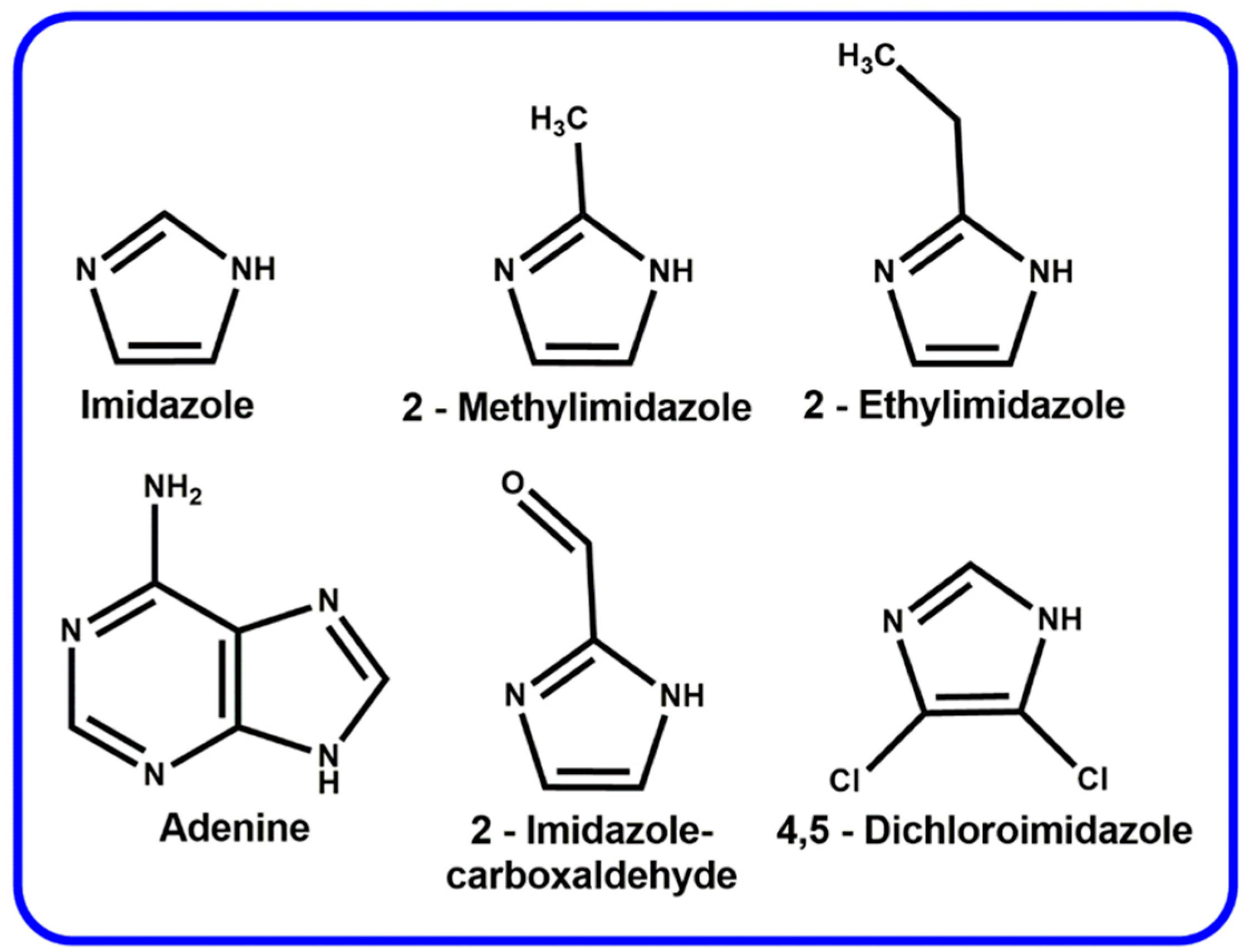
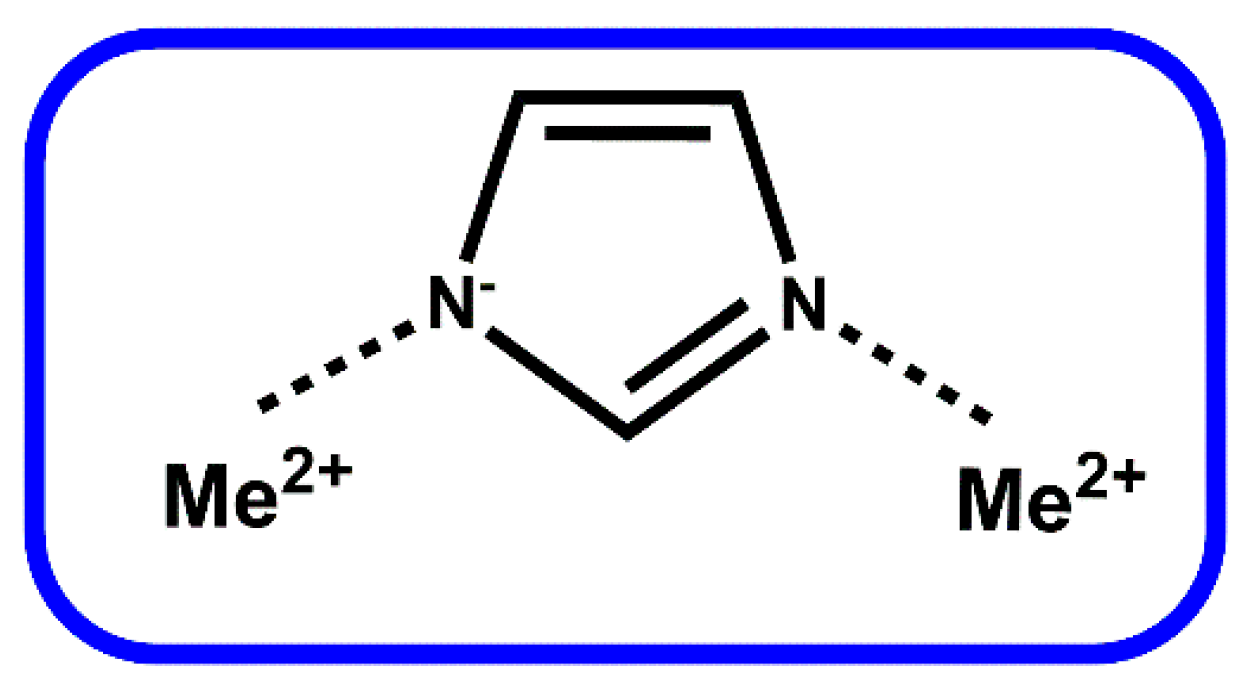
2. Zeolitic Imidazolate Frameworks (ZIFs)
3. Biomedical Applications of MOFs
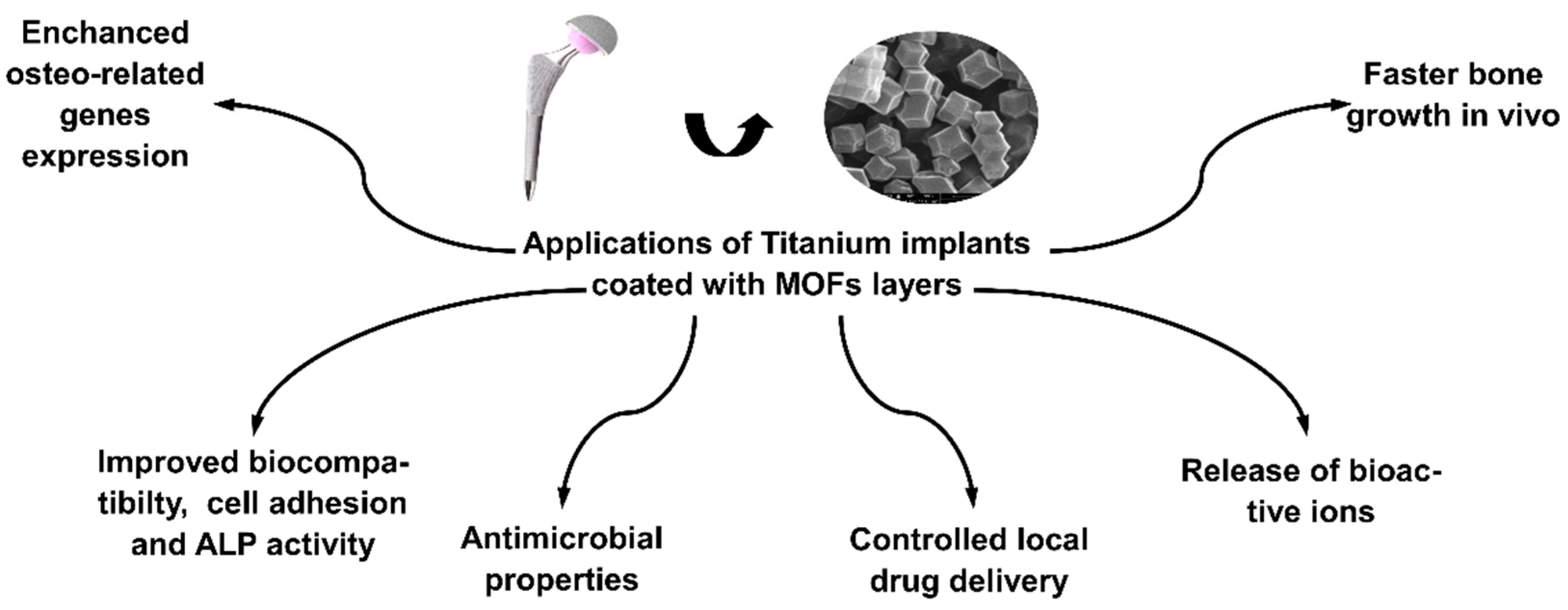
4. MOFs in Modification of Titanium Alloy
5. Conclusions and Possible Development Directions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, A.; Bueno-Perez, R.; Wiggin, S.; Fairen-Jimenez, D. Enabling Efficient Exploration of Metal-Organic Frameworks in the Cambridge Structural Database. CrystEngComm 2020, 22, 7152–7161. [Google Scholar] [CrossRef]
- Bohrman, J.A.; Carreon, M.A. Synthesis and CO2/CH4 Separation Performance of Bio-MOF-1 Membranes. Chem. Commun. 2012, 48, 5130–5132. [Google Scholar] [CrossRef]
- Gan, Y.-L.; Huang, K.-R.; Li, Y.-G.; Qin, D.-P.; Zhang, D.-M.; Zong, Z.-A.; Cui, L.-S. Synthesis, Structure and Fluorescent Sensing for Nitrobenzene of a Zn-Based MOF. J. Mol. Struct. 2021, 1223, 129217. [Google Scholar] [CrossRef]
- Zha, Q.; Yuan, F.; Qin, G.; Ni, Y. Cobalt-Based MOF-on-MOF Two-Dimensional Heterojunction Nanostructures for Enhanced Oxygen Evolution Reaction Electrocatalytic Activity. Inorg. Chem. 2020, 59, 1295–1305. [Google Scholar] [CrossRef] [PubMed]
- Rojas, S.; Devic, T.; Horcajada, P. Metal Organic Frameworks Based on Bioactive Components. J. Mater. Chem. B 2017, 5, 2560–2573. [Google Scholar] [CrossRef] [PubMed]
- Cooper, L.; Hidalgo, T.; Gorman, M.; Lozano-Fernández, T.; Simón-Vázquez, R.; Olivier, C.; Guillou, N.; Serre, C.; Martineau, C.; Taulelle, F.; et al. A Biocompatible Porous Mg-Gallate Metal-Organic Framework as an Antioxidant Carrier. Chem. Commun. 2015, 51, 5848–5851. [Google Scholar] [CrossRef]
- Can, M.; Demirci, S.; Sunol, A.K.; Sahiner, N. An Amino Acid, l-Glutamic Acid-Based Metal-Organic Frameworks and Their Antibacterial, Blood Compatibility, Biocompatibility, and Sensor Properties. Microporous Mesoporous Mater. 2020, 309, 110533. [Google Scholar] [CrossRef]
- Dong, X.; Shi, Z.; Li, D.; Li, Y.; An, N.; Shang, Y.; Sakiyama, H.; Muddassir, M.; Si, C. The Regulation Research of Topology and Magnetic Exchange Models of CPs through Co(II) Concentration Adjustment. J. Solid State Chem. 2023, 318, 123713. [Google Scholar] [CrossRef]
- Qin, L.; Li, Y.; Liang, F.; Li, L.; Lan, Y.; Li, Z.; Lu, X.; Yang, M.; Ma, D. A Microporous 2D Cobalt-Based MOF with Pyridyl Sites and Open Metal Sites for Selective Adsorption of CO2. Microporous Mesoporous Mater. 2022, 341, 112098. [Google Scholar] [CrossRef]
- Qin, L.; Liang, F.; Li, Y.; Wu, J.; Guan, S.; Wu, M.; Xie, S.; Luo, M.; Ma, D. A 2D Porous Zinc-Organic Framework Platform for Loading of 5-Fluorouracil. Inorganics 2022, 10, 202. [Google Scholar] [CrossRef]
- Hönicke, I.M.; Senkovska, I.; Bon, V.; Baburin, I.A.; Bönisch, N.; Raschke, S.; Evans, J.D.; Kaskel, S. Balancing Mechanical Stability and Ultrahigh Porosity in Crystalline Framework Materials. Angew. Chem. Int. Ed. 2018, 57, 13780–13783. [Google Scholar] [CrossRef] [PubMed]
- Furukawa, H.; Cordova, K.E.; O’Keeffe, M.; Yaghi, O.M. The Chemistry and Applications of Metal-Organic Frameworks. Science 2013, 341, 1230444. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.-S.; Li, M.; Peng, Y.-L.; Zhang, Z.; Chen, W.; Huang, X.-C. An Ultrastable Metal Azolate Framework with Binding Pockets for Optimal Carbon Dioxide Capture. Angew. Chem. Int. Ed. 2019, 58, 16071–16076. [Google Scholar] [CrossRef] [PubMed]
- Almáši, M.; Zeleňák, V.; Palotai, P.; Beňová, E.; Zeleňáková, A. Metal-Organic Framework MIL-101(Fe)-NH2 Functionalized with Different Long-Chain Polyamines as Drug Delivery System. Inorg. Chem. Commun. 2018, 93, 115–120. [Google Scholar] [CrossRef]
- Trushina, D.B.; Sapach, A.Y.; Burachevskaia, O.A.; Medvedev, P.V.; Khmelenin, D.N.; Borodina, T.N.; Soldatov, M.A.; Butova, V.V. Doxorubicin-Loaded Core-Shell UiO-66@SiO2 Metal-Organic Frameworks for Targeted Cellular Uptake and Cancer Treatment. Pharmaceutics 2022, 14, 1325. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Cui, X.; O’Nolan, D.; Wen, H.-M.; Jiang, M.; Krishna, R.; Wu, H.; Lin, R.-B.; Chen, Y.-S.; Yuan, D.; et al. An Ideal Molecular Sieve for Acetylene Removal from Ethylene with Record Selectivity and Productivity. Adv. Mater. 2017, 29, 1704210. [Google Scholar] [CrossRef]
- Sun, Y.; Xue, Z.; Liu, Q.; Jia, Y.; Li, Y.; Liu, K.; Lin, Y.; Liu, M.; Li, G.; Su, C.-Y. Modulating Electronic Structure of Metal-Organic Frameworks by Introducing Atomically Dispersed Ru for Efficient Hydrogen Evolution. Nat. Commun. 2021, 12, 1369. [Google Scholar] [CrossRef]
- Umapathi, R.; Ghoreishian, S.M.; Sonwal, S.; Rani, G.M.; Huh, Y.S. Portable Electrochemical Sensing Methodologies for On-Site Detection of Pesticide Residues in Fruits and Vegetables. Coord. Chem. Rev. 2022, 453, 214305. [Google Scholar] [CrossRef]
- Venkateswara Raju, C.; Hwan Cho, C.; Mohana Rani, G.; Manju, V.; Umapathi, R.; Suk Huh, Y.; Pil Park, J. Emerging Insights into the Use of Carbon-Based Nanomaterials for the Electrochemical Detection of Heavy Metal Ions. Coord. Chem. Rev. 2023, 476, 214920. [Google Scholar] [CrossRef]
- Ratnamala, A.; Reddy, G.D.; Noorjahaan, M.; Manjunatha, H.; Janardan, S.; Kumar, N.S.; Chandra Babu Naidu, K.; Khan, A.; Asiri, A.M. Chapter 3—Titanium-Based Metal-Organic Frameworks for Photocatalytic Applications. In Metal-Organic Frameworks for Chemical Reactions; Khan, A., Verpoort, F., Asiri, A.M., Hoque, M.E., Bilgrami, A.L., Azam, M., Naidu, K.C.B., Eds.; Elsevier: Amsterdam, The Netherlands, 2021; pp. 37–63. [Google Scholar]
- Yang, D.; Gates, B.C. Catalysis by Metal Organic Frameworks: Perspective and Suggestions for Future Research. ACS Catal. 2019, 9, 1779–1798. [Google Scholar] [CrossRef]
- Beydaghdari, M.; Hooriabad Saboor, F.; Babapoor, A.; Karve, V.V.; Asgari, M. Recent Advances in MOF-Based Adsorbents for Dye Removal from the Aquatic Environment. Energies 2022, 15, 2023. [Google Scholar] [CrossRef]
- Chen, J.; Cheng, F.; Luo, D.; Huang, J.; Ouyang, J.; Nezamzadeh-Ejhieh, A.; Khan, M.S.; Liu, J.; Peng, Y. Recent Advances in Ti-Based MOFs in Biomedical Applications. Dalton Trans. 2022, 51, 14817–14832. [Google Scholar] [CrossRef] [PubMed]
- Keskin, S.; Kızılel, S. Biomedical Applications of Metal Organic Frameworks. Ind. Eng. Chem. Res. 2011, 50, 1799–1812. [Google Scholar] [CrossRef]
- Al Sharabati, M.; Sabouni, R.; Husseini, G.A. Biomedical Applications of Metal-Organic Frameworks for Disease Diagnosis and Drug Delivery: A Review. Nanomaterials 2022, 12, 277. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Zhu, Y.; Kaskel, S. Porphyrin-Based Metal-Organic Frameworks for Biomedical Applications. Angew. Chem. Int. Ed. 2021, 60, 5010–5035. [Google Scholar] [CrossRef]
- Sun, Y.; Zheng, L.; Yang, Y.; Qian, X.; Fu, T.; Li, X.; Yang, Z.; Yan, H.; Cui, C.; Tan, W. Metal-Organic Framework Nanocarriers for Drug Delivery in Biomedical Applications. Nano-Micro Lett. 2020, 12, 103. [Google Scholar] [CrossRef]
- Chen, B.; Yang, Z.; Zhu, Y.; Xia, Y. Zeolitic Imidazolate Framework Materials: Recent Progress in Synthesis and Applications. J. Mater. Chem. A 2014, 2, 16811–16831. [Google Scholar] [CrossRef]
- McCarthy, M.C.; Varela-Guerrero, V.; Barnett, G.V.; Jeong, H.-K. Synthesis of Zeolitic Imidazolate Framework Films and Membranes with Controlled Microstructures. Langmuir 2010, 26, 14636–14641. [Google Scholar] [CrossRef]
- Shieh, F.-K.; Wang, S.-C.; Leo, S.-Y.; Wu, K.C.-W. Water-Based Synthesis of Zeolitic Imidazolate Framework-90 (ZIF-90) with a Controllable Particle Size. Chem.–Eur. J. 2013, 19, 11139–11142. [Google Scholar] [CrossRef]
- Huang, X.-C.; Lin, Y.-Y.; Zhang, J.-P.; Chen, X.-M. Ligand-Directed Strategy for Zeolite-Type Metal-Organic Frameworks: Zinc(II) Imidazolates with Unusual Zeolitic Topologies. Angew. Chem. Int. Ed. 2006, 45, 1557–1559. [Google Scholar] [CrossRef]
- Phan, A.; Doonan, C.J.; Uribe-Romo, F.J.; Knobler, C.B.; O’Keeffe, M.; Yaghi, O.M. Synthesis, Structure, and Carbon Dioxide Capture Properties of Zeolitic Imidazolate Frameworks. Acc. Chem. Res. 2010, 43, 58–67. [Google Scholar] [CrossRef] [PubMed]
- Nune, S.K.; Thallapally, P.K.; Dohnalkova, A.; Wang, C.; Liu, J.; Exarhos, G.J. Synthesis and Properties of Nano Zeolitic Imidazolate Frameworks. Chem. Commun. 2010, 46, 4878–4880. [Google Scholar] [CrossRef] [PubMed]
- Park, K.S.; Ni, Z.; Côté, A.P.; Choi, J.Y.; Huang, R.; Uribe-Romo, F.J.; Chae, H.K.; O’Keeffe, M.; Yaghi, O.M. Exceptional Chemical and Thermal Stability of Zeolitic Imidazolate Frameworks. Proc. Natl. Acad. Sci. USA 2006, 103, 10186–10191. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Liang, F.; Bux, H.; Yang, W.; Caro, J. Zeolitic Imidazolate Framework ZIF-7 Based Molecular Sieve Membrane for Hydrogen Separation. J. Membr. Sci. 2010, 354, 48–54. [Google Scholar] [CrossRef]
- Schulte, Z.M.; Kwon, Y.H.; Han, Y.; Liu, C.; Li, L.; Yang, Y.; Jarvi, A.G.; Saxena, S.; Veser, G.; Johnson, J.K.; et al. H2/CO2 Separations in Multicomponent Metal-Adeninate MOFs with Multiple Chemically Distinct Pore Environments. Chem. Sci. 2020, 11, 12807–12815. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.-L.; Liu, B.; Akita, T.; Haruta, M.; Sakurai, H.; Xu, Q. Au@ZIF-8: CO Oxidation over Gold Nanoparticles Deposited to Metal-Organic Framework. J. Am. Chem. Soc. 2009, 131, 11302–11303. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Yang, Y.-W. Metal-Organic Frameworks for Biomedical Applications. Small 2020, 16, 1906846. [Google Scholar] [CrossRef]
- Kaur, H.; Mohanta, G.C.; Gupta, V.; Kukkar, D.; Tyagi, S. Synthesis and Characterization of ZIF-8 Nanoparticles for Controlled Release of 6-Mercaptopurine Drug. J. Drug Deliv. Sci. Technol. 2017, 41, 106–112. [Google Scholar] [CrossRef]
- Zheng, C.; Wang, Y.; Phua, S.Z.F.; Lim, W.Q.; Zhao, Y. ZnO-DOX@ZIF-8 Core–Shell Nanoparticles for PH-Responsive Drug Delivery. ACS Biomater. Sci. Eng. 2017, 3, 2223–2229. [Google Scholar] [CrossRef]
- Gong, M.; Yang, J.; Li, Y.; Gu, J. Glutathione-Responsive Nanoscale MOFs for Effective Intracellular Delivery of the Anticancer Drug 6-Mercaptopurine. Chem. Commun. 2020, 56, 6448–6451. [Google Scholar] [CrossRef]
- Yang, X.; Tang, Q.; Jiang, Y.; Zhang, M.; Wang, M.; Mao, L. Nanoscale ATP-Responsive Zeolitic Imidazole Framework-90 as a General Platform for Cytosolic Protein Delivery and Genome Editing. J. Am. Chem. Soc. 2019, 141, 3782–3786. [Google Scholar] [CrossRef] [PubMed]
- Lan, G.; Ni, K.; Lin, W. Nanoscale Metal-Organic Frameworks for Phototherapy of Cancer. Coord. Chem. Rev. 2019, 379, 65–81. [Google Scholar] [CrossRef] [PubMed]
- Lu, K.; He, C.; Lin, W. Nanoscale Metal-Organic Framework for Highly Effective Photodynamic Therapy of Resistant Head and Neck Cancer. J. Am. Chem. Soc. 2014, 136, 16712–16715. [Google Scholar] [CrossRef]
- Lu, J.; Yang, L.; Zhang, W.; Li, P.; Gao, X.; Zhang, W.; Wang, H.; Tang, B. Photodynamic Therapy for Hypoxic Solid Tumors via Mn-MOF as a Photosensitizer. Chem. Commun. 2019, 55, 10792–10795. [Google Scholar] [CrossRef]
- Sharma, S.; Mittal, D.; Verma, A.K.; Roy, I. Copper-Gallic Acid Nanoscale Metal-Organic Framework for Combined Drug Delivery and Photodynamic Therapy. ACS Appl. Bio Mater. 2019, 2, 2092–2101. [Google Scholar] [CrossRef]
- Ryu, U.; Yoo, J.; Kwon, W.; Choi, K.M. Tailoring Nanocrystalline Metal-Organic Frameworks as Fluorescent Dye Carriers for Bioimaging. Inorg. Chem. 2017, 56, 12859–12865. [Google Scholar] [CrossRef]
- Rieter, W.J.; Taylor, K.M.L.; An, H.; Lin, W.; Lin, W. Nanoscale Metal-Organic Frameworks as Potential Multimodal Contrast Enhancing Agents. J. Am. Chem. Soc. 2006, 128, 9024–9025. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.; Wu, H.; Feng, R.; Wang, D.; Xu, P.; Wang, H.; Guo, Z.; Chen, Q. Bimetallic Zeolitic Imidazolate Framework as an Intrinsic Two-Photon Fluorescence and PH-Responsive MR Imaging Agent. ACS Omega 2018, 3, 9790–9797. [Google Scholar] [CrossRef]
- Guerrero, F.; Pulido, V.; Hamad, S.; Aljama, P.; Martín-Malo, A.; Carrillo-Carrión, C. Incorporating Zeolitic-Imidazolate Framework-8 Nanoparticles into Kidney Scaffolds: A First Step towards Innovative Renal Therapies. Nanoscale 2022, 14, 17543–17549. [Google Scholar] [CrossRef]
- Karakeçili, A.; Topuz, B.; Korpayev, S.; Erdek, M. Metal-Organic Frameworks for on-Demand PH Controlled Delivery of Vancomycin from Chitosan Scaffolds. Mater. Sci. Eng. C 2019, 105, 110098. [Google Scholar] [CrossRef]
- Han, L.; Huang, Z.; Zhu, M.; Zhu, Y.; Li, H. Drug-Loaded Zeolite Imidazole Framework-8-Functionalized Bioglass Scaffolds with Antibacterial Activity for Bone Repair. Ceram. Int. 2022, 48, 6890–6898. [Google Scholar] [CrossRef]
- Soldatkina, O.V.; Kucherenko, I.S.; Soldatkin, O.O.; Pyeshkova, V.M.; Dudchenko, O.Y.; Akata Kurç, B.; Dzyadevych, S.V. Development of Electrochemical Biosensors with Various Types of Zeolites. Appl. Nanosci. 2019, 9, 737–747. [Google Scholar] [CrossRef]
- Sheta, S.M.; El-Sheikh, S.M.; Osman, D.I.; Salem, A.M.; Ali, O.I.; Harraz, F.A.; Shousha, W.G.; Shoeib, M.A.; Shawky, S.M.; Dionysiou, D.D. A Novel HCV Electrochemical Biosensor Based on a Polyaniline@Ni-MOF Nanocomposite. Dalton Trans. 2020, 49, 8918–8926. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Bai, J.; Huo, Y.; Ning, B.; Peng, Y.; Li, S.; Han, D.; Kang, W.; Gao, Z. A Zirconium-Porphyrin MOF-Based Ratiometric Fluorescent Biosensor for Rapid and Ultrasensitive Detection of Chloramphenicol. Biosens. Bioelectron. 2020, 149, 111801. [Google Scholar] [CrossRef] [PubMed]
- Kurup, A.; Dhatrak, P.; Khasnis, N. Surface Modification Techniques of Titanium and Titanium Alloys for Biomedical Dental Applications: A Review. Mater. Today Proc. 2021, 39, 84–90. [Google Scholar] [CrossRef]
- Jaafar, A.; Hecker, C.; Árki, P.; Joseph, Y. Sol-Gel Derived Hydroxyapatite Coatings for Titanium Implants: A Review. Bioengineering 2020, 7, 127. [Google Scholar] [CrossRef] [PubMed]
- Souza, J.C.M.; Sordi, M.B.; Kanazawa, M.; Ravindran, S.; Henriques, B.; Silva, F.S.; Aparicio, C.; Cooper, L.F. Nano-Scale Modification of Titanium Implant Surfaces to Enhance Osseointegration. Acta Biomater. 2019, 94, 112–131. [Google Scholar] [CrossRef] [PubMed]
- Olmo, J.A.-D.; Ruiz-Rubio, L.; Pérez-Alvarez, L.; Sáez-Martínez, V.; Vilas-Vilela, J.L. Antibacterial Coatings for Improving the Performance of Biomaterials. Coatings 2020, 10, 139. [Google Scholar] [CrossRef]
- Stewart, P.S.; Bjarnsholt, T. Risk Factors for Chronic Biofilm-Related Infection Associated with Implanted Medical Devices. Clin. Microbiol. Infect. 2020, 26, 1034–1038. [Google Scholar] [CrossRef]
- Wang, Q.; Zhou, P.; Liu, S.; Attarilar, S.; Ma, R.L.-W.; Zhong, Y.; Wang, L. Multi-Scale Surface Treatments of Titanium Implants for Rapid Osseointegration: A Review. Nanomaterials 2020, 10, 1244. [Google Scholar] [CrossRef]
- Yan, Y.; Sun, J.; Han, Y.; Li, D.; Cui, K. Microstructure and Bioactivity of Ca, P and Sr Doped TiO2 Coating Formed on Porous Titanium by Micro-Arc Oxidation. Surf. Coat. Technol. 2010, 205, 1702–1713. [Google Scholar] [CrossRef]
- Guo, S.; Yu, D.; Xiao, X.; Liu, W.; Wu, Z.; Shi, L.; Zhao, Q.; Yang, D.; Lu, Y.; Wei, X.; et al. A Vessel Subtype Beneficial for Osteogenesis Enhanced by Strontium-Doped Sodium Titanate Nanorods by Modulating Macrophage Polarization. J. Mater. Chem. B 2020, 8, 6048–6058. [Google Scholar] [CrossRef]
- Sandomierski, M.; Jakubowski, M.; Ratajczak, M.; Voelkel, A. Drug Distribution Evaluation Using FT-IR Imaging on the Surface of a Titanium Alloy Coated with Zinc Titanate with Potential Application in the Release of Drugs for Osteoporosis. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2022, 281, 121575. [Google Scholar] [CrossRef]
- Sandomierski, M.; Zielińska, M.; Buchwald, T.; Patalas, A.; Voelkel, A. Controlled Release of the Drug for Osteoporosis from the Surface of Titanium Implants Coated with Calcium Titanate. J. Biomed. Mater. Res. B Appl. Biomater. 2022, 110, 431–437. [Google Scholar] [CrossRef]
- Sandomierski, M.; Zielińska, M.; Voelkel, A. A Long-Term Controlled Release of the Drug for Osteoporosis from the Surface of Titanium Implants Coated with Calcium Zeolite. Mater. Chem. Front. 2021, 5, 5718–5725. [Google Scholar] [CrossRef]
- Gittens, R.A.; Scheideler, L.; Rupp, F.; Hyzy, S.L.; Geis-Gerstorfer, J.; Schwartz, Z.; Boyan, B.D. A Review on the Wettability of Dental Implant Surfaces II: Biological and Clinical Aspects. Acta Biomater. 2014, 10, 2907–2918. [Google Scholar] [CrossRef] [PubMed]
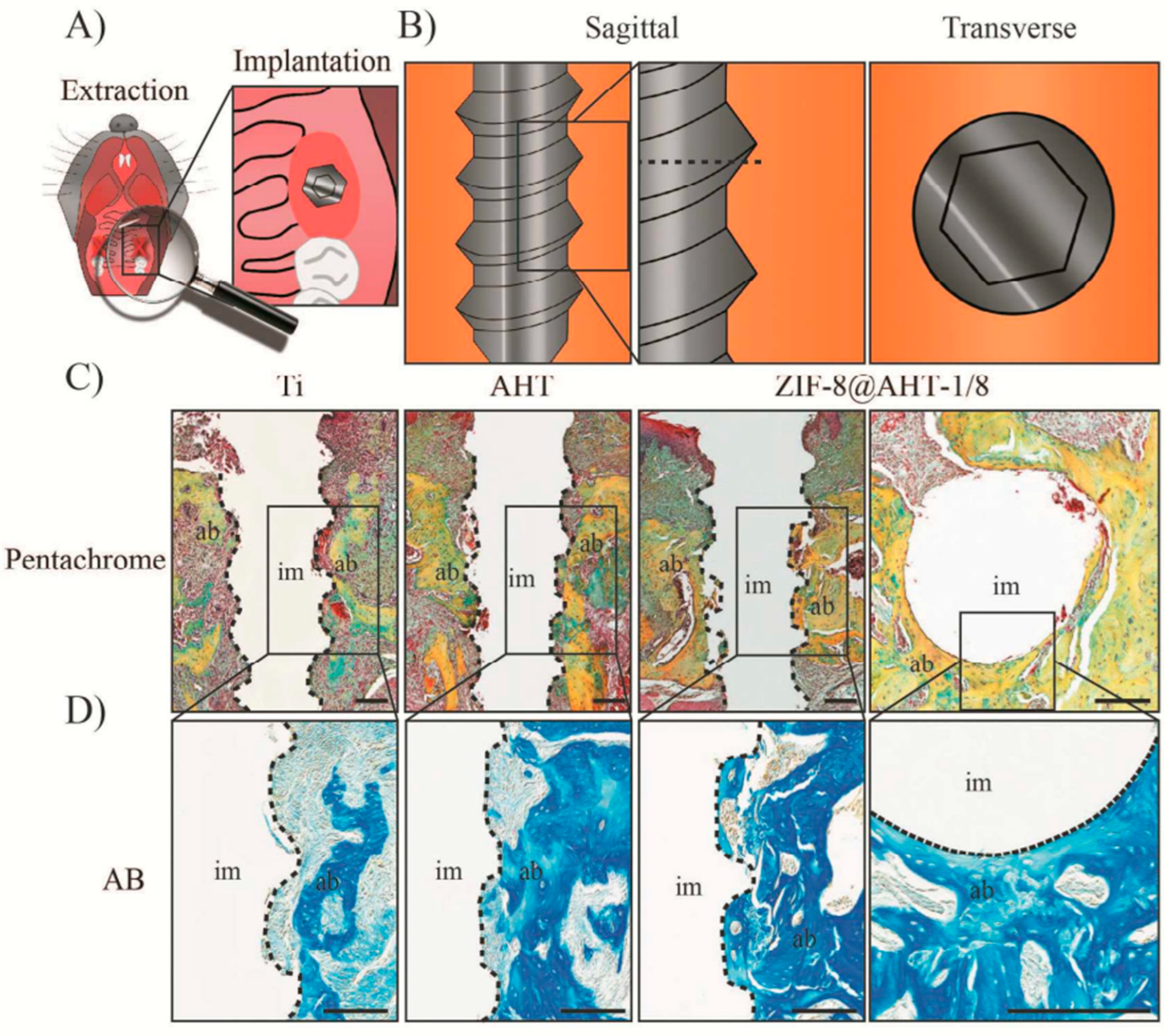
- Zhang, X.; Chen, J.; Pei, X.; Wang, J.; Wan, Q.; Jiang, S.; Huang, C.; Pei, X. Enhanced Osseointegration of Porous Titanium Modified with Zeolitic Imidazolate Framework-8. ACS Appl. Mater. Interfaces 2017, 9, 25171–25183. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Zhang, X.; Huang, C.; Cai, H.; Hu, S.; Wan, Q.; Pei, X.; Wang, J. Osteogenic Activity and Antibacterial Effect of Porous Titanium Modified with Metal-Organic Framework Films. J. Biomed. Mater. Res. A 2017, 105, 834–846. [Google Scholar] [CrossRef]
- Teng, W.; Zhang, Z.; Wang, Y.; Ye, Y.; Yinwang, E.; Liu, A.; Zhou, X.; Xu, J.; Zhou, C.; Sun, H.; et al. Iodine Immobilized Metal-Organic Framework for NIR-Triggered Antibacterial Therapy on Orthopedic Implants. Small 2021, 17, 2102315. [Google Scholar] [CrossRef]
- Sandomierski, M.; Jakubowski, M.; Ratajczak, M.; Voelkel, A. Zeolitic Imidazolate Framework-8 (ZIF-8) Modified Titanium Alloy for Controlled Release of Drugs for Osteoporosis. Sci. Rep. 2022, 12, 9103. [Google Scholar] [CrossRef]
- Tao, B.; Zhao, W.; Lin, C.; Yuan, Z.; He, Y.; Lu, L.; Chen, M.; Ding, Y.; Yang, Y.; Xia, Z.; et al. Surface Modification of Titanium Implants by ZIF-8@Levo/LBL Coating for Inhibition of Bacterial-Associated Infection and Enhancement of In Vivo Osseointegration. Chem. Eng. J. 2020, 390, 124621. [Google Scholar] [CrossRef]
- Shen, X.; Zhang, Y.; Ma, P.; Sutrisno, L.; Luo, Z.; Hu, Y.; Yu, Y.; Tao, B.; Li, C.; Cai, K. Fabrication of Magnesium/Zinc-Metal Organic Framework on Titanium Implants to Inhibit Bacterial Infection and Promote Bone Regeneration. Biomaterials 2019, 212, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Tao, B.; Lin, C.; He, Y.; Yuan, Z.; Chen, M.; Xu, K.; Li, K.; Guo, A.; Cai, K.; Chen, L. Osteoimmunomodulation Mediating Improved Osteointegration by OGP-Loaded Cobalt-Metal Organic Framework on Titanium Implants with Antibacterial Property. Chem. Eng. J. 2021, 423, 130176. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, Y.; Zhang, S.; Yao, K.; Sun, Y.; Liu, Y.; Wang, X.; Huang, W. Synthesis of Rare Earth Doped MOF Base Coating on TiO2nanotubes Arrays by Electrochemical method Using as Antibacterial Implant Material. Inorg. Chem. Commun. 2021, 127, 108484. [Google Scholar] [CrossRef]
- Wu, J.; Jiang, S.; Xie, W.; Xue, Y.; Qiao, M.; Yang, X.; Zhang, X.; Wan, Q.; Wang, J.; Chen, J.; et al. Surface Modification of the Ti Surface with Nanoscale Bio-MOF-1 for Improving Biocompatibility and Osteointegration in Vitro and in Vivo. J. Mater. Chem. B 2022, 10, 8535–8548. [Google Scholar] [CrossRef]
- Li, M.; Wei, Y.; Ma, B.; Hu, Y.; Li, D.; Cui, X. Synthesis and Antibacterial Properties of ZIF-8/Ag-Modified Titanium Alloy. J. Bionic Eng. 2022, 19, 507–515. [Google Scholar] [CrossRef]
- Ran, J.; Zeng, H.; Cai, J.; Jiang, P.; Yan, P.; Zheng, L.; Bai, Y.; Shen, X.; Shi, B.; Tong, H. Rational Design of a Stable, Effective, and Sustained Dexamethasone Delivery Platform on a Titanium Implant: An Innovative Application of Metal Organic Frameworks in Bone Implants. Chem. Eng. J. 2018, 333, 20–33. [Google Scholar] [CrossRef]
- Li, M.; He, P.; Wu, Y.; Zhang, Y.; Xia, H.; Zheng, Y.; Han, Y. Stimulatory Effects of the Degradation Products from Mg-Ca-Sr Alloy on the Osteogenesis through Regulating ERK Signaling Pathway. Sci. Rep. 2016, 6, 32323. [Google Scholar] [CrossRef]
- Matlinska, M.A.; Ha, M.; Hughton, B.; Oliynyk, A.O.; Iyer, A.K.; Bernard, G.M.; Lambkin, G.; Lawrence, M.C.; Katz, M.J.; Mar, A.; et al. Alkaline Earth Metal-Organic Frameworks with Tailorable Ion Release: A Path for Supporting Biomineralization. ACS Appl. Mater. Interfaces 2019, 11, 32739–32745. [Google Scholar] [CrossRef]
- Hidalgo, T.; Cooper, L.; Gorman, M.; Lozano-Fernández, T.; Simón-Vázquez, R.; Mouchaham, G.; Marrot, J.; Guillou, N.; Serre, C.; Fertey, P.; et al. Crystal Structure Dependent in Vitro Antioxidant Activity of Biocompatible Calcium Gallate MOFs. J. Mater. Chem. B 2017, 5, 2813–2822. [Google Scholar] [CrossRef]
- Hu, H.; Zhao, P.; Liu, J.; Ke, Q.; Zhang, C.; Guo, Y.; Ding, H. Lanthanum Phosphate/Chitosan Scaffolds Enhance Cytocompatibility and Osteogenic Efficiency via the Wnt/β-Catenin Pathway. J. Nanobiotechnol. 2018, 16, 98. [Google Scholar] [CrossRef] [PubMed]

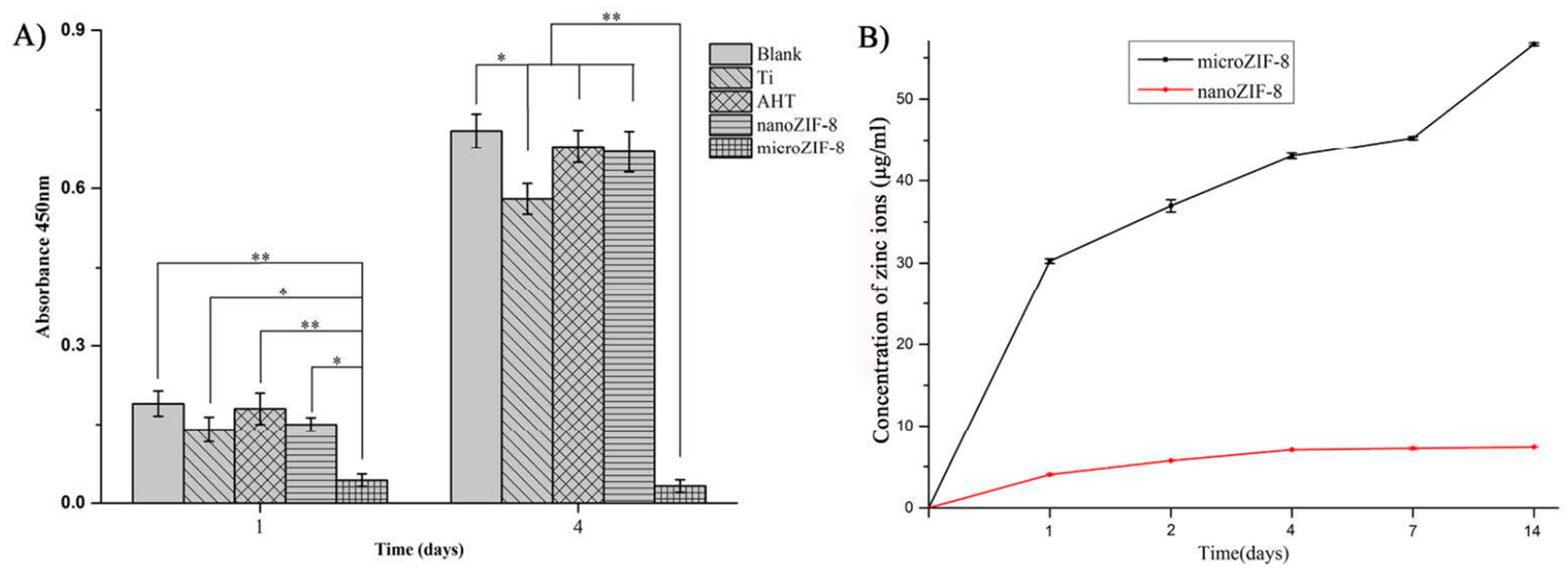
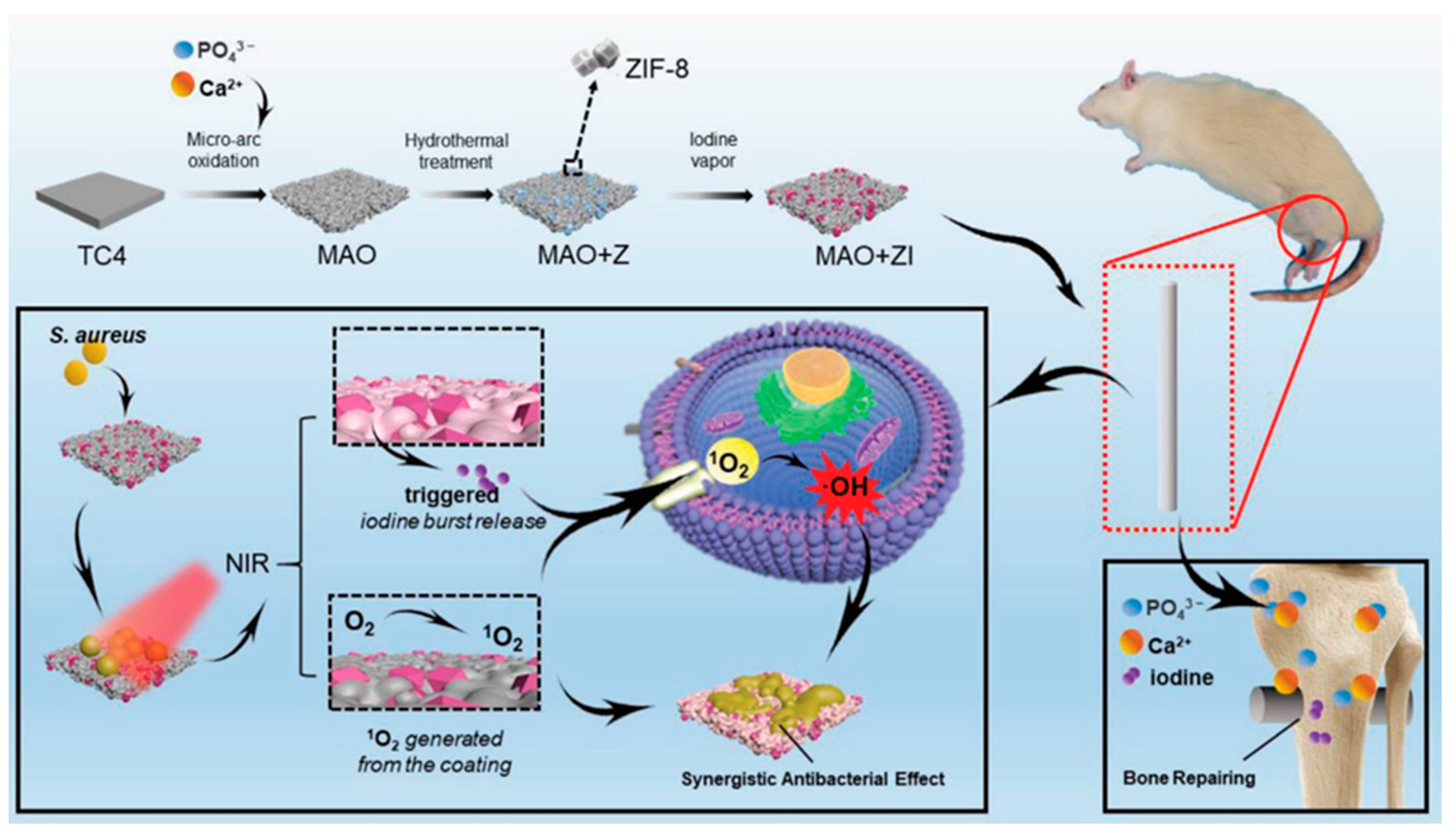
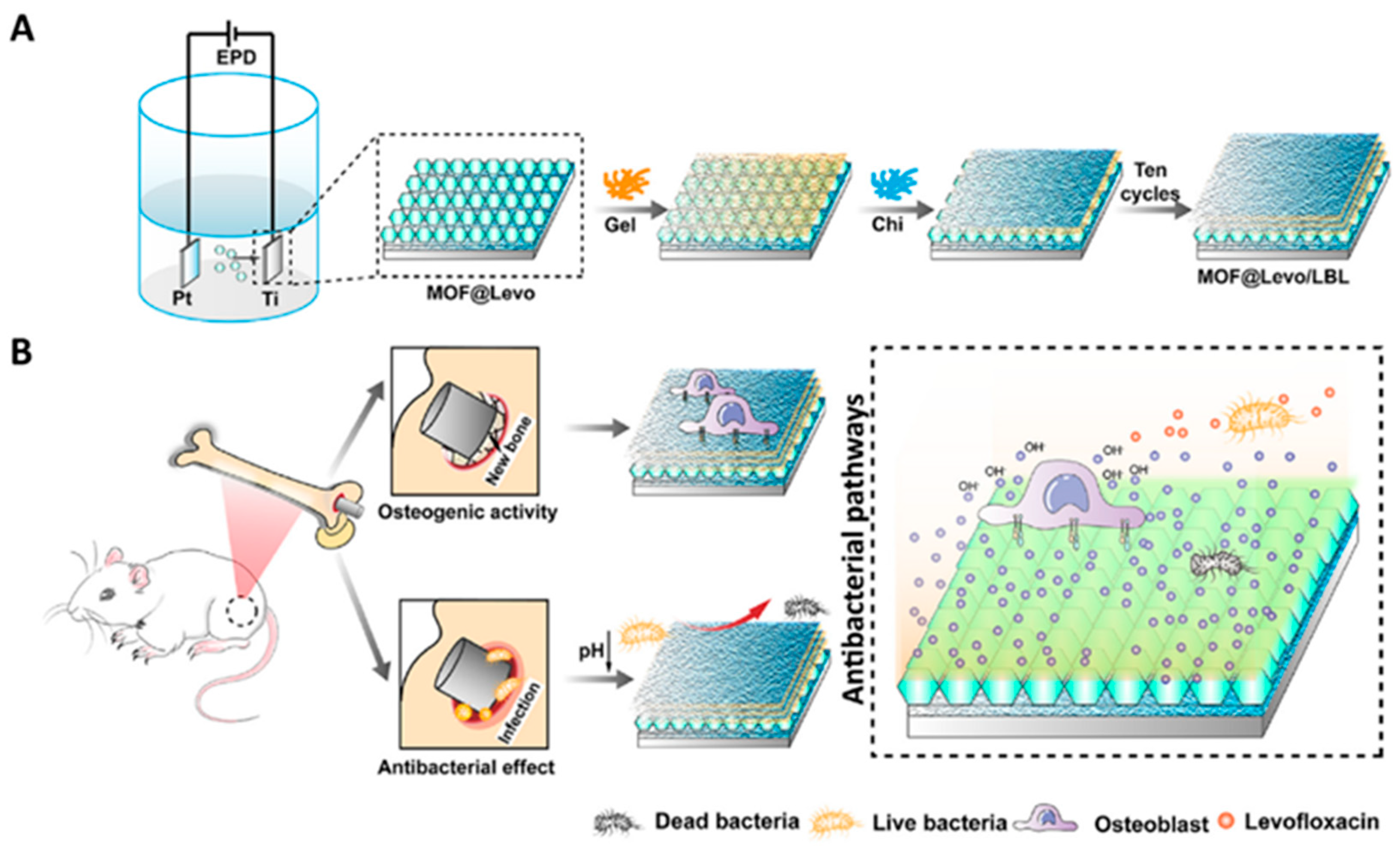
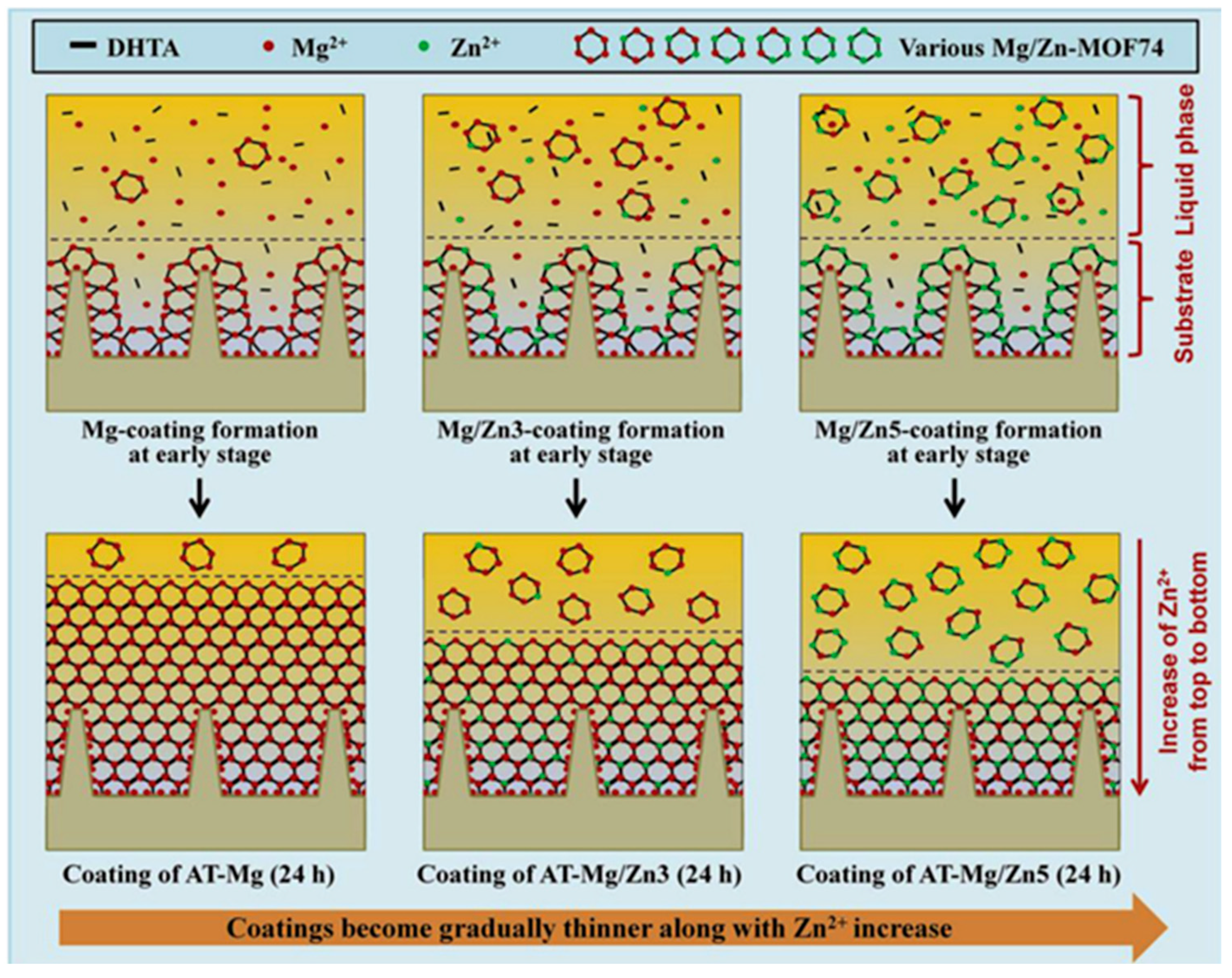
| Type of MOF | Influence of Modification on Material Properties | Ref. |
|---|---|---|
| ZIF-8 | Biocompatibility, Zn2+ release, increased collagen production, improved extracellular matrix mineralization and alkaline phosphatase activity, faster bone growth in vivo. | [68] |
| ZIF-8 | Biocompatibility, Zn2+ release, better cell adhesion, antibacterial activity | [69] |
| ZIF-8 | Biocompatibility, NIR triggered iodine release, antibacterial effect | [70] |
| ZIF-8 | Local controlled risedronate delivery | [71] |
| ZIF-8 | Controlled levofloxacin delivery, improved osteo-related genes expression, biocompatibility, antibacterial | [72] |
| ZIF-8 | Ag+ release, improved antibacterial effect, biocompatibility better corrosion resistance | [77] |
| ZIF-8 | Controlled dexamethasone delivery, biocompatibility, enhanced ALP activity | [78] |
| MOF-74 | Antibacterial, Zn2+ release, enhanced osteo-related genes expression, biocompatibility | [73] |
| ZIF-67 | Osteogenic growth peptide delivery, Co2+ release, biocompatibility, antibacterial | [74] |
| Bio-MOF-1 | Enhanced osteo-related genes expression, improved ALP activity, better cell proliferation, and faster bone growth in vivo. | [76] |
| MIL-125-Ti | Improved corrosion resistance, biocompatibility, Cerium release, Ca and P release, antibacterial | [75] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jakubowski, M.; Domke, A.; Voelkel, A.; Sandomierski, M. Biomedical Applications of Titanium Alloys Modified with MOFs—Current Knowledge and Further Development Directions. Crystals 2023, 13, 257. https://doi.org/10.3390/cryst13020257
Jakubowski M, Domke A, Voelkel A, Sandomierski M. Biomedical Applications of Titanium Alloys Modified with MOFs—Current Knowledge and Further Development Directions. Crystals. 2023; 13(2):257. https://doi.org/10.3390/cryst13020257
Chicago/Turabian StyleJakubowski, Marcel, Aleksandra Domke, Adam Voelkel, and Mariusz Sandomierski. 2023. "Biomedical Applications of Titanium Alloys Modified with MOFs—Current Knowledge and Further Development Directions" Crystals 13, no. 2: 257. https://doi.org/10.3390/cryst13020257
APA StyleJakubowski, M., Domke, A., Voelkel, A., & Sandomierski, M. (2023). Biomedical Applications of Titanium Alloys Modified with MOFs—Current Knowledge and Further Development Directions. Crystals, 13(2), 257. https://doi.org/10.3390/cryst13020257










