Carbonate and Oxalate Crystallization Effected by the Metabolism of Fungi and Bacteria in Various Trophic Conditions: The Case of Penicillium chrysogenum and Penicillium chrysogenum with Bacillus subtilis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Bacterial and Fungal Strain Identification
2.2. Bioinspired Syntheses
2.3. Methods and Approaches
2.3.1. Powder X-ray Diffraction (PXRD)
2.3.2. Scanning Electron Microscopy (SEM) and Energy-Dispersive X-ray Spectroscopy (EDXS)
2.3.3. Chromatography–Mass Spectrometry (GC-MS Analysis)
2.3.4. Determination of Biomass, EPS Content and pH Values
3. Results
3.1. The Growth of the Biomass on Marble Surface
3.1.1. P. chrysogenum
3.1.2. P. chrysogenum–B. subtilis Association
3.2. The Metabolism of Microorganisms and the pH of Liquid Medium
3.2.1. P. chrysogenum
3.2.2. P. chrysogenum–B. subtilis association
3.3. The Phase Composition and the Morphology of Crystals
3.3.1. Experiments with P. chrysogenum
3.3.2. Experiments with P. chrysogenum–B. subtilis Association
4. Discussion
4.1. The Effect of Trophic Conditions on the Growth and Metabolism of P. chrysogenum and the P. chrysogenum–B. subtilis Association
4.2. The Effect of the Microbial Metabolism on the pH
4.3. The Effect of Trophic Conditions on the Crystallization by P. chrysogenum and the P. chrysogenum–B. subtilis Association on the Marble Surface
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rousk, J.; Bengtson, P. Microbial regulation of global biogeochemical cycles. Front. Microbiol. 2014, 5, 103. [Google Scholar] [CrossRef] [Green Version]
- Gadd, G.M. Metals, minerals and microbes: Geomicrobiology and bioremediation. Microbiology 2010, 156, 609–643. [Google Scholar] [CrossRef]
- Boniek, D.; MendesIsolda, I.C.; Abreu, C.M.; Abreu, O.C.; Show, O.; Stoianoff, M.A.R. Ecology and identification of environmental fungi and metabolic processes involved in the biodeterioration of Brazilian soapstone historical monuments. Lett. Appl. Microbiol. 2017, 65, 431–438. [Google Scholar] [CrossRef]
- Warscheid, T.; Braams, J. Biodeterioration of stone: A review. Int. Biodeterior. Biodegrad. 2000, 46, 343–368. [Google Scholar] [CrossRef]
- Bravo, D.; Martin, G.M.; Cailleau, G.; Verrecchia, E.; Junier, P. Identification of active oxalotrophic bacteria by Bromodeoxyuridine DNA-labeling in a microcosm soil experiments. FEMS Microbiol. Lett. 2013, 348, 103–111. [Google Scholar] [CrossRef] [Green Version]
- Syed, S.; Buddolla, V.; Lian, B. Oxalate Carbonate Pathway—Conversion and Fixation of Soil Carbon—A Potential Scenario for Sustainability. Front. Plant Sci. 2020, 11, 591297. [Google Scholar] [CrossRef]
- Verrecchia, E.P.; Braissant, O.; Cailleau, G. The oxalate–carbonate pathway in soil carbon storage: The role of fungi and oxalotrophic bacteria. In Fungi in Biogeochemical Cycles; Gadd, G.M., Ed.; Cambridge University Press: Cambridge, UK, 2006; pp. 289–310. [Google Scholar] [CrossRef]
- Gupta, A.; Gupta, R.; Singh, R.L. Microbes and Environment. Princ. Appl. Environ. Biotechnol. A Sustain. Future 2016, 15, 43–84. [Google Scholar] [CrossRef]
- Sazanova, K.V.; Frank-Kamenetskaya, O.V.; Vlasov, D.Y.; Zelenskaya, M.S.; Vlasov, A.D.; Rusakov, A.V.; Petrova, M.A. Carbonate and Oxalate Crystallization by Interaction of Calcite Marble with Bacillus subtilis and Bacillus subtilis–Aspergillus niger Association. Crystals 2020, 10, 756. [Google Scholar] [CrossRef]
- Konopka, A. What is microbial community ecology? ISME J. 2009, 3, 1223–1230. [Google Scholar] [CrossRef]
- Bengtsson-Palme, J. Microbial model communities: To understand complexity, harness the power of simplicity. Comput. Struct. Biotechnol. J. 2020, 18, 3987–4001. [Google Scholar] [CrossRef]
- Gralka, M.; Szabo, R.; Stocker, R.; Cordero, O.X. Trophic Interactions and the Drivers of Microbial Community Assembly. Curr. Biol. 2020, 30, 1176–1188. [Google Scholar] [CrossRef]
- Achal, V.; Mukherjee, A. A review of microbial precipitation for sustainable construction. Constr. Build. Mater. 2015, 93, 1224–1235. [Google Scholar] [CrossRef]
- Wang, R.; Qian, C. In situ restoration of the surface defects on cement-based materials by bacteria mineralization with spraying method. J. Wuhan Univ. Technol.-Mat. Sci. Ed. 2014, 29, 518–526. [Google Scholar] [CrossRef]
- Dejong, J.T.; Fritzges, M.B.; Nüsslein, K. Microbially induced cementation to control sand response to undrained shear. J. Geotech. Geoenviron. Eng. 2006, 132, 1381–1392. [Google Scholar] [CrossRef]
- Reeder, R.J.; Lamble, G.M.; Northrup, P.A. XAFS study of the coordination and local relaxation around Co2+, Zn2+, Pb2+, and Ba2+, trace elements in calcite. Am. Mineral. 1999, 84, 1049–1060. [Google Scholar] [CrossRef]
- Kumari, D.; Quan, X.-Y.; Pan, X.; Achal, V.; Li, Q.; Gadd, G.M. Microbially-induced Carbonate Precipitation for Immobilization of Toxic Metals. Adv. Appl. Microbiol. 2016, 94, 79–108. [Google Scholar] [CrossRef] [Green Version]
- Lin, W.; Huang, Z.; Li, X.; Liu, M.; Cheng, Y. Bio-remediation of acephate-Pb(II) Compound Contaminants by Bacillus Subtilis FZUL-33. J. Environ. Sci. 2016, 45, 94–99. [Google Scholar] [CrossRef]
- Sturm, E.V.; Frank-Kamenetskaya, O.V.; Vlasov, D.Y.; Zelenskaya, M.S.; Sazanova, K.V.; Rusakov, A.V.; Kniep, R. Crystallization of calcium oxalate hydrates by interaction of calcite marble with fungus Aspergillus niger. Am. Mineral. 2015, 100, 2559–2565. [Google Scholar] [CrossRef]
- Dutton, M.V.; Evants, C.S. Oxalate production by fungi: Its role in pathogenicity and ecology in the soil environment. Can. J. Microbiol. 1996, 42, 881–895. [Google Scholar] [CrossRef]
- Minir, E.; Yoon, J.; Tokimatsu, T.; Shimada, M. A physiological role for oxalic acid biosynthesis in the wood-rotting basidiomycete Fomitopsis palustris. Proc. Natl. Acad. Sci. USA 2001, 98, 11126–11130. [Google Scholar] [CrossRef] [Green Version]
- Schilling, J.S.; Jellison, J. Oxalate regulation by two brown rot fungi decaying oxalate-amended and non-amended wood. Holzforschung 2005, 59, 681–688. [Google Scholar] [CrossRef]
- Sazanova, K.V.; Vlasov, D.Y.; Osmolovskay, N.G.; Schiparev, S.M.; Rusakov, A.V. Significance and regulation of acids production by rock-inhabited fungi. In Biogenic-Abiogenic Interactions in Natural and Anthropogenic Systems; Frank-Kamenetskaya, O.V., Panova, E.G., Vlasov, D.Y., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 379–392. [Google Scholar] [CrossRef]
- Guggiari, M.; Bloque, R.; Aragno, M.; Verrecchia, E.; Job, D.; Junier, P. Experimental calcium-oxalate crystal production and dissolution by selected wood-rot fungi. Int. Biodeterior. Biodegrad. 2011, 65, 803–809. [Google Scholar] [CrossRef] [Green Version]
- Zhu, T.; Dittrich, M. Carbonate Precipitation through Microbial Activities in Natural environment, and Their Potential in Biotechnology: A Review. Front. Bioeng. Biotechnol. 2016, 4, 4. [Google Scholar] [CrossRef] [Green Version]
- Anbu, P.; Kang, C.-H.; Shin, Y.-J.; Socorresponding, J.-S. Formations of calcium carbonate minerals by bacteria and its multiple applications. Springerplus 2016, 5, 250. [Google Scholar] [CrossRef] [Green Version]
- Sazanova, K.; Osmolovskaya, N.; Schiparev, S.; Yakkonen, K.; Kuchaeva, L.; Vlasov, D. Organic acids induce tolerance to zinc- and copper-exposed fungi under various growth conditions. Curr. Microbiol. 2015, 70, 520–527. [Google Scholar] [CrossRef]
- Fang, C.; Kumari, D.; Zhu, X.; Achal, V. Role of fungal-mediated mineralization in biocementation of sand and its improved compressive strength. Int. Biodeterior. Biodegrad. 2018, 133, 216–220. [Google Scholar] [CrossRef]
- Mahapatra, S.; Banerjee, D. Fungal exopolysaccharide: Production, composition and applications. Microbiol. Insights 2013, 6, 1MBI-S10957. [Google Scholar] [CrossRef] [Green Version]
- Doyle, J.J.; Doyle, J.L. A rapid DNA isolation procedure for small quantities of fresh leaf tissue. Phytochem. Bull. 1987, 19, 11–15. [Google Scholar]
- White, T.J.; Bruns, T.; Lee, S.; Taylor, J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In PCR Protocols: A Guide to Methods and Applications; Innis, M.A., Gelfand, D.H., Sninsky, J.J., White, T.J., Eds.; Academic Press: London, UK, 1990; pp. 315–322. [Google Scholar]
- Santamaria, F.; Reyes, F. Proteases produced during autolysis of filamentous fungi. Trans. Br. Mycol. Soc. 1988, 91, 217–220. [Google Scholar] [CrossRef]
- Ghorbani, Y.; Oliazadeh, M.; Shahvedi, A.; Roohi, R.; Pirayehgar, A. Use of some isolated fungi in biological Leaching of Aluminum from low grade bauxite. Afr. J. Biotechnol. 2007, 6, 1284–1288. [Google Scholar]
- Kuz’mina, M.A.; Rusakov, A.V.; Frank-Kamenetskaya, O.V.; Vlasov, D.Y. The influence of inorganic and organic components of biofilms with microscopic fungi on the phase composition and morphology of crystallizing calcium oxalates. Crystallogr. Rep. 2019, 64, 161–167. [Google Scholar] [CrossRef]
- Frank-Kamenetskaya, O.V.; Ivanyuk, G.Y.; Zelenskaya, M.S.; Izatulina, A.R.; Kalashnikov, A.O.; Vlasov, D.Y.; Polyanskaya, E.I. Calcium Oxalates in Lichens on Surface of Apatite-Nepheline Ore (Kola Peninsula, Russia). Minerals 2019, 9, 656. [Google Scholar] [CrossRef] [Green Version]
- Rusakov, A.; Kuzmina, M.; Frank-Kamenetskaya, O. Biofilm Medium Chemistry and Calcium Oxalate Morphogenesis. Molecules 2021, 26, 5030. [Google Scholar] [CrossRef]
- Frank-Kamenetskaya, O.V.; Zelenskaya, M.S.; Izatulina, A.R.; Vereshchagin, O.S.; Vlasov, D.Y.; Himelbrant, D.E.; Pankin, D.V. Copper oxalate formation by lichens and fungi. Sci. Rep. 2021, 11, 24239. [Google Scholar] [CrossRef]
- Frank-Kamenetskaya, O.V.; Zelenskaya, M.S.; Izatulina, A.R.; Gurzhiy, V.V.; Rusakov, A.V.; Vlasov, D.Y. Oxalate formation by Aspergillus niger on manganese ore minerals. Am. Mineral. 2022, 7, 100–109. [Google Scholar] [CrossRef]
- Alkan, N.; Espeso, E.A.; Prusky, D. Virulence Regulation of Phytopathogenic Fungi by pH. Antioxid. Redox Signal. 2013, 19, 1012–1025. [Google Scholar] [CrossRef]
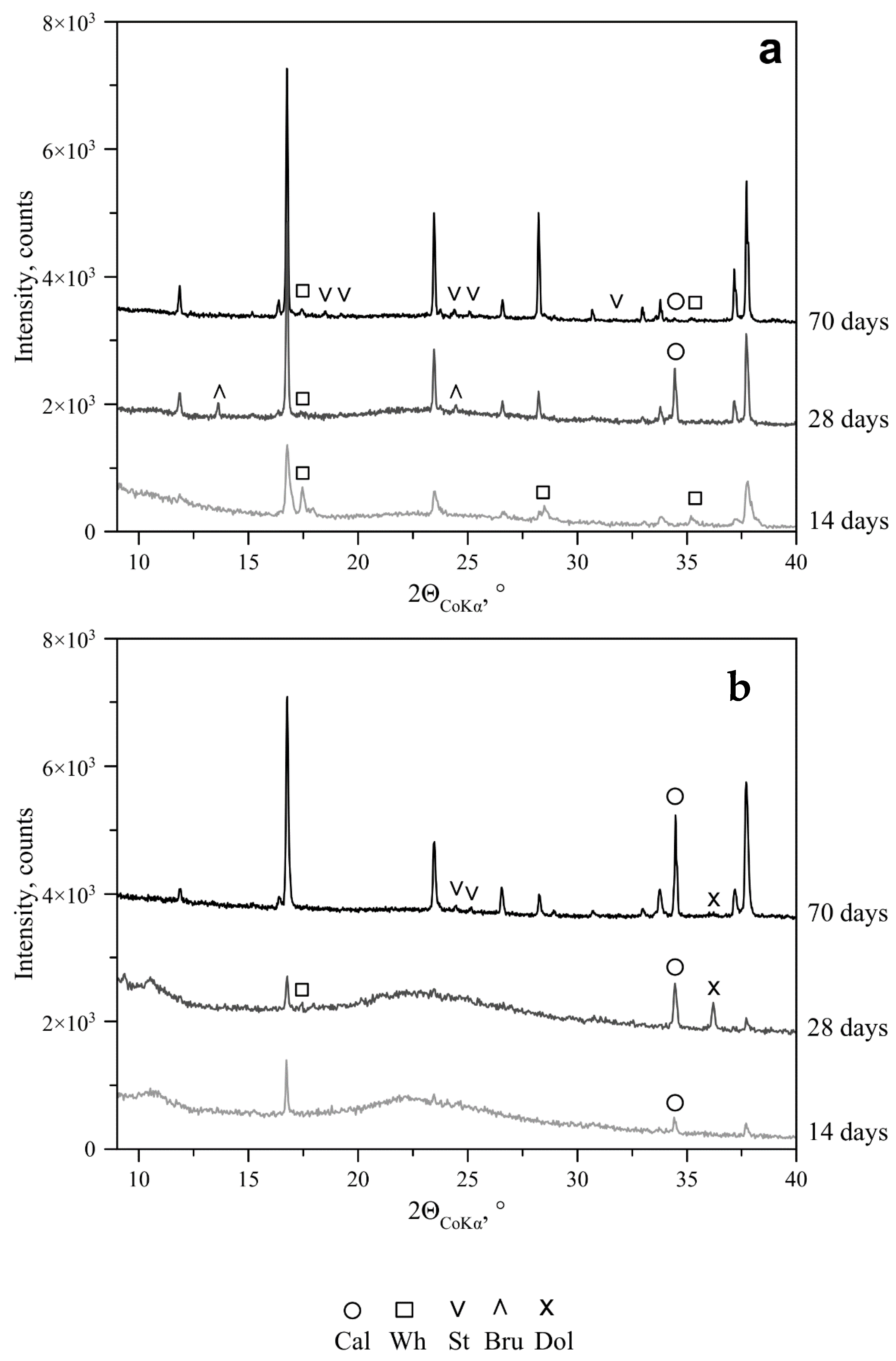

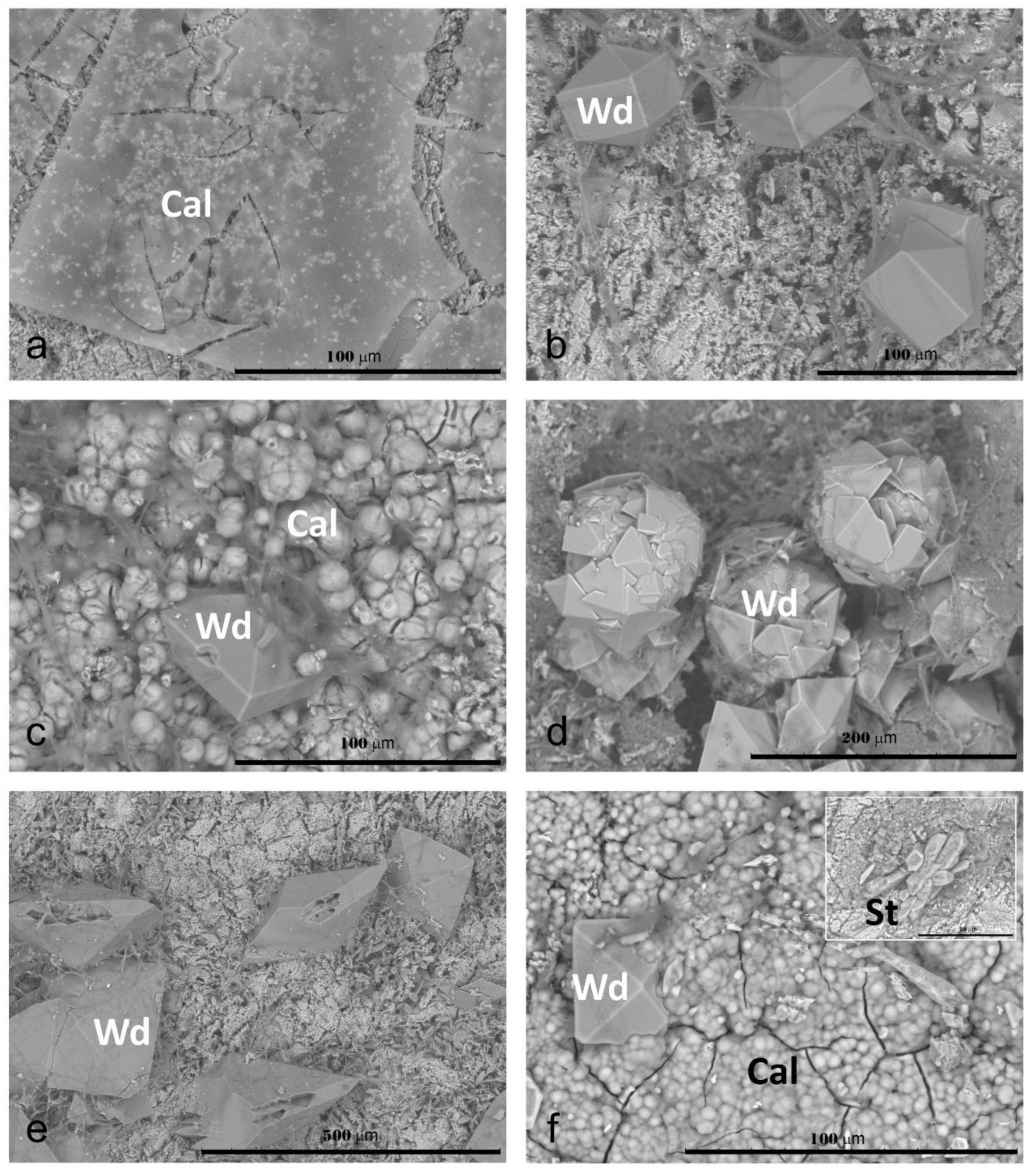
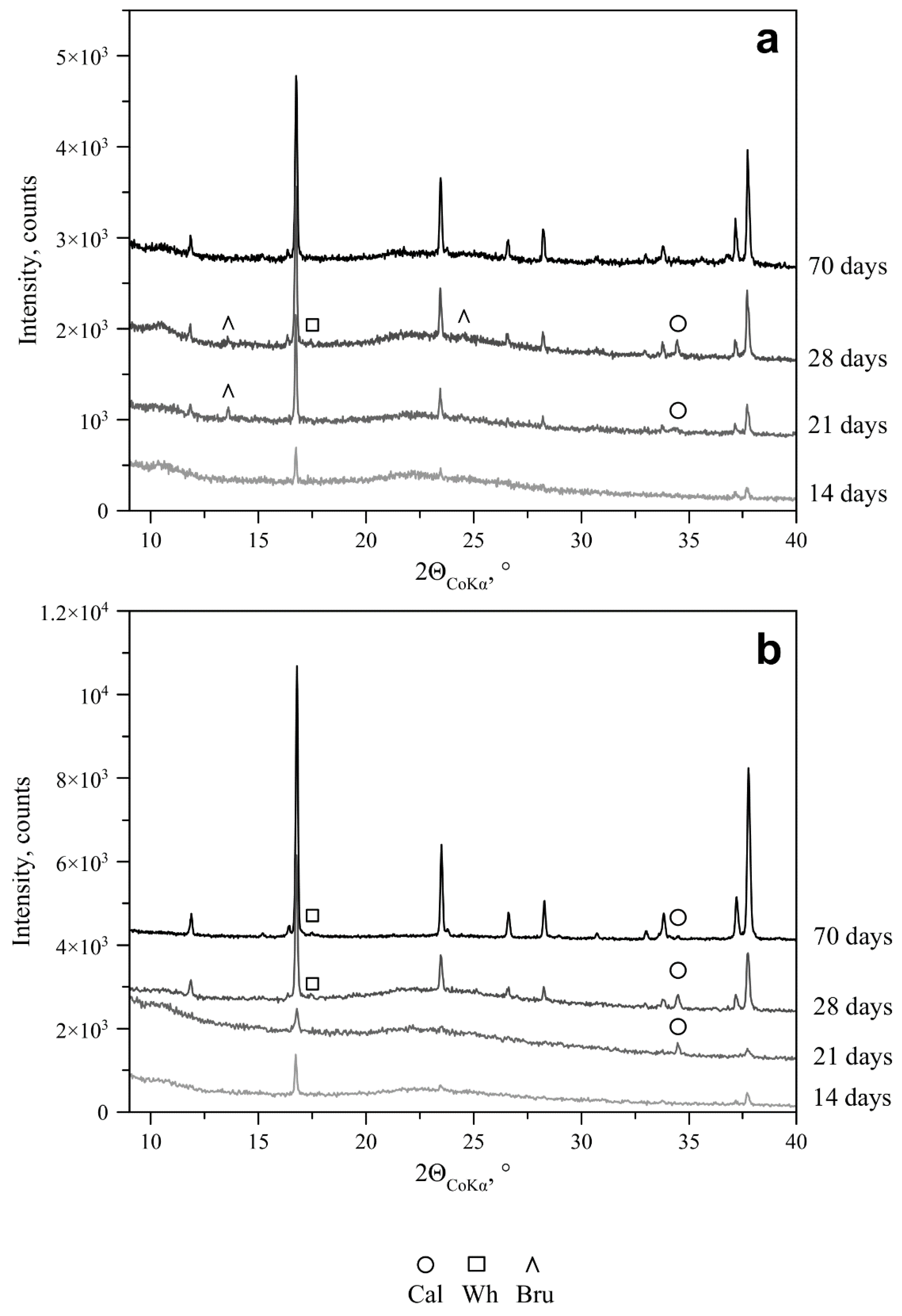

 —initial pH of Czapek–Dox medium.
—initial pH of Czapek–Dox medium.
 —initial pH of Czapek–Dox medium.
—initial pH of Czapek–Dox medium.
| Days | Glucose Concentrations, g/L | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 10 | 30 | ||||||||||
| Biomass, g/100 mL | EPS, μg/mL | Oxalic acid, μg/mL | pH | Biomass, g/100 mL | EPS, μg/mL | Oxalic Acid, μg/mL | pH | Biomass, g/100 mL | EPS, μg/mL | Oxalic Acid, μg/mL | pH | |
| 7 | 0.03 | trace | trace | 6.5 | 0.28 | trace | 32.4 ± 7.4 | 6.2 | 0.23 | 1080 ± 96 | 64.1 ± 0.6 | 4.5 |
| 14 | 0.08 | 0.03 | 3.8 ± 0.4 | 6.5 | 0.41 | trace | 82.6 ± 9.2 | 6.0 | 0.50 | 1550 ± 62 | 386.5 ± 12.9 | 4.2 |
| 21 | 0.16 | 0.05 | 5.8 ± 0.9 | 6.5 | 0.54 | 510 ± 29 | 153.3 ± 11.13 | 6.0 | 0.75 | 1680 ± 85 | 452.6 ± 22.9 | 3.8 |
| 28 | 0.23 | 220 ± 18 | 9.9 ± 0.6 | 6.0 | 0.56 | 550 ± 35 | 224.7 ± 9.0 | 5.5 | 0.82 | 30,240 ± 2004 | 474.8 ± 34.1 | 3.9 |
| 70 | 0.27 | 388 ± 58 | 38.4 ± 3.8 | 6.0 | 0.46 | 1183 ± 2083 | 311.3 ± 27.0 | 6.5 | 0.74 | 58,046 ± 4008 | 436.0 ± 24.8 | 7.0 |
| Days | Glucose Concentrations, g/L | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 10 | 30 | ||||||||||
| Biomass, g/100 mL | EPS, μg/mL | Oxalic Acid, μg/mL | pH | Biomass, g/100 mL | EPS, μg/mL | Oxalic Acid, μg/mL | pH | Biomass, g/100 mL | EPS, μg/mL | Oxalic Acid, μg/mL | pH | |
| 7 | 0.02 | 330 ± 16 | 0.05 | 7.0 | 0.23 | 690 ± 37 | 44.6 ± 6.8 | 5.5 | 0.24 | 1180 ± 54 | 74.1 ± 1.5 | 4.5 |
| 14 | 0.08 | 360 ± 21 | 4.7 ± 0.7 | 6.5 | 0.48 | 810 ± 51 | 92.3 ± 7.9 | 5.7 | 0.58 | 2450 ± 71 | 414.8 ± 18.1 | 5.5 |
| 21 | 0.09 | 460 ± 28 | 6.3 ± 0.4 | 7.2 | 0.58 | 950 ± 44 | 184.2 ± 9.2 | 6.5 | 0.78 | 3930 ± 144 | 464.1 ± 24.7 | 5.0 |
| 28 | 0.29 | 570 ± 33 | 10.2 ± 0.6 | 7.0 | 0.61 | 870 ± 58 | 286.2 ± 12.9 | 7.2 | 0.83 | 55,400 ± 3008 | 516.7 ± 38.8 | 6.2 |
| 70 | 0.30 | 601 ± 52 | 41.1 ± 8.5 | 7.0 | 0.58 | 1834 ± 178 | 334.6 ± 18.8 | 8.0 | 0.80 | 64,300 ± 8734 | 480.1 ± 32.2 | 7.0 |
| Days | Culture | Acid Concentrations, μg/mL * | ||||
|---|---|---|---|---|---|---|
| Gluconic | Citric | Fumaric | Malic | Succinic | ||
| 7 | P.chrysogenum | 90.1 ± 7.1 | 7.8 ± 0.4 | 1.2 ± 0.3 | trace | 3.4 ± 0.4 |
| P. chrysogenum–B. subtilis | 120.8 ± 9.1 | 9.4 ± 1.1 | 3.1 ± 0.4 | trace | 8.4 ± 0.9 | |
| 14 | P.chrysogenum | 40.8 ± 3.4 | 20.8 ± 3.0 | 9.5 ± 0.8 | 1.8 ± 0.3 | 17.4 ± 1.1 |
| P. chrysogenum–B. subtilis | 64.4 ± 5.0 | 18.7 ± 4.4 | 14.8 ± 0.9 | 1.7 ± 0.3 | 32.5 ± 4.0 | |
| 21 | P.chrysogenum | 0.21 ± 0.04 | 1.8 ± 0.04 | 1.8 ± 0.3 | 3.8 ± 0.4 | 3.3 ± 0.4 |
| P. chrysogenum–B. subtilis | 1.9 ± 0.3 | 5.2 ± 0.08 | 1.1 ± 0.2 | 3.4 ± 0.7 | 1.4 ± 0.2 | |
| 28 | P.chrysogenum | 0.09 ± 0.02 | 1.3 ± 0.04 | 1.0 ± 0.1 | 4.0 ± 0.3 | 0.4 ± 0.07 |
| P. chrysogenum–B. subtilis | 0.14 ± 0.03 | 0.11 ± 0.02 | 0.3 ± 0.02 | 4.4 ± 0.5 | 0.13 ± 0.03 | |
| 70 | P.chrysogenum | not found | not found | not found | not found | not found |
| P. chrysogenum–B. subtilis | not found | not found | not found | not found | not found | |
| Days | Glucose Content, g/L | |||||
|---|---|---|---|---|---|---|
| 1 | 10 | 30 | ||||
| Phase Composition | pH | Phase Composition | pH | Phase Composition | pH | |
| 7 | not found | 6.5 | Wd | 6.25 | not found | 4.5 |
| 14 | Bru | 6.5 | Wd > Wh | 6 | Wd, Bru | 4.25 |
| 21 | Bru, Cal | 6.5 | Wd, Bru, Cal (trace) | 6.0 | Wd, Bru, Cal | 3.75 |
| 28 | Bru, Cal | 6.0 | Wd > Wh, Cals, Bru | 5.5 | Wd > Wh, Bru (trace), Cal, Dol | 3.75 |
| 70 | Bru, Wh (trace) | 6.0 | Wd > Wh, Cals, St | 6.5 | Wd, Cals, Dol, Bru, St | 7.0 |
| Days | Glucose Content, g/L | |||||
|---|---|---|---|---|---|---|
| 1 | 10 | 30 | ||||
| Phase Composition | pH | Phase Composition | pH | Phase Composition | pH | |
| 7 | Br | 7.0 | Wd (trace) | 5.5 | Not found | 4.5 |
| 14 | Cals * | 6.5 | Wd, Bru | 5.75 | Wd > Wh * | 5.5 |
| 21 | Cals, Bru, St | 7.25 | Wd > Wh *, Bru, Cals (trace) | 6.5 | Wd, Bru, Cal (trace) | 5.0 |
| 28 | Cals | 7.0 | Wd > Wh, Bru, Cals | 7.25 | Wd > Wh, Cals * | 6.25 |
| 70 | Br | 7.0 | Wd, Cals *, St (trace) | 8.0 | Wd > Wh, Cals (trace), Bru, St (trace) | 7.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sazanova, K.V.; Zelenskaya, M.S.; Izatulina, A.R.; Korneev, A.V.; Vlasov, D.Y.; Frank-Kamenetskaya, O.V. Carbonate and Oxalate Crystallization Effected by the Metabolism of Fungi and Bacteria in Various Trophic Conditions: The Case of Penicillium chrysogenum and Penicillium chrysogenum with Bacillus subtilis. Crystals 2023, 13, 94. https://doi.org/10.3390/cryst13010094
Sazanova KV, Zelenskaya MS, Izatulina AR, Korneev AV, Vlasov DY, Frank-Kamenetskaya OV. Carbonate and Oxalate Crystallization Effected by the Metabolism of Fungi and Bacteria in Various Trophic Conditions: The Case of Penicillium chrysogenum and Penicillium chrysogenum with Bacillus subtilis. Crystals. 2023; 13(1):94. https://doi.org/10.3390/cryst13010094
Chicago/Turabian StyleSazanova, Katerina V., Marina S. Zelenskaya, Alina R. Izatulina, Anatoliy V. Korneev, Dmitry Yu. Vlasov, and Olga V. Frank-Kamenetskaya. 2023. "Carbonate and Oxalate Crystallization Effected by the Metabolism of Fungi and Bacteria in Various Trophic Conditions: The Case of Penicillium chrysogenum and Penicillium chrysogenum with Bacillus subtilis" Crystals 13, no. 1: 94. https://doi.org/10.3390/cryst13010094






