Hexagonal Nanocrystal Growth of Mg or Zn from Incorporation in GaN Powders Obtained through Pyrolysis of a Viscous Complex Compound and Its Nitridation
Abstract
:1. Introduction
2. Materials and Methods
Characterizations
3. Results and Discussion
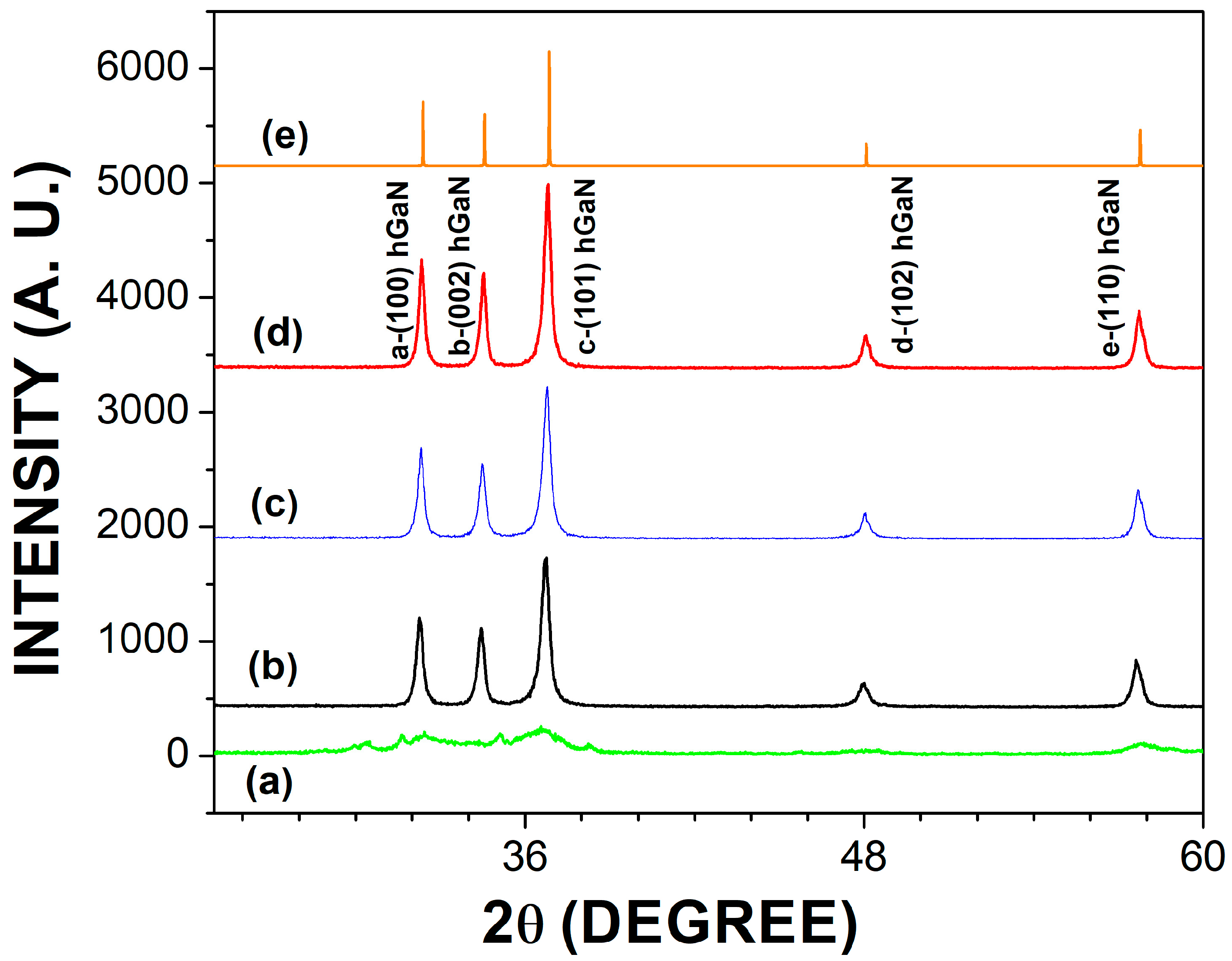
3.1. Structural Analysis
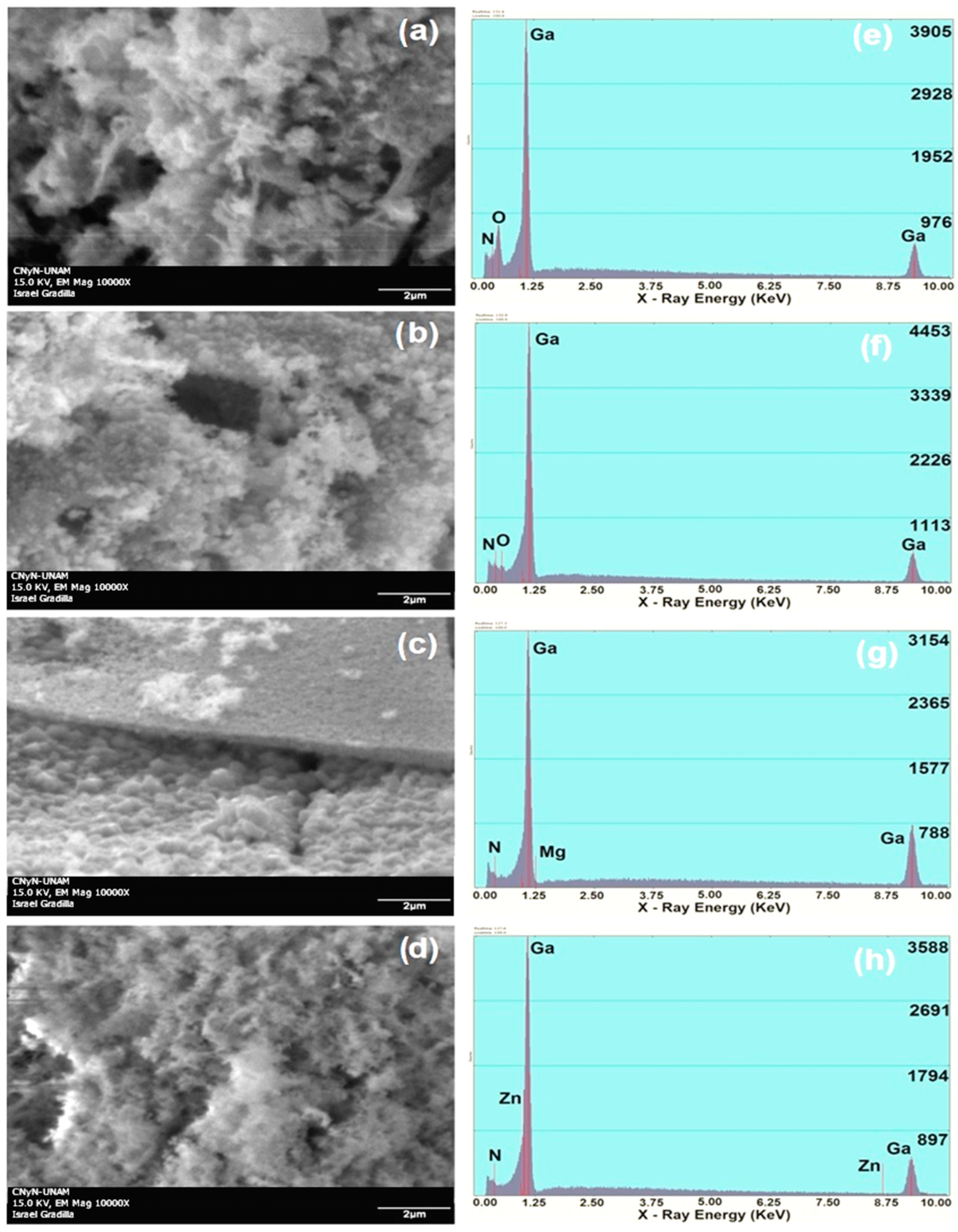
3.2. Electron Microscopy
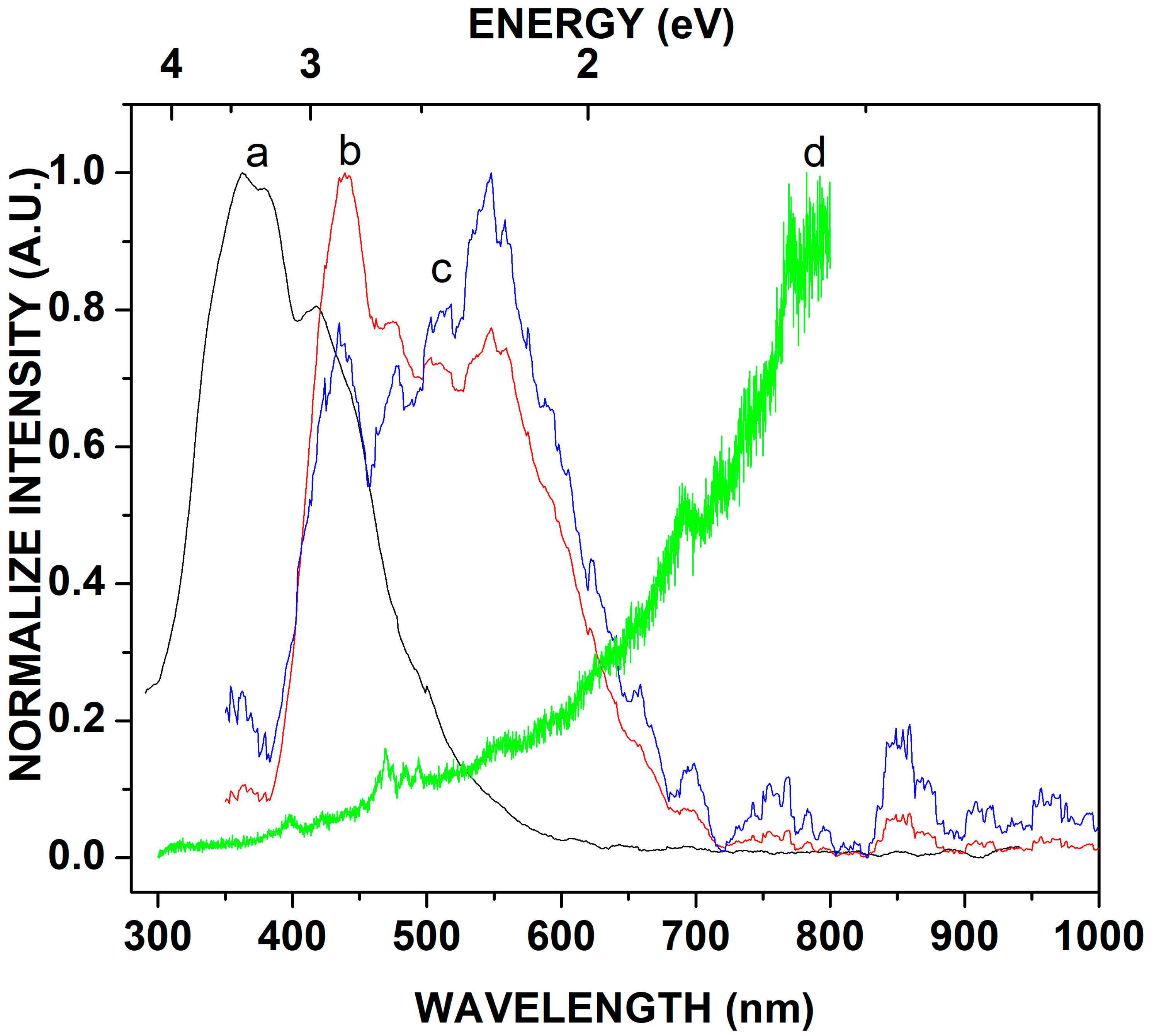
3.3. Photoluminescence
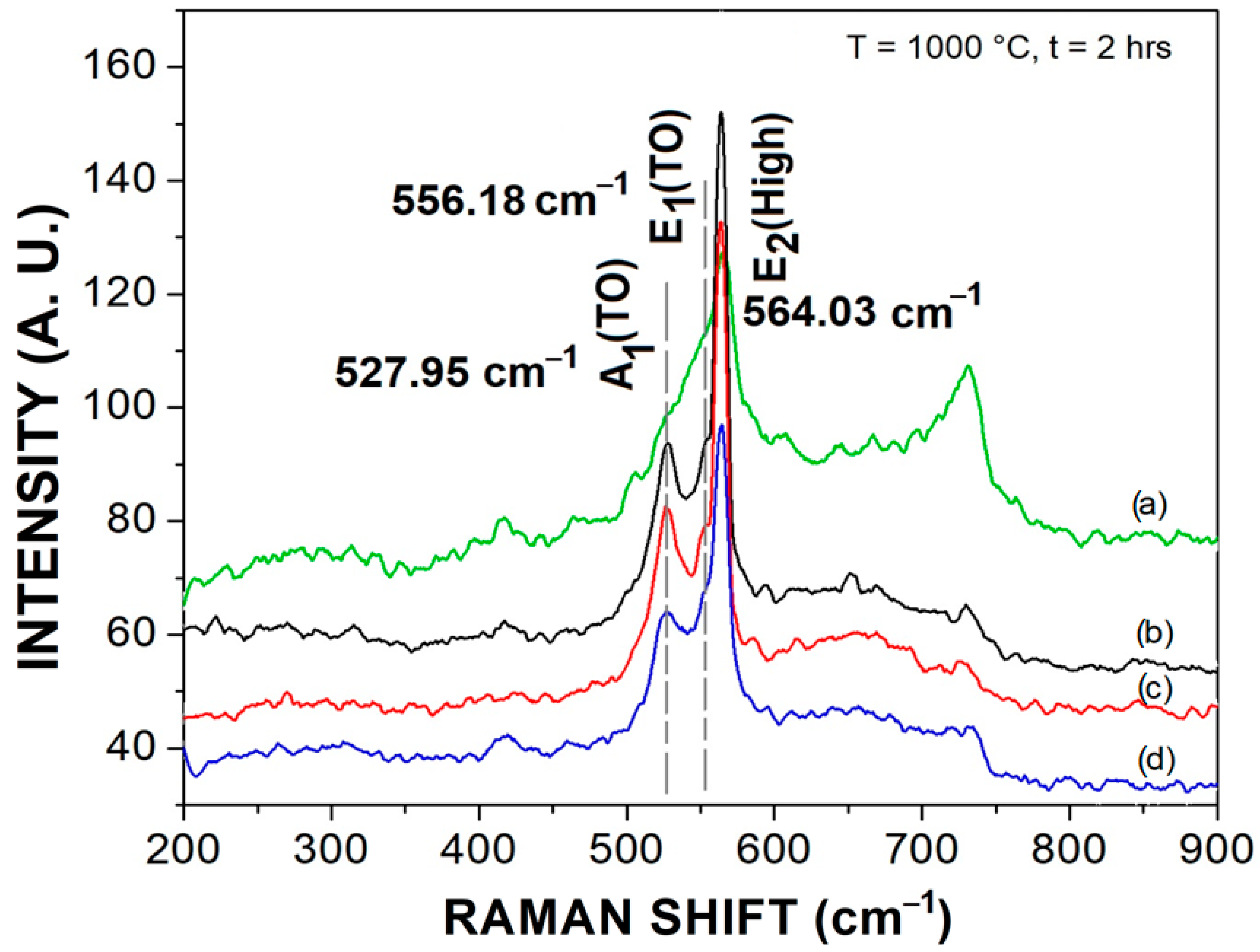
3.4. Raman Scattering
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Garcia, R.; Hirata, G.A.; Thomas, A.C.; Ponce, F.A. Structure and luminescence of nanocrystalline gallium nitride synthesized by a novel polymer pyrolysis route. Opt. Mater. 2006, 29, 19–23. [Google Scholar] [CrossRef]
- Gastellou, E.; Morales, C.; García, R.; García, G.; Hirata, G.A.; Galeazzi, R.; Herrera, A.M.; Rosendo, E.; Díaz, T.; Ramos, J.R.; et al. Enhanced crystalline size of undoped GaN powders obtained by nitridation of metallic gallium. Opt. Mater. 2018, 83, 154–196. [Google Scholar] [CrossRef]
- Benzarti, Z.; Sekrafi, T.; Bougrioua, Z.; Khalfallah, A.; El Jani, B. Effect of SiN Treatment on Optical Properties of InxGa1−xN/GaN MQW Blue LEDs. J. Electron. Mater. 2017, 46, 4312–4320. [Google Scholar] [CrossRef]
- Benzarti, Z.; Khalfallah, A.; Bougrioua, Z.; Evaristo, M.; Cavaleiro, A. Understanding the influence of physical properties on the mechanical characteristics of Mg-doped GaN thin films. Mater. Chem. Phys. 2023, 307, 128182. [Google Scholar] [CrossRef]
- Escobosa, A.; Sánchez-R, V.M.; To, M.; Navarro, A.G. Photoluminescence studies of GaN: To comparison between layers grown on sapphire and nitridated GaAs layers. Phys. Status Solidi B 2005, 242, 1883–1886. [Google Scholar] [CrossRef]
- Sim, J.K.; Um, D.Y.; Kim, J.W.; Kim, J.S.; Jeong, K.U.; Lee, C.R. Improvement in the performance of CIGS solar cells by introducing GaN nanowires on the absorber layer. J. Alloys Compd. 2019, 779, 643–647. [Google Scholar] [CrossRef]
- Amano, H.; Baines, Y.; Beam, E.; Borga, M.; Bouchet, T.; Chalker, P.R.; Charles, M.; Chen, K.J.; Chowdhury, N.; Chu, R.; et al. The 2018 GaN power electronics roadmap. J. Phys. D Appl. Phys. 2018, 51, 163001. [Google Scholar] [CrossRef]
- Maruyama, T.; Miyake, H. Gallium nitride thin films deposited by radio-frequency magnetron sputtering. J. Vac. Sci. Technol. A 2006, 4, 1096–1099. [Google Scholar] [CrossRef]
- Langford, I.; Wilson, A.J.C. Sherrer after sixty years: A survey and some new results in the determination of crystallite size. J. Appl. Crystallogr. 1978, 11, 102–113. [Google Scholar] [CrossRef]
- Ilegems, M.; Dingle, R. Luminescence of Be- and Mg-doped GaN. J. Appl. Phys. 1973, 44, 4234–4235. [Google Scholar] [CrossRef]
- Pankove, J.I.; Berkeyheiser, J.E.; Miller, E.A. Properties of Zn-doped GaN. I. Photoluminescence. J. Appl. Phys. 1974, 45, 1280–1286. [Google Scholar] [CrossRef]
- Reshchikov, M.A.; Morkoç, H. Luminescence properties of defects in GaN. J. Appl. Phys. 2005, 97, 061301. [Google Scholar] [CrossRef]
- Kumar, M.S.; Kumar, J. XRD, XPS, SEM, PL and Raman scattering analysis ofsynthesized GaN powder. Mater. Chem. Phys. 2002, 77, 341–345. [Google Scholar] [CrossRef]
- Kuball, M. Raman spectroscopy of GaN, AlGaN and AlN for process and growth monitoring/control. Surf. Interface Anal. 2001, 31, 987–999. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gastellóu, E.; García, R.; Herrera, A.M.; Ramos, A.; García, G.; Hirata, G.A.; Luna, J.A.; Carrillo, R.C.; Rodríguez, J.A.; Robles, M.; et al. Hexagonal Nanocrystal Growth of Mg or Zn from Incorporation in GaN Powders Obtained through Pyrolysis of a Viscous Complex Compound and Its Nitridation. Crystals 2023, 13, 1421. https://doi.org/10.3390/cryst13101421
Gastellóu E, García R, Herrera AM, Ramos A, García G, Hirata GA, Luna JA, Carrillo RC, Rodríguez JA, Robles M, et al. Hexagonal Nanocrystal Growth of Mg or Zn from Incorporation in GaN Powders Obtained through Pyrolysis of a Viscous Complex Compound and Its Nitridation. Crystals. 2023; 13(10):1421. https://doi.org/10.3390/cryst13101421
Chicago/Turabian StyleGastellóu, Erick, Rafael García, Ana M. Herrera, Antonio Ramos, Godofredo García, Gustavo A. Hirata, José A. Luna, Roberto C. Carrillo, Jorge A. Rodríguez, Mario Robles, and et al. 2023. "Hexagonal Nanocrystal Growth of Mg or Zn from Incorporation in GaN Powders Obtained through Pyrolysis of a Viscous Complex Compound and Its Nitridation" Crystals 13, no. 10: 1421. https://doi.org/10.3390/cryst13101421






