Corrosion and Wear-Resistant Composite Zirconium Nitride Layers Produced on the AZ91D Magnesium Alloy in Hybrid Process Using Hydrothermal Treatment
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
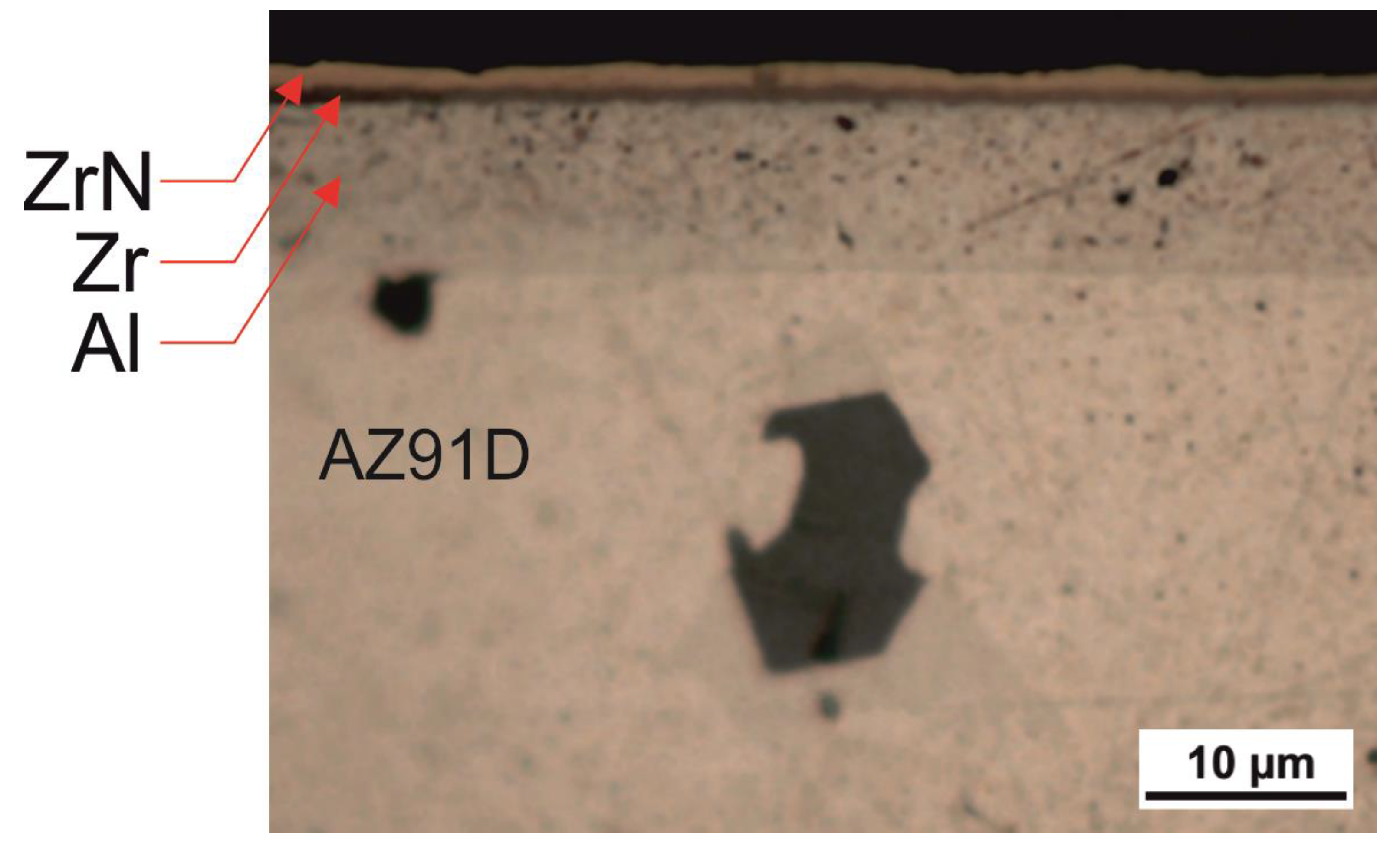
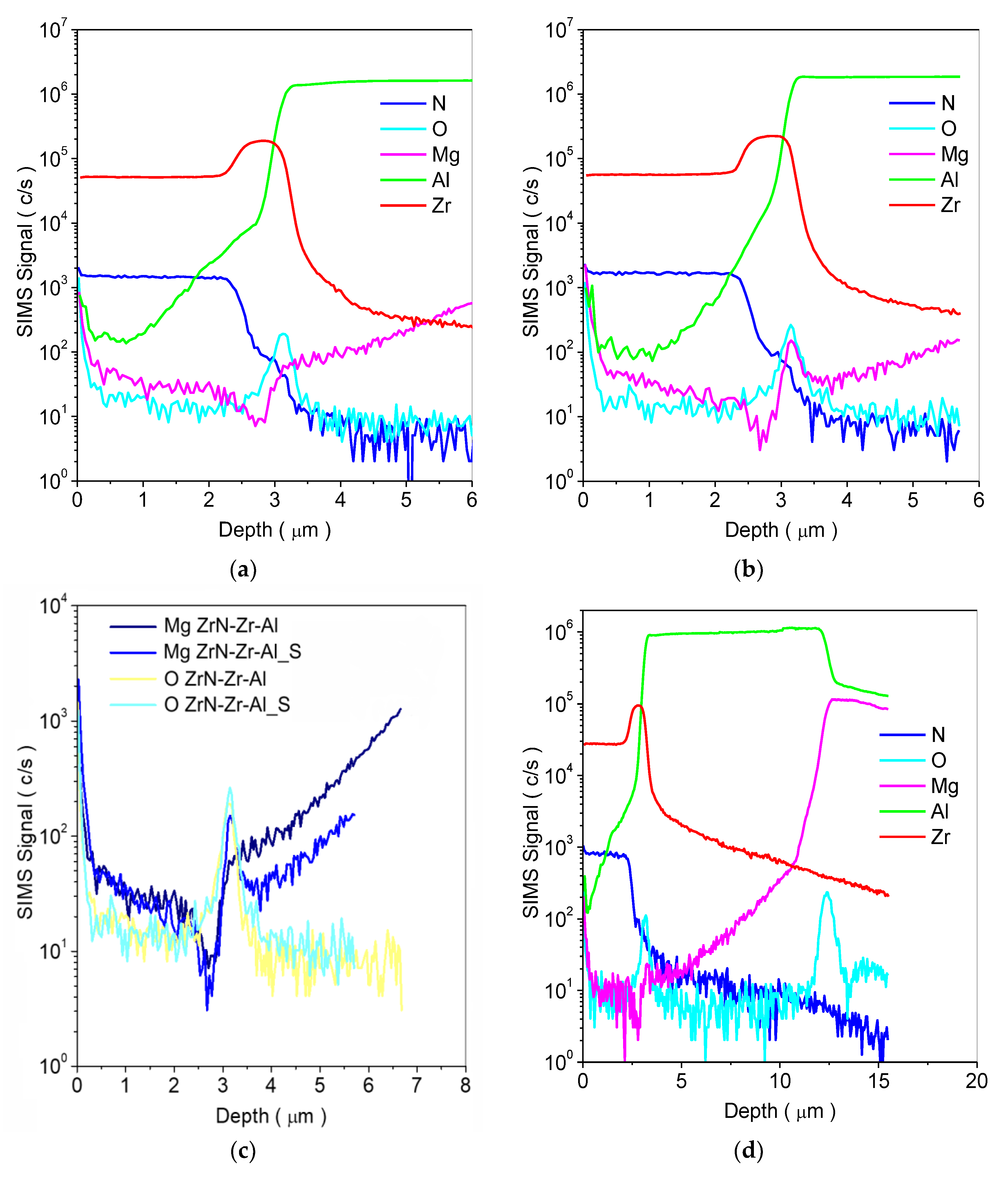
3.1. Microstructure, Chemical and Phase Composition of the Layer
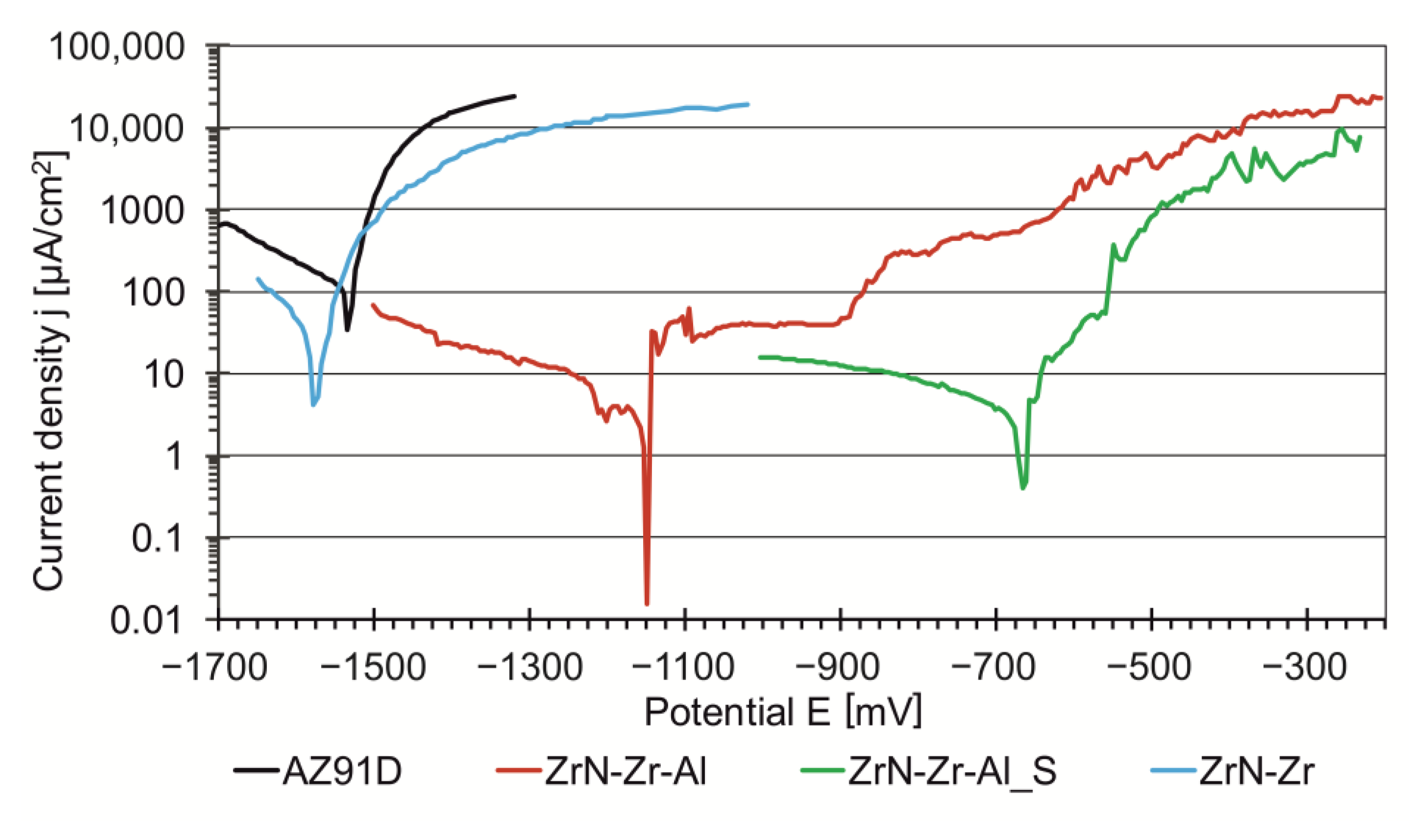
3.2. Corrosion Resistance
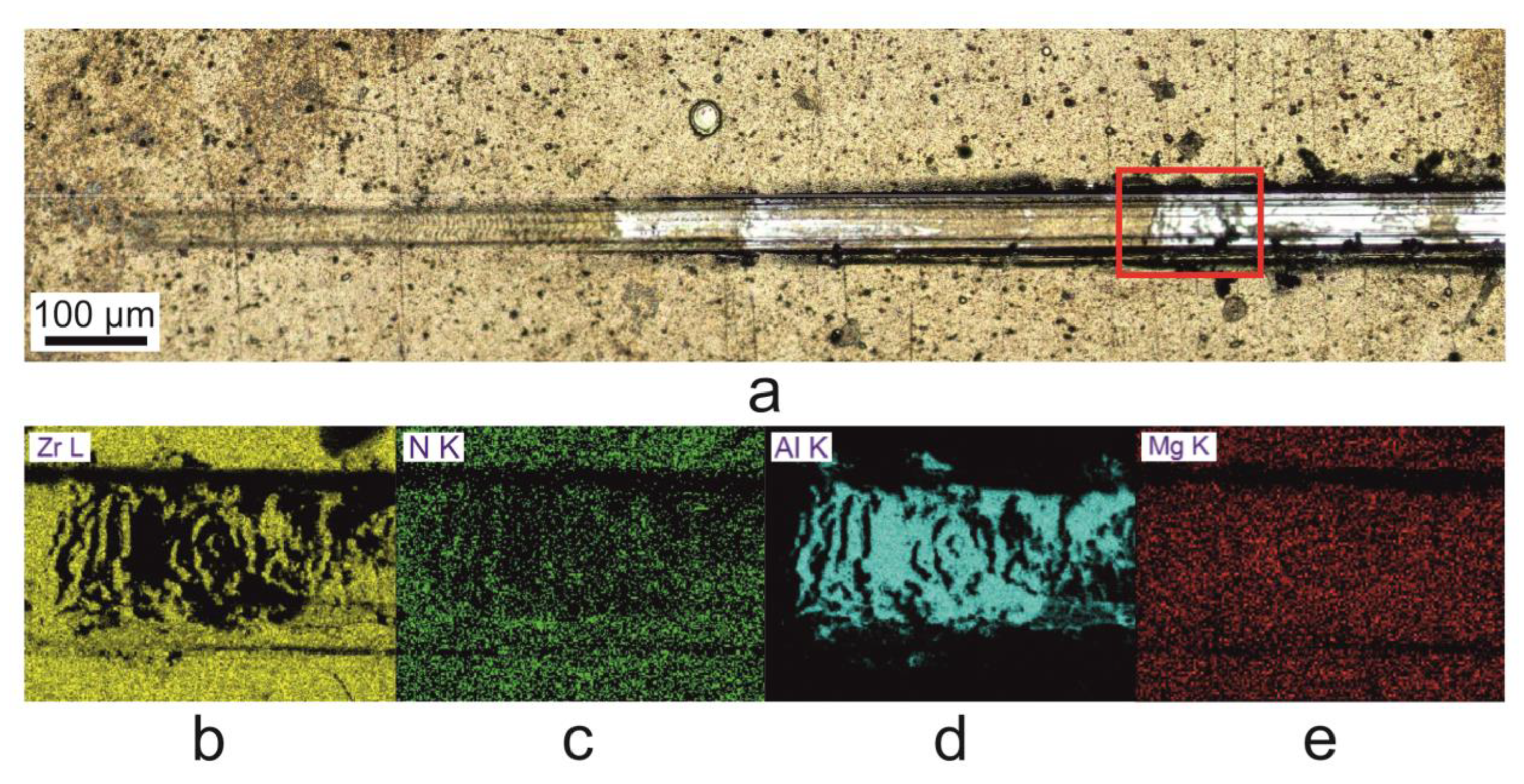
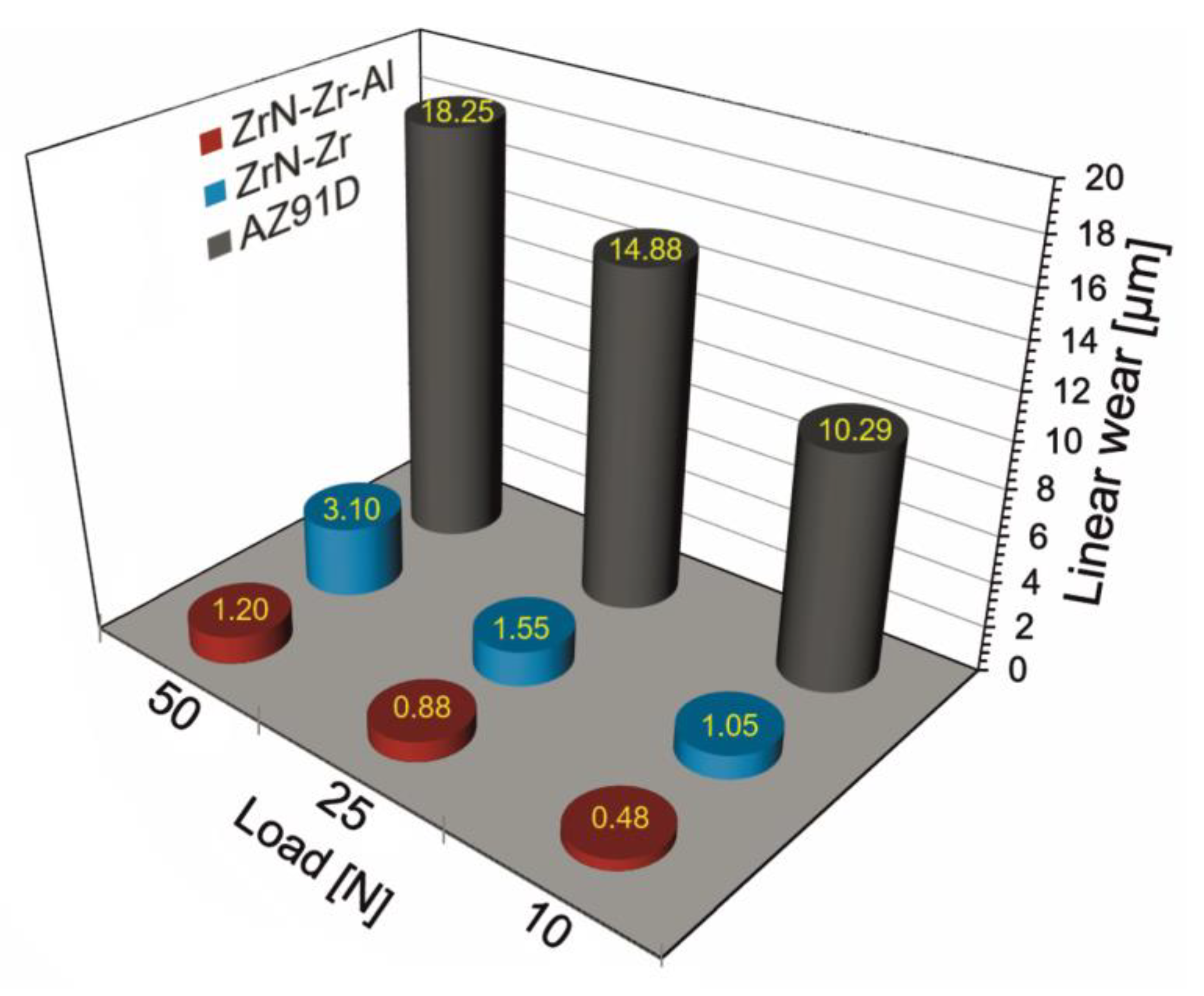
3.3. Mechanical Properties
4. Conclusions
- The effect of the zirconium nitride composite layer of the ZrN-Zr-Al type with zirconium and aluminum intermediate layers produced on the magnesium alloy AZ91D using PVD methods sealed in the final hydrothermal treatment process is a significant improvement in corrosion resistance in the 0.5M sodium chloride environment, manifested in the potentiodynamic test shift of the corrosion potential towards positive values by ΔEcorr = 865 mV in comparison to the alloy without the layer (Ecorr = −1530 ± 1.4 mV). The unsealed ZrN-Zr-Al layer improves the corrosion resistance to a much lower extent (ΔEcorr = 385 mV). On the other hand, the zirconium nitride layer of the ZrN-Zr type, produced directly on the AZ91D alloy, reduces the corrosion resistance of the alloy with a negative shift of the corrosion potential to the value of Ecorr = −1577 ± 0.3 mV. The unfavorable effect of the ZrN-Zr-type zirconium nitride layer on the corrosion resistance of the AZ91D alloy is the result of the presence of inevitable defects in the layer structure, typical for PVD methods, in the form of droplets and craters, which are the source of micro-discontinuities of the nitride layer. This leads to the formation of corrosion cells between the cathodic zirconium nitride coating and the highly chemically active magnesium alloy, and consequently results in accelerated galvanic corrosion. Hence, the key role of the hydrothermal final sealing treatment of the composite layer of zirconium nitride with an intermediate layer of zirconium and aluminum plays a key role in achieving maximum tightness.
- The production of a composite layer of zirconium nitride on the intermediate zirconium and aluminum sublayer of the ZrN-Zr-Al type on the AZ91D magnesium alloy resulted in a more than two-fold increase in surface hardness. The effect of hardening the surface of the AZ91D alloy with a layer of zirconium nitride results in a significant, approximately more than one order of magnitude increase in friction wear resistance in the load range up to 50 N in the modified Amsler roll on block test, while the outer layer of zirconium nitride is not worn through. The increase in resistance observed for the ZrN-Zr layer without the aluminum sublayer is almost 2.5 times lower. The composite zirconium nitride layer of the ZrN-Zr-Al type in the Vickers hardness indentation test carried out using the Vickers HV1 indentation showed resistance to mechanical damage under the influence of concentrated loads. This layer also showed favorable behavior in the scratch test, because damage to the external layer of zirconium nitride during scratching, in the form of cracks, its local or even complete exfoliation, did not expose the substrate made of magnesium alloy AZ91D, but only the aluminum sublayer. During the scratching progresses and the load increases, the sublayer is not subject to cracking or exfoliation of its fragments, but due to the plasticity of aluminum, it is only gradually worn, protecting the magnesium alloy substrate from the corrosive environment effectively. Mechanical damage to the outer layer of zirconium nitride in the ZrN-Zr-Al layer only leads to a decrease in corrosion resistance, and not to accelerated galvanic corrosion, as in the case of ZrN-Zr layers without an aluminum sublayer. As a result, a composite layer of the ZrN-Zr-Al type promises prospectively well in terms of its service durability of AZ91D magnesium alloy products, not only in corrosive, but also mechanical conditions.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Song, J.; She, J.; Chen, D.; Pan, F. Latest research advances on magnesium and magnesium alloys worldwide. J. Magnes. Alloys 2020, 8, 1–41. [Google Scholar] [CrossRef]
- Ramalingam, V.V.; Ramasamy, P.; Kovukkal, M.D.; Myilsamy, G. Research and Development in Magnesium Alloys for Industrial and Biomedical Applications: A Review. Met. Mater. Int. 2020, 26, 409–430. [Google Scholar] [CrossRef]
- Gray, J.E.; Luan, B. Protective Coatings on Magnesium and Its Alloys—A Critical Review. J. Alloys Compd. 2002, 336, 88–113. [Google Scholar] [CrossRef]
- Wang, X.; Zhao, Z.; Chen, J.; Zhou, X.; Zong, Y. Microstructure, Wear and Corrosion Behaviors of Electrodeposited Ni-Diamond Micro-Composite Coatings. Coatings 2022, 12, 1391. [Google Scholar] [CrossRef]
- Wan, X.; Tian, C.; Li, Y.; Zhou, J.; Qian, S.; Su, L.; Wang, L. Effect of Y2O3 Addition on Microstructure and Properties of Laser Cladded Al-Si Coatings on AZ91D Magnesium Alloy. Materials 2023, 16, 338. [Google Scholar] [CrossRef] [PubMed]
- Štrbák, M.; Kajánek, D.; Knap, V.; Florková, Z.; Pastorková, J.; Hadzima, B.; Goraus, M. Effect of plasma electrolytic oxidation on the short-term corrosion behaviour of AZ91 magnesium alloy in aggressive chloride environment. Coatings 2022, 12, 566. [Google Scholar] [CrossRef]
- Altun, H.; Sen, S. The effect of DC magnetron sputtering AlN coatings on the corrosion behaviour of magnesium alloys. Surf. Coat. Technol. 2005, 197, 193–200. [Google Scholar] [CrossRef]
- Hoche, H.; Blawert, C.; Broszeit, E.; Berger, C. Galvanic corrosion properties of differently PVD-treated magnesium die cast alloy AZ91. Surf. Coat. Technol. 2005, 193, 223–229. [Google Scholar] [CrossRef]
- Hoche, H.; Schroeder, H.J.; Scheerer, H.; Broszeit, E.; Berger, C. Tribological Studies of CrN-coated Magnesium AZ91 at Temperatures up to 250 °C. Adv. Eng. Mater. 2002, 4, 42–51. [Google Scholar] [CrossRef]
- Reiners, G.; Griepentrog, M. Hard coatings on magnesium alloys by sputter deposition using a pulsed DC bias voltage. Surf. Coat. Technol. 1995, 76–77, 809–814. [Google Scholar] [CrossRef]
- Zeng, X.; Wu, G.; Yao, S. Formation by reactive magnetron sputtering of TiN coating on Ti-implanted. Mater. Lett. 2006, 60, 2252–2255. [Google Scholar] [CrossRef]
- Kiahosseini, S.R.; Mojtahedzadeh Larijani, M. Effects of nitrogen gas ratio on the structural and corrosion properties of ZrN thin films grown on biodegradable magnesium alloy by ion-beam sputtering. Appl. Phys. A 2017, 123, 759. [Google Scholar] [CrossRef]
- Jin, W.; Zhou, H.; Li, J.; Ruan, Q.; Li, J.; Peng, X.; Li, W.; Chu, P.K. Zirconium-based nanostructured coating on the Mg-4Y-3RE alloy for corrosion retardation. Chem. Phys. Lett. 2020, 756, 137824. [Google Scholar] [CrossRef]
- Kiahosseini, S.R.; Afshar, A.; Mojtahedzadeh Larijani, M.; Yousefpour, M. Structural and corrosion characterization of hydroxyapatite/zirconium nitride-coated AZ91 magnesium alloy by ion beam sputtering. Appl. Surf. Sci. 2017, 401, 172–180. [Google Scholar] [CrossRef]
- Miao, Q.; Cui, C.E.; Pan, J.D. CrN–TiN multilayer coating on magnesium alloy AZ91 by arc-glow plasma depositing process. Surf. Coat. Technol. 2007, 201, 5077–5080. [Google Scholar] [CrossRef]
- Hoche, H.; Allebrandt, D.; Scheerer, H.; Berger, C. Engineering and design of wear and corrosion resistant PVD coatings regarding the exceptional properties of magnesium substrates. Plasma Process. Polym. 2007, 4, 568–573. [Google Scholar] [CrossRef]
- Hoche, H.; Schmidt, J.; Groß, S.; Troßmann, T.; Berger, C. PVD coating and substrate pretreatment concepts for corrosion and wear protection of magnesium alloys. Surf. Coat. Technol. 2011, 205, 145–150. [Google Scholar] [CrossRef]
- Hoche, H.; Groß, S.; Troßmann, T.; Schmidt, J.; Oechsner, M. PVD coating and substrate pretreatment concepts for magnesium alloys by multinary coatings based on Ti(X)N. Surf. Coat. Technol. 2013, 228, S336–S341. [Google Scholar] [CrossRef]
- Hoche, H.; Groß, S.; Oechsner, M. Development of new PVD coatings for magnesium alloys with improved corrosion properties. Surf. Coat. Technol. 2014, 259, 102–108. [Google Scholar] [CrossRef]
- Vasylyev, M.A.; Mordyuk, B.N.; Sidorenko, S.I.; Voloshko, S.M.; Burmak, A.P.; Kruhlov, I.O.; Zakiev, V.I. Characterization of ZrN coating low-temperature deposited on the preliminary Ar+ ions treated 2024 Al-alloy. Surf. Coat. Technol. 2019, 361, 413–424. [Google Scholar] [CrossRef]
- Tacikowski, M.; Banaszek, M.; Smolik, J. Corrosion-resistant composite titanium nitride layers produced on the AZ91D magnesium alloy by a hybrid method. Vacuum 2014, 99, 298–302. [Google Scholar] [CrossRef]
- Fenker, M.; Balzer, M.; Kappl, H. Corrosion behaviour of decorative and wear resistant coatings on steel deposited by reactive sputtering—Tests and improvements. Thin Solid Films 2006, 515, 27–32. [Google Scholar] [CrossRef]
- Tacikowski, M.; Kamiński, J.; Rożniatowski, K.; Pisarek, M.; Jakieła, R.; Marchlewski, P.; Wierzchoń, T. Improving the Properties of Composite Titanium Nitride Layers on the AZ91D Magnesium Alloy Using Hydrothermal Treatment. Materials 2021, 14, 5903. [Google Scholar] [CrossRef]
- Tacikowski, M.; Grzonka, J.; Płociński, T.; Jakieła, R.; Pisarek, M.; Wierzchoń, T. Composite titanium nitride layers produced on the AZ91D magnesium alloy by a hybrid method including hydrothermal modification of the layer. Appl. Surf. Sci. 2015, 346, 394–405. [Google Scholar] [CrossRef]
- Jakieła, R. The Role of Atmospheric Elements in the Wide Band-Gap Semiconductors. Acta Phys. Pol. A 2019, 136, 916–939. [Google Scholar] [CrossRef]



| Variant Denotation | Type of Coating Type of PVD Method Coating Thickness [µm] | Treatment | ||
|---|---|---|---|---|
| Al | Zr | ZrN | ||
| MS 1 | MS 1 | AE 2 | ||
| AZ91D | – | – | – | As delivered |
| ZrN-Zr-Al | 9 | 1 | 2 | As deposited |
| ZrN-Zr-Al_S | 9 | 1 | 2 | Hydrothermal sealing |
| ZrN-Zr * | – | 1 | 2 | As deposited |
| Processing Parameters | Deposition of Al Magnetron Sputtering | Deposition of Zr Magnetron Sputtering | Deposition of ZrN Arc Evaporation |
|---|---|---|---|
| Working atmosphere | Ar | Ar | N2 |
| Pressure | 5 × 10−3 mbar | 5 × 10−3 mbar | 1.2 × 10−2 mbar |
| Gas flow | 200 mL/min | 200 mL/min | 72 mL/min |
| Substrate temperature | <200 °C | <200 °C | <230 °C |
| Bias voltage | −100 V | −50 V | −120 V |
| Bias current | 65 A | 65 A | 4 × 65 A |
| AZ91D | ZrN-Zr-Al | ZrN-Zr-Al_S | ZrN-Zr | |
|---|---|---|---|---|
| Potential E [mV] | −1530 ± 1.4 | −1147 ± 0.7 | −665 ± 0.9 | −1577 ± 0.3 |
| Corrosion current density j [µA/cm2] | 99.72 ± 2.39 | 5.17 ± 0.59 | 3.24 ± 0.26 | 22.48 ± 0.61 |
| Layer Type | Lc1 | Lc2 | Lc3 | |
|---|---|---|---|---|
| ZrN-Zr-Al | Road [mm] | 0.28 ± 0.01 | 0.53 ± 0.01 | 1.47 ± 0.01 |
| Force [N] | 1.88 ± 0.019 | 2.69 ± 0.027 | 5.66 ± 0.057 | |
| ZrN-Zr | Road [mm] | 0.53 ± 0.01 | 1.34 ± 0.01 | 2.56 ± 0.01 |
| Force [N] | 2.64 ± 0.026 | 5.11 ± 0.051 | 8.93 ± 0.089 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tacikowski, M.; Karpiniak, P.; Marciniak, S.; Słoma, J.; Smolik, J.; Jakieła, R. Corrosion and Wear-Resistant Composite Zirconium Nitride Layers Produced on the AZ91D Magnesium Alloy in Hybrid Process Using Hydrothermal Treatment. Crystals 2023, 13, 1455. https://doi.org/10.3390/cryst13101455
Tacikowski M, Karpiniak P, Marciniak S, Słoma J, Smolik J, Jakieła R. Corrosion and Wear-Resistant Composite Zirconium Nitride Layers Produced on the AZ91D Magnesium Alloy in Hybrid Process Using Hydrothermal Treatment. Crystals. 2023; 13(10):1455. https://doi.org/10.3390/cryst13101455
Chicago/Turabian StyleTacikowski, Michał, Piotr Karpiniak, Szymon Marciniak, Jacek Słoma, Jerzy Smolik, and Rafał Jakieła. 2023. "Corrosion and Wear-Resistant Composite Zirconium Nitride Layers Produced on the AZ91D Magnesium Alloy in Hybrid Process Using Hydrothermal Treatment" Crystals 13, no. 10: 1455. https://doi.org/10.3390/cryst13101455






