Morphological, Structural, and Optical Features of Thermally Annealed Slag Powders Generated from the Iron and Steel Industry: A Source of Disordered Iron Oxide Composites
Abstract
:1. Introduction
2. Materials and Method
2.1. Sample Collection and Preparation
2.2. Techniques for Characterization
3. Results and Discussion
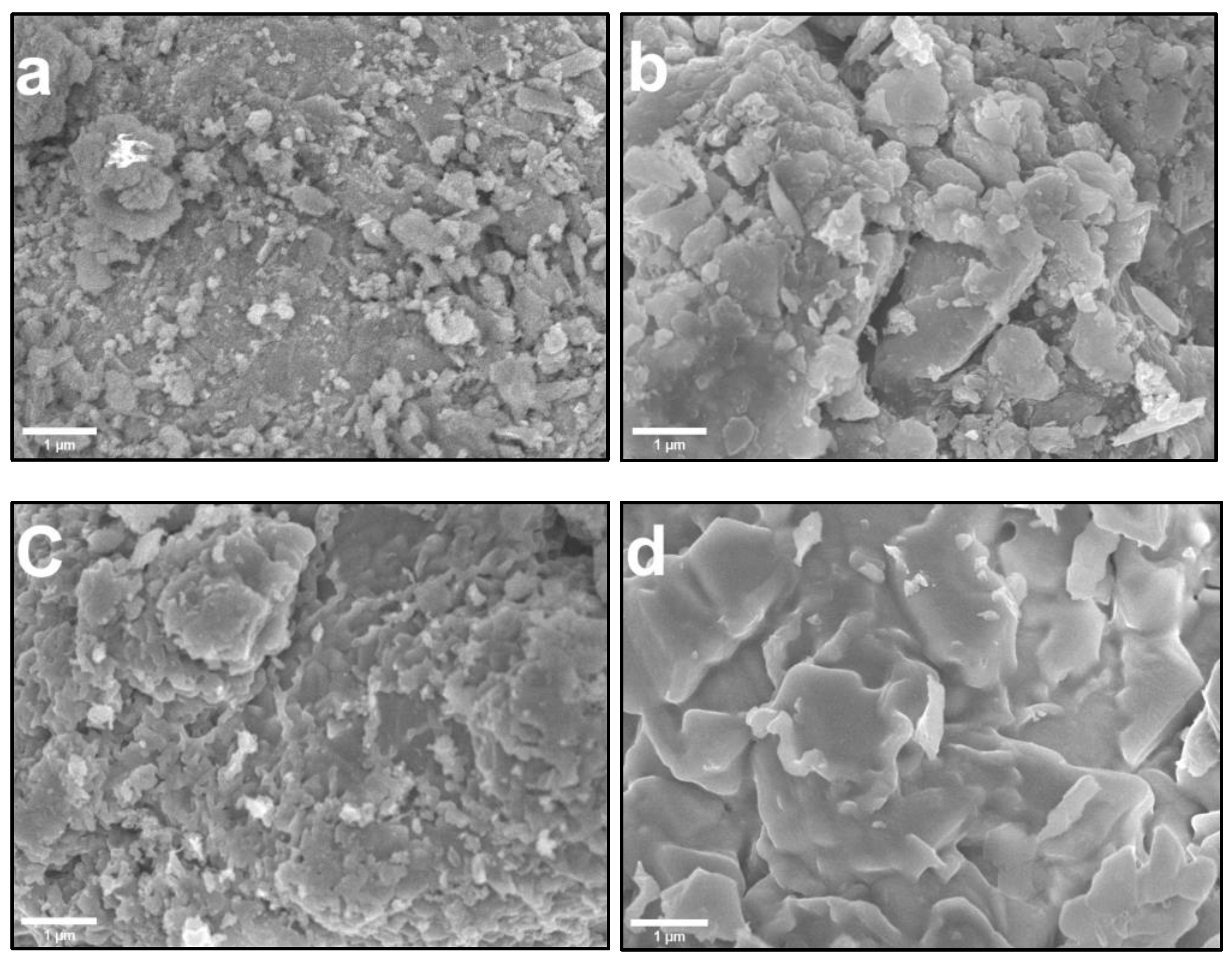
3.1. Morphological and Chemical Composition
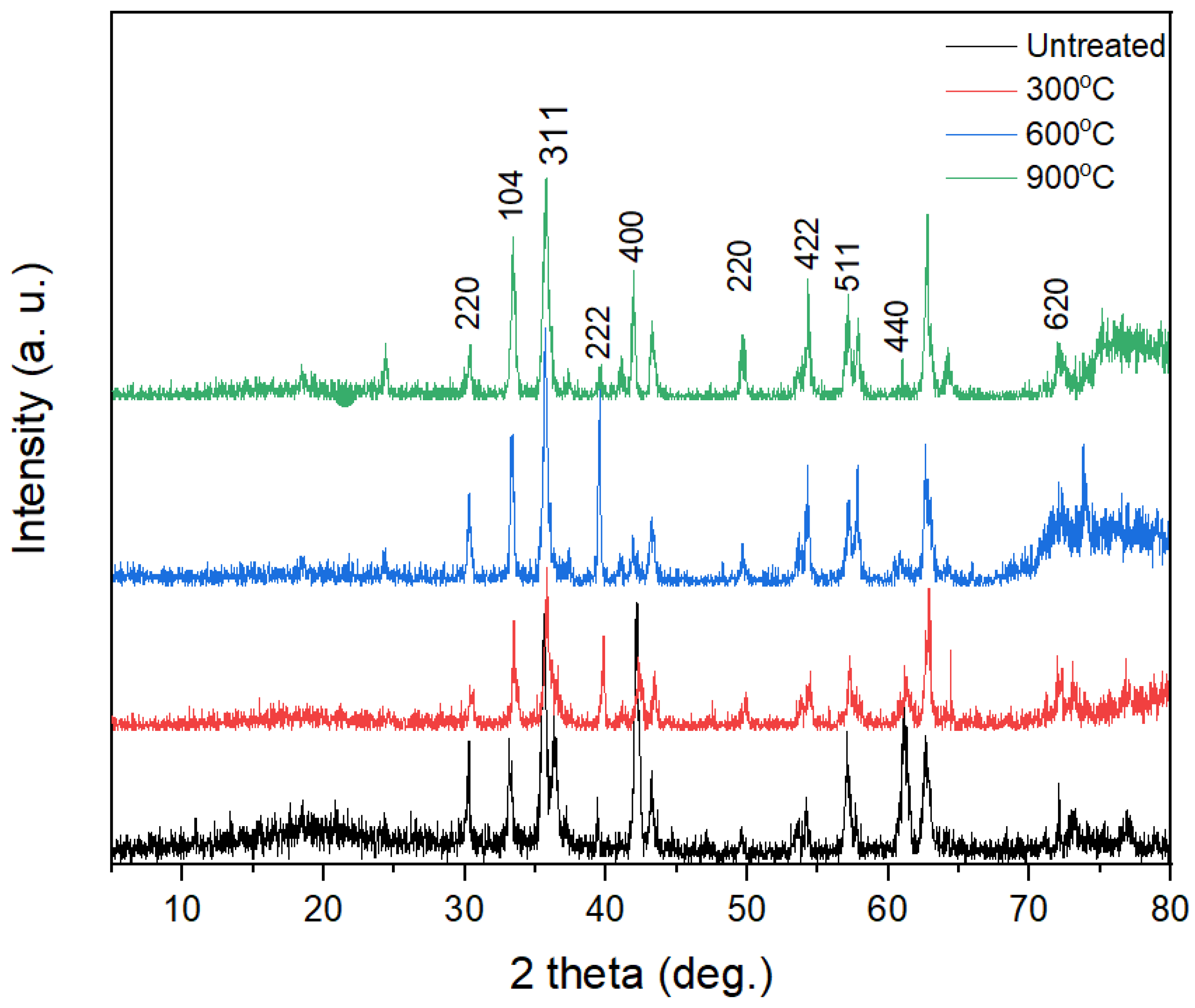
3.2. X-ray Diffractometry Patterns
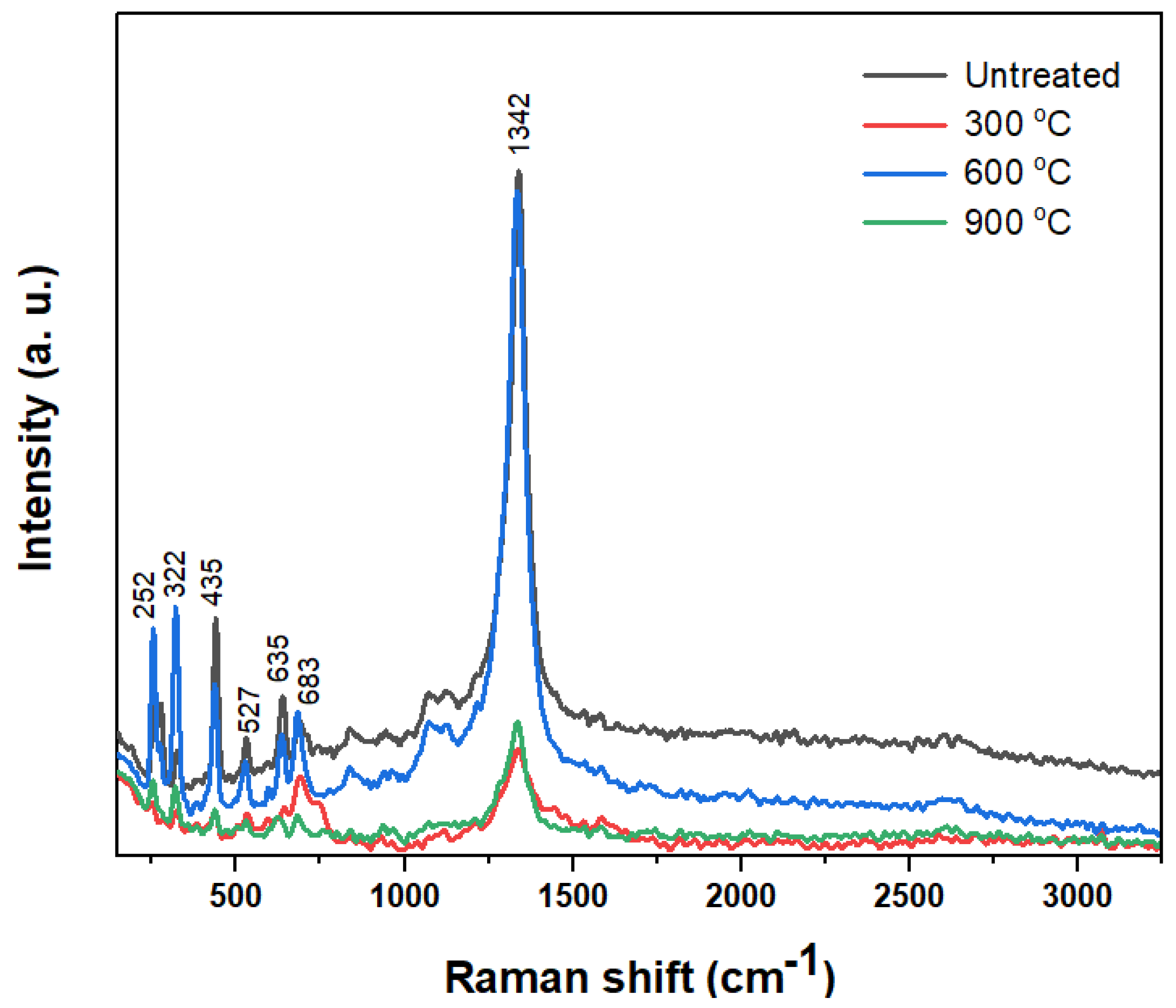
3.3. Raman Analysis
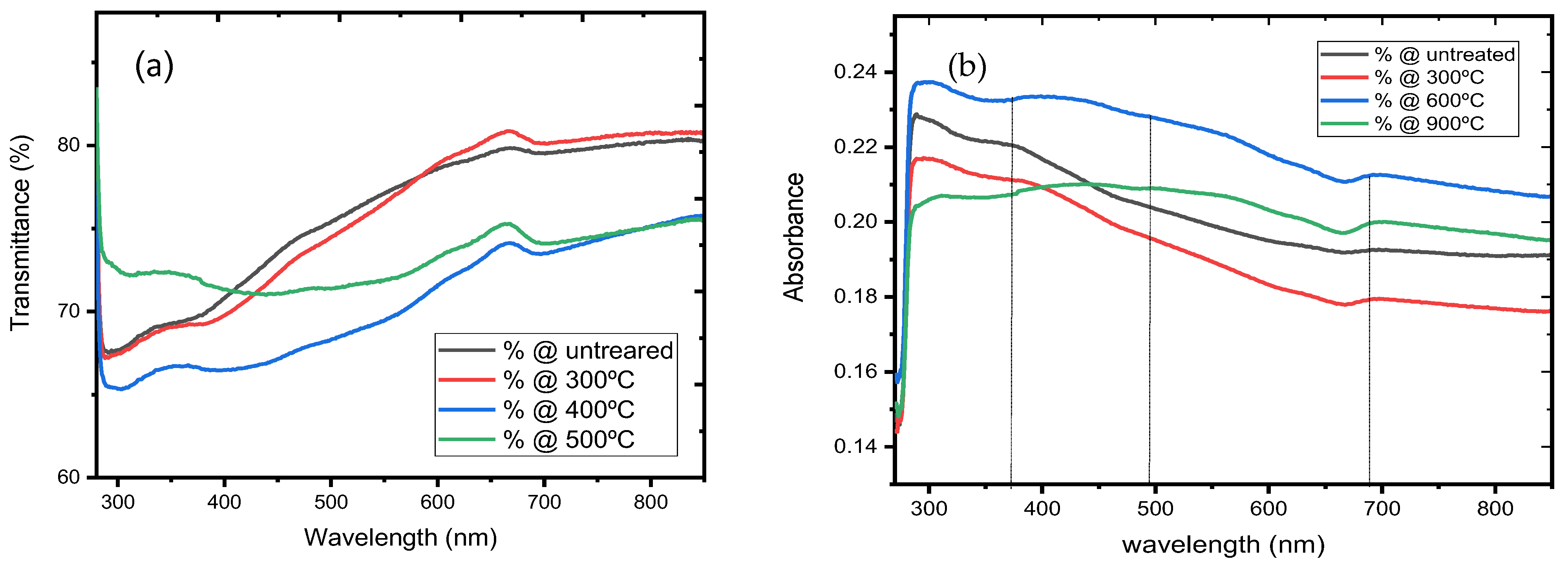
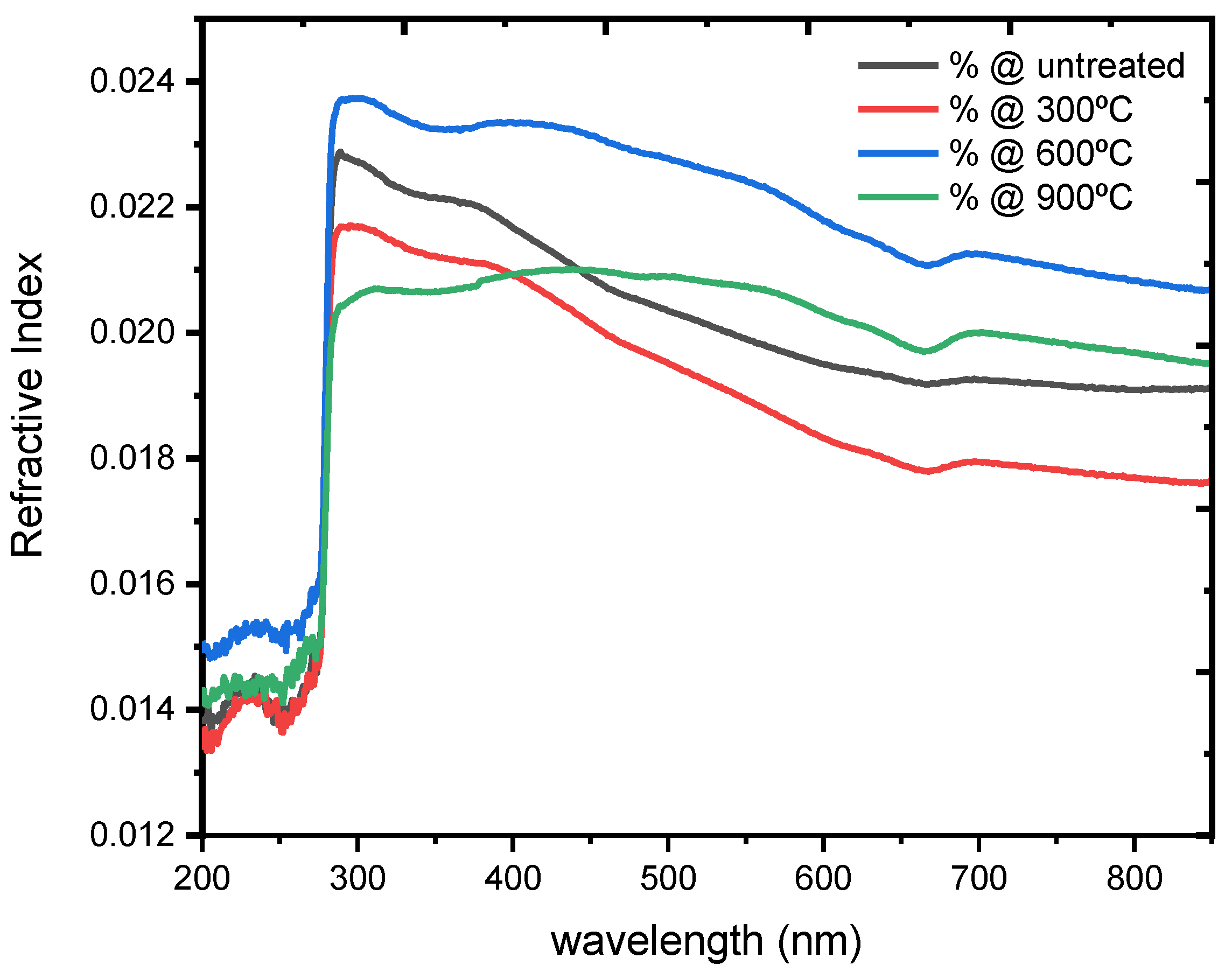
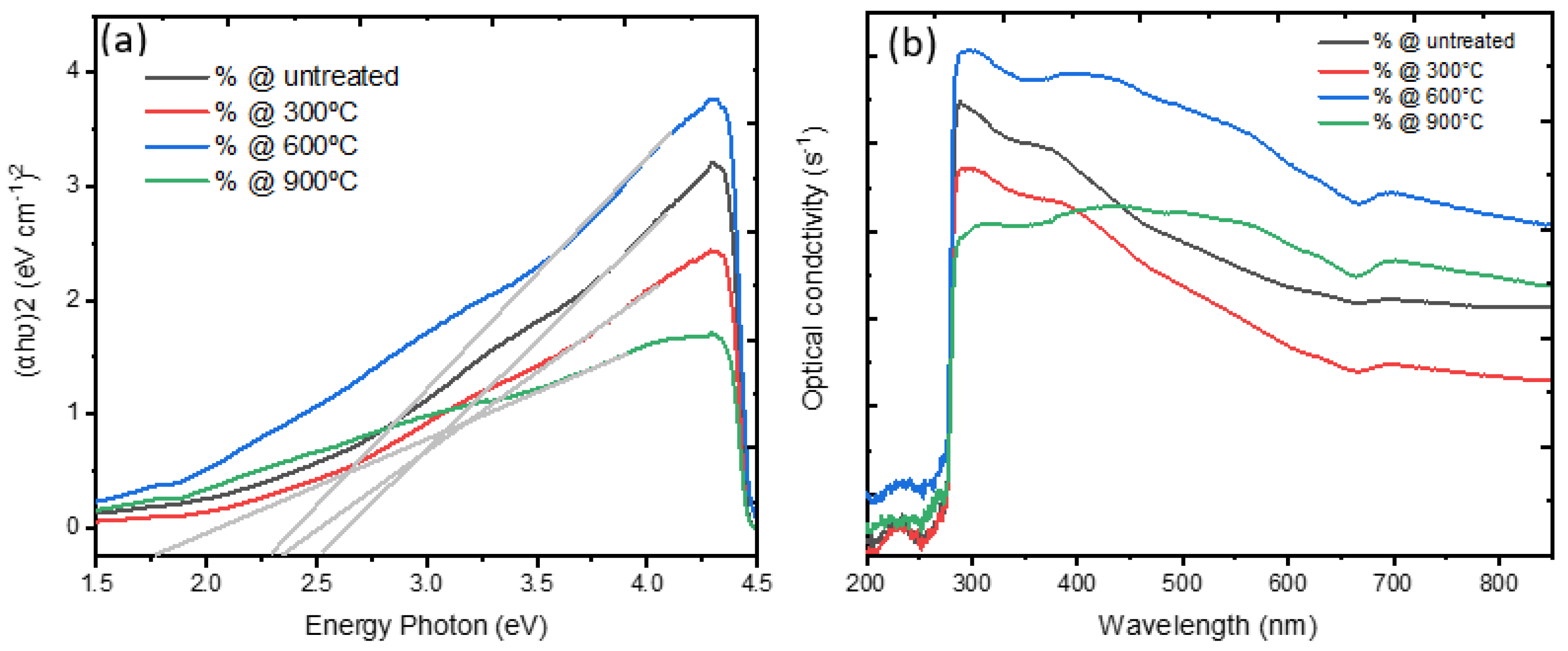
3.4. Optical Properties
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cabra, I.P.; Sharma, S.; Callahan, C.; Jani, K.; Kumar, Y.; Toetz, V.; Shuster, E. Market analysis for the integration of new power technologies: A case study of the deployment of hybrid fossil-based generator plus energy storage (ES-FE). Appl. Energy 2023, 351, 121861. [Google Scholar] [CrossRef]
- Ibrahim, H.; Ilinca, A.; Perron, J. Energy storage systems—Characteristics and comparisons. Renew. Sustain. Energy Rev. 2008, 12, 1221–1250. [Google Scholar] [CrossRef]
- Liu, C.; Li, F.; Ma, L.-P.; Cheng, H.-M. Advanced materials for energy storage. Adv. Mater. 2010, 22, E28–E62. [Google Scholar] [CrossRef]
- Koohi-Fayegh, S.; Rosen, M.A. A review of energy storage types, applications and recent developments. J. Energy Storage 2020, 27, 101047. [Google Scholar] [CrossRef]
- Block, T.; Schmücker, M. Metal oxides for thermochemical energy storage: A comparison of several metal oxide systems. Sol. Energy 2016, 126, 195–207. [Google Scholar] [CrossRef]
- Guo, J.; Bao, Y.; Wang, M. Steel slag in China: Treatment, recycling, and management. Waste Manag. 2018, 78, 318–330. [Google Scholar] [CrossRef]
- Ying, L.; Honghui, F.; Lijun, L. Research Progress of Steel Slag Treatment and Comprehensive Utilization Technology. Chem. Eng. Equip. 2014, 9, 190–192. [Google Scholar]
- Chen, J.; Qian, L.; Fu, Z.; Li, G.; Zeng, Y. Recycling of iron from vanadium titanium magnetite tailings and its application in an asymmetric supercapacitor. New J. Chem. 2023, 47, 9383–9391. [Google Scholar] [CrossRef]
- Yao, J.; Yang, Y.; Li, Y.; Jiang, J.; Xiao, S.; Yang, J. Interconnected α-Fe2O3 nanoparticles prepared from leaching liquor of tin ore tailings as anode materials for lithium-ion batteries. J. Alloys Compd. 2021, 855, 157288. [Google Scholar] [CrossRef]
- Jadhav, D.A.; Ghadge, A.N.; Ghangrekar, M.M. Enhancing the power generation in microbial fuel cells with effective utilization of goethite recovered from mining mud as anodic catalyst. Bioresour. Technol. 2015, 191, 110–116. [Google Scholar] [CrossRef]
- Chen, Z.; Yun, S.; Wu, L.; Zhang, J.; Shi, X.; Wei, W.; Liu, Y.; Zheng, R.; Han, N.; Ni, B.-J. Waste-Derived Catalysts for Water Electrolysis: Circular Economy-Driven Sustainable Green Hydrogen Energy. Nano Micro Lett. 2023, 15, 4. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.; Zhou, W.; Lyu, X.; Liu, X.; Su, H.; Li, C.; Wang, H. Comprehensive utilization of steel slag: A review. Powder Technol. 2023, 422, 118449. [Google Scholar] [CrossRef]
- Baalamurugan, J.; Kumar, V.G.; Padmapriya, R.; Raja, V.K.B. Recent applications of steel slag in construction industry. Environ. Dev. Sustain. 2023, 1–32. [Google Scholar] [CrossRef]
- Wang, J.; Huang, Y. Exploration of steel slag for thermal energy storage and enhancement by Na2CO3 modification. J. Clean. Prod. 2023, 395, 136289. [Google Scholar] [CrossRef]
- Batra, A.; Kassu, A.; Curley, M.; Sampson, J.; Palwai, S.; Showe, A. Optical parametric investigation on a ploed PVDF thin-film for optoelctronic devices. Sensores Transducers 2019, 236, 1–6. [Google Scholar]
- Wadatkar, N.; Waghuley, S. Complex optical studies on conducting polyindole as-synthesized through chemical route. Egypt. J. Basic Appl. Sci. 2015, 2, 19–24. [Google Scholar] [CrossRef]
- Ferber, N.L.; Al Naimi, K.M.; Hoffmann, J.-F.; Al-Ali, K.; Calvet, N. Development of an electric arc furnace steel slag-based ceramic material for high temperature thermal energy storage applications. J. Energy Storage 2022, 51, 104408. [Google Scholar] [CrossRef]
- Núñez, F.C.; Sanz, J.L.; Zaversky, F. Analysis of steel making slag pebbles as filler material for thermocline tanks in a hybrid thermal energy storage system. Sol. Energy 2019, 188, 1221–1231. [Google Scholar] [CrossRef]
- Haunstetter, J.; Krüger, M.; Zunft, S. Experimental Studies on Thermal Performance and Thermo-Structural Stability of Steelmaking Slag as Inventory Material for Thermal Energy Storage. Appl. Sci. 2023, 13, 124. [Google Scholar] [CrossRef]
- Zhiwei, L. Technical Basic Research on the Recovery of Iron Components from Steel Slag and the Preparation of Cement Materials from Tailings of Electric Furnace; Beijing University of Science and Technology: Beijing, China, 2015. [Google Scholar]
- Xingwu, S. Characteristics and Comprehensive Utilization Technology of Steel Slag. Des. Res. Nonferrous Metall. 2007, 5, 31–34. [Google Scholar]
- Subo, Y. Extraction and Recovery of Vanadium from Steel Slag of Converter Containing Vanadium. Iron Steel 2005, 40, 72–75. [Google Scholar]
- Dhoble, Y.N.; Ahmed, S. Review on the innovative uses of steel slag for waste minimization. J. Mater. Cycles Waste Manag. 2018, 20, 1373–1382. [Google Scholar] [CrossRef]
- Ashraf, M.; Khan, I.; Usman, M.; Khan, A.; Shah, S.S.; Khan, A.Z.; Saeed, K.; Yaseen, M.; Ehsan, M.F.; Tahir, M.N.; et al. Hematite and Magnetite Nanostructures for Green and Sustainable Energy Harnessing and Environmental Pollution Control: A Review. Chem. Res. Toxicol. 2020, 33, 1292–1311. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.C.; Choi, S.Q.; Park, J. Asymmetric Supercapacitors Using Porous Carbons and Iron Oxide Electrodes Derived from a Single Fe Metal-Organic Framework (MIL-100 (Fe)). Nanomaterials 2023, 13, 1824. [Google Scholar] [CrossRef] [PubMed]
- Guo, F.; Chen, Q.; Liu, Z.; Cheng, D.; Han, N.; Chen, Z. Repurposing Mining and Metallurgical Waste as Electroactive Materials for Advanced Energy Applications: Advances and Perspectives. Appl. Sci. 2020, 10, 931. [Google Scholar] [CrossRef]
- Tossavainen, M.; Engstrom, F.; Yang, Q.; Menad, N.; Larsson, M.L.; Bjorkman, B. Characteristics of steel slag under different cooling conditions. Waste Manag. 2007, 27, 1335–1344. [Google Scholar] [CrossRef]
- Tawfik, E.K.; Eisa, W.H.; Okasha, N.; Ashry, H.A. Influence of annealing temperature of α- Fe2O3 nanoparticles on Structure and Optical Properties. J. Sci. Res. Sci. 2020, 37, 73–91. [Google Scholar]
- Baalamurugan, J.; Kumar, V.G.; Stalin Dhas, T.; Taran, S.; Nalini, S.; Karthick, V.; Ravil, M.; Govindaraju, K. Utilization of induction furnace steel slag based iron oxide nanocomposites for antibacterial studies. SN Appl. Sci. 2021, 3, 295. [Google Scholar] [CrossRef]
- Al Naimi, K.M.; Delclos, T.; Calvet, N. Industrial waste produced in the UAE, valuable high-temperature materials for thermal energy storage applications. Energy Procedia 2015, 75, 2087–2092. [Google Scholar] [CrossRef]
- Arora, G.; Sharma, S. A Review on Monolithic and Hybrid Metal–Matrix Composites Reinforced with Industrial-Agro Wastes. J. Braz. Soc. Mech. Sci. Eng. 2017, 39, 4819–4835. [Google Scholar] [CrossRef]
- Song, H.-K.; Sonkaria, S.; Khare, V.; Dong, K.; Lee, H.-T.; Ahn, S.-H.; Kim, H.-K.; Kang, H.-J.; Lee, S.-H.; Jung, S.P.; et al. Pond Sediment Magnetite Grains Show a Distinctive Microbial Community. Microb. Ecol. 2015, 70, 168–174. [Google Scholar] [CrossRef] [PubMed]
- Buema, G.; Borhan, A.I.; Herea, D.D.; Stoian, G.; Chiriac, H.; Lupu, N.; Roman, T.; Pui, A.; Harja, M.; Gherca, D. Magnetic solid-phase extraction of cadmium ions by hybrid self-assembled multicore type nanobeads. Polymers 2021, 13, 229. [Google Scholar] [CrossRef] [PubMed]
- Peng, H.; Pearce, C.I.; Huang, W.; Zhu, Z.; N’Diaye, A.T.; Rosso, K.M.; Liu, J. Reversible Fe(II) uptake/release by magnetite nanoparticles. Environ. Sci. Nano 2018, 5, 1545–1555. [Google Scholar] [CrossRef]
- Ashraf, M.A.; Wiener, J.; Farooq, A.; Saskova, J.; Noman, M.T. Development of Maghemite Glass Fibre Nanocomposite for Adsorptive Removal of Methylene Blue. Fibers Polym. 2018, 19, 1735–1746. [Google Scholar] [CrossRef]
- Sajid, M.; Ihsanullah, I. Magnetic layered double hydroxide-based composites as sustainable adsorbent materials for water treatment applications: Progress, challenges, and outlook. Sci. Total Environ. 2023, 880, 163299. [Google Scholar] [CrossRef]
- Marius, C.; Kiss, M.L.; Ieta, A.; Ercuta, A. Synthesis of Micrometric Single Crystalline Magnetite with Superparamagnetic Properties for Biomedical Ap-plications. NSTI-Nanotech 2013, 1, 378–381. [Google Scholar]
- Gherca, D.; Borhan, A.I.; Mihai, M.M.; Herea, D.-D.; Stoian, G.; Roman, T.; Chiriac, H.; Lupu, N.; Buema, G. Magnetite-induced topological transformation of 3D hierarchical MgAl layered double hydroxides to highly dispersed 2D magnetic hetero-nanosheets for effective removal of cadmium ions from aqueous solutions. Mater. Chem. Phys. 2022, 284, 126047. [Google Scholar] [CrossRef]
- Song, Q.; Guo, M.-Z.; Wang, L.; Ling, T.-C. Use of steel slag as sustainable construction materials: A review of accelerated carbonation treatment. Resour. Conserv. Recycl. 2021, 173, 105740. [Google Scholar] [CrossRef]
- López-Sánchez, J.; del Campo, A.; Román-Sánchez, S.; de la Fuente, R.; Carmona, N.; Serrano, A. Large Two-Magnon Raman Hysteresis Observed in a Magnetically Uncompensated Hematite Coating across the Morin Transition. Coatings 2022, 12, 540. [Google Scholar] [CrossRef]
- Chen, L.; Liu, D.; Yang, P. Preparation of α-Fe2O3/RGO Composites toward Supercapacitor Applications. RSC Adv. 2019, 9, 12793–12800. [Google Scholar] [CrossRef]
- Najafi, M.; Bellani, S.; Galli, V.; Zappia, M.I.; Bagheri, A.; Safarpour, M.; Beydaghi, H.; Eredia, M.; Pasquale, L.; Carzino, R.; et al. Carbon- α-Fe2O3 Composite Active Material for High-Capacity Electrodes with High Mass Loading and Flat Current Collector for Quasi-Symmetric Supercapacitors. Electrochem 2022, 3, 463–478. [Google Scholar] [CrossRef]
- Leon, Y.; Lofrumento, C.; Zoppi, A.; Carles, R.; Castellucci, E.M. Micro-Raman investigation of terra sigillata slips: A comparative study of central Italian and southern Gaul pro-ductions. J. Raman Spectrosc. 2010, 41, 1550–1555. [Google Scholar] [CrossRef]
- Tong, Y.; Zhang, Y.; Tong, N.; Zhang, Z.; Wang, Y.; Zhang, X.; Zhu, S.; Li, F.; Wang, X. HZSM-5 zeolites containing impurity iron species for the photocatalytic reduction of CO2 with H2O. Catal. Sci. Technol. 2016, 6, 7579. [Google Scholar] [CrossRef]
- Zhang, M.; Luo, W.; Li, Z.; Yu, T.; Zou, Z. Improved photoelectrochemical responses of Si and Ti codoped α-Fe2O3 photoanode films. Appl. Phys. Lett. 2010, 97, 042105. [Google Scholar] [CrossRef]
- Ajmi, A.; Karoui, K.; Khirouni, K.; Rhaiem, A.B. Optical and dielectric properties of NaCoPO4 in the three phases α, β and γ. RSC Adv. 2019, 9, 14772. [Google Scholar] [CrossRef]
- Dalal, J.; Sinha, N.; Kumar, B. Structural, optical and dielectric studies of novel non-linear bisglycine lithium nitrate piezoelectric single crystal. Opt. Mater. 2014, 37, 457–463. [Google Scholar] [CrossRef]
- Wu, K.; Nie, F.; Zhao, H.; Qi, X.; Li, P.; Wu, M. Morphology-dependent catalysis on nanostructures Fe2O3@MWCNT as Pt-free counter electrode for dye-sensitized solar cells. Int. J. Hydrogen Energy 2023, 48, 33571–33579. [Google Scholar] [CrossRef]
- Rehani, D.; Saxena, M.; Dhakate, S.R.; Sharma, S.N. Iron-Doped Titania for Magneto-Opto-Electronic Device Applications. J. Electron. Mater. 2023, 52, 2895–2903. [Google Scholar] [CrossRef]
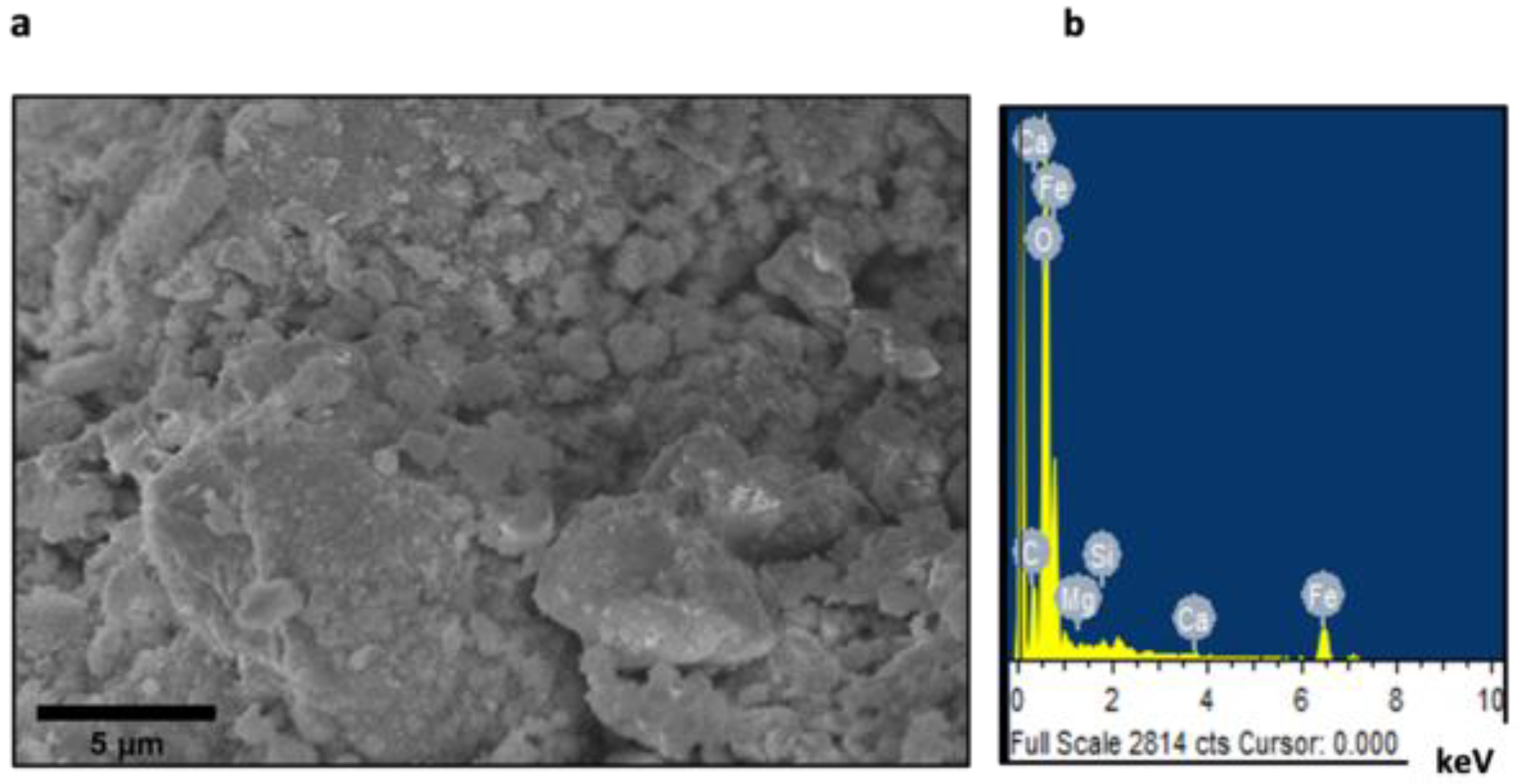

| Element | Weight% | Atomic% |
|---|---|---|
| C K | 8.20 | 18.55 |
| O K | 29.95 | 50.85 |
| Mg K | 0.28 | 0.31 |
| Si K | 0.46 | 0.44 |
| Ca K | 0.65 | 0.44 |
| Fe L | 60.46 | 29.41 |
| Totals | 100.00 |
| Elements | Untreated | 300 °C | 600 °C | 900 °C | ||||
|---|---|---|---|---|---|---|---|---|
| Weight (%) | Atomic (%) | Weight (%) | Atomic (%) | Weight (%) | Atomic (%) | Weight (%) | Atomic (%) | |
| C K | 8.20 | 18.55 | 5.96 | 13.79 | 4.31 | 10.69 | 0.00 | 0.00 |
| O K | 29.95 | 50.85 | 31.62 | 54.88 | 28.82 | 53.65 | 30.02 | 59.96 |
| Mg K | 0.28 | 0.31 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Si K | 0.46 | 0.44 | 0.62 | 0.62 | 0.00 | 0.00 | 0.00 | 0.00 |
| Ca K | 0.65 | 0.44 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Fe L | 60.46 | 29.41 | 61.79 | 30.72 | 66.86 | 35.69 | 69.98 | 40.04 |
| Total | 100 | 100 | 100 | 100 | ||||
| No. | Temperature (°C) | Band Gap Energy (eV) | Refractive Index @ 300 nm | Optical Conductivity (S−1) @ 300 nm |
|---|---|---|---|---|
| 1 | RT | 2.5 | 0.138 | 1.9 × 106 |
| 2 | 300 | 2.4 | 0.135 | 1.5 × 106 |
| 3 | 600 | 2.2 | 0.142 | 2.1 × 106 |
| 4 | 900 | 1.8 | 0.131 | 1.2 × 106 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saeedi, A.M.; Almarri, H.M.; Alabdallah, N.M.; Alamri, M.A.; Albaqawi, H.S.; Algamdi, A.R.; Alfayez, F.A.; Alluqmani, S.M. Morphological, Structural, and Optical Features of Thermally Annealed Slag Powders Generated from the Iron and Steel Industry: A Source of Disordered Iron Oxide Composites. Crystals 2023, 13, 1601. https://doi.org/10.3390/cryst13111601
Saeedi AM, Almarri HM, Alabdallah NM, Alamri MA, Albaqawi HS, Algamdi AR, Alfayez FA, Alluqmani SM. Morphological, Structural, and Optical Features of Thermally Annealed Slag Powders Generated from the Iron and Steel Industry: A Source of Disordered Iron Oxide Composites. Crystals. 2023; 13(11):1601. https://doi.org/10.3390/cryst13111601
Chicago/Turabian StyleSaeedi, Ahmad M., Hana M. Almarri, Nadiyah M. Alabdallah, Mohammed A. Alamri, Hissah Saedoon Albaqawi, Amira R. Algamdi, Fayez A. Alfayez, and Saleh M. Alluqmani. 2023. "Morphological, Structural, and Optical Features of Thermally Annealed Slag Powders Generated from the Iron and Steel Industry: A Source of Disordered Iron Oxide Composites" Crystals 13, no. 11: 1601. https://doi.org/10.3390/cryst13111601






