Electrochemical Characterization Method of Laves Phase in 9Cr Martensitic Heat-Resistant Steel and Creep Life Prediction
Abstract
:1. Introduction
2. Materials and Methods
3. Results
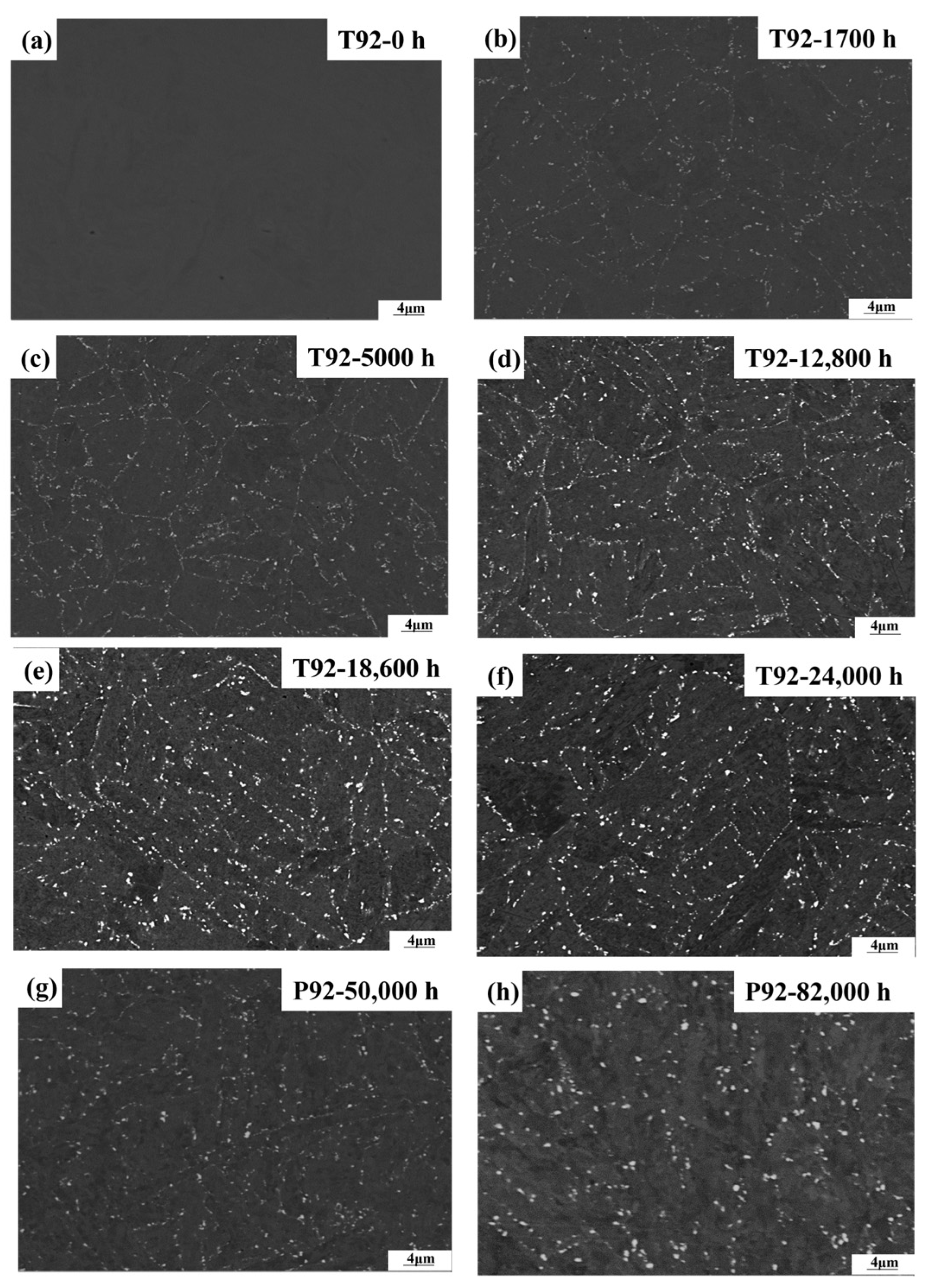
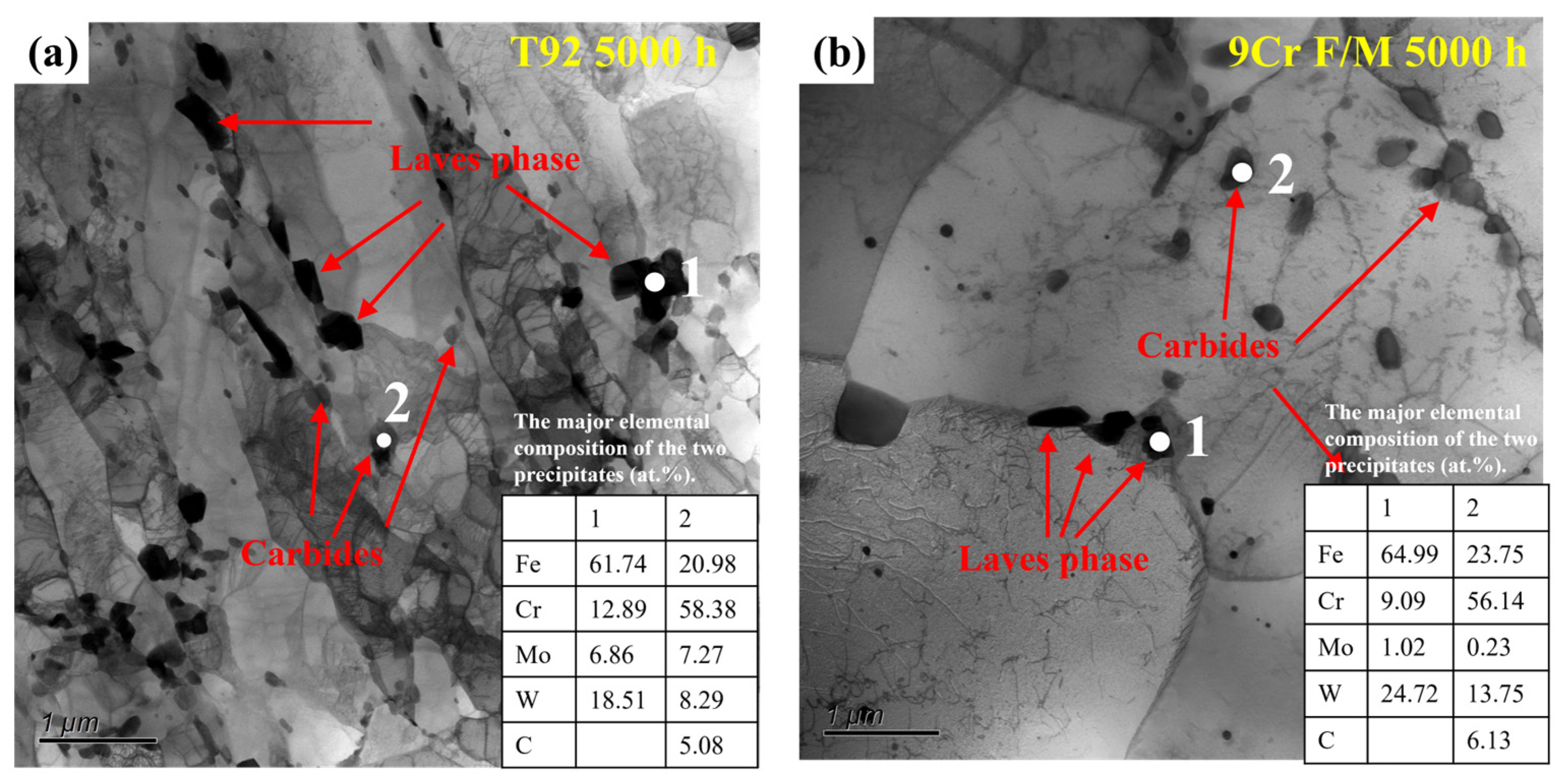
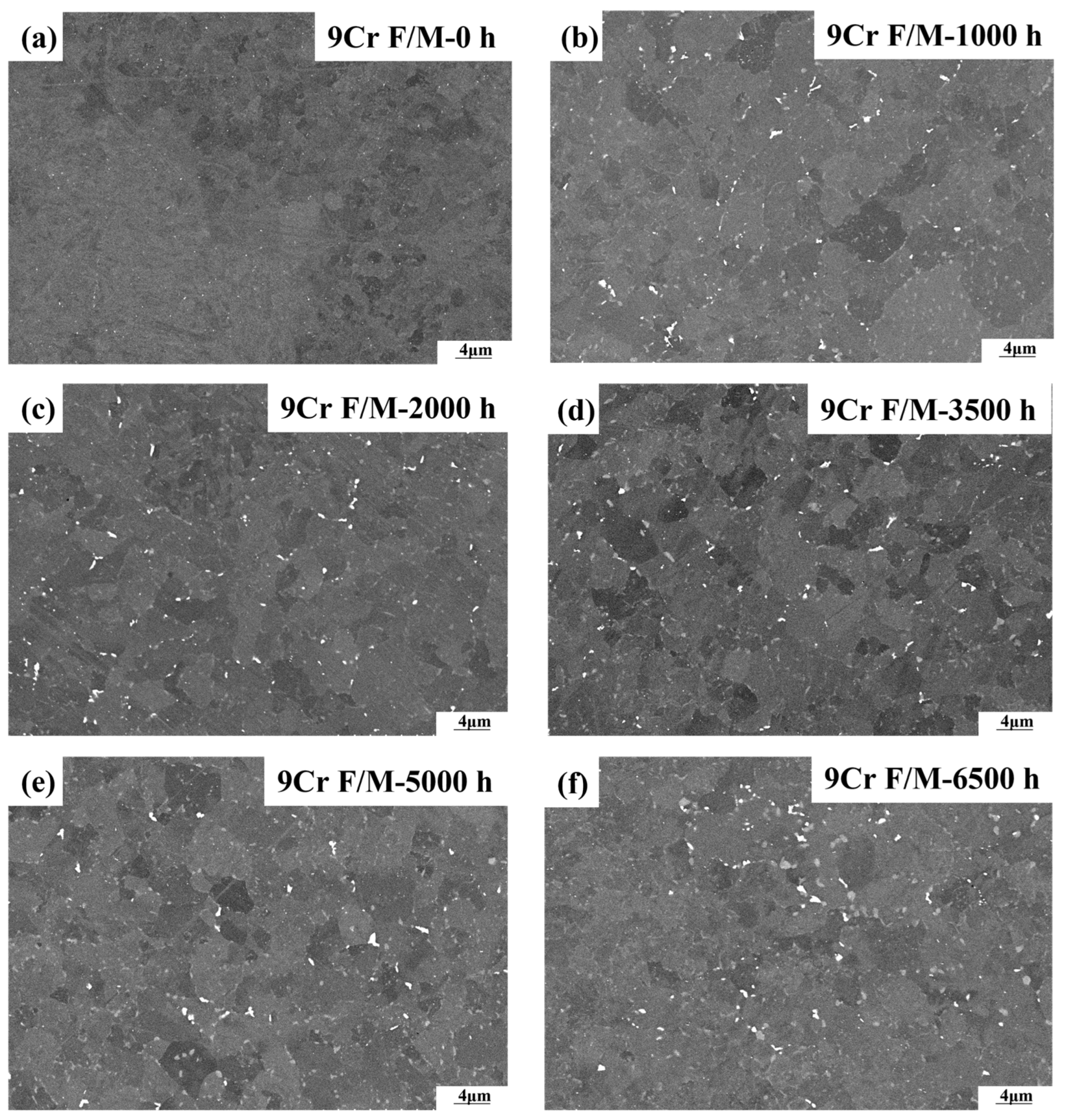
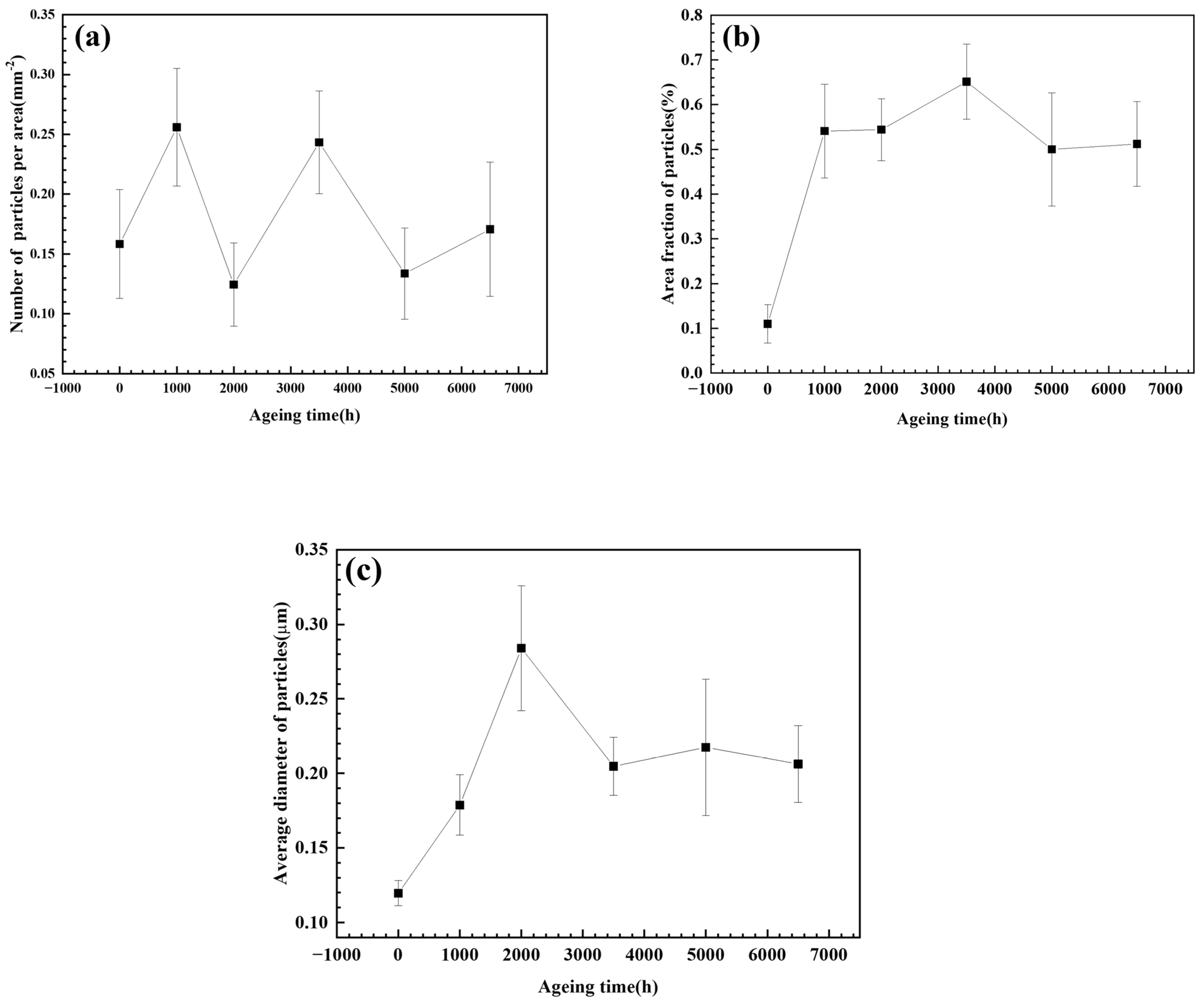
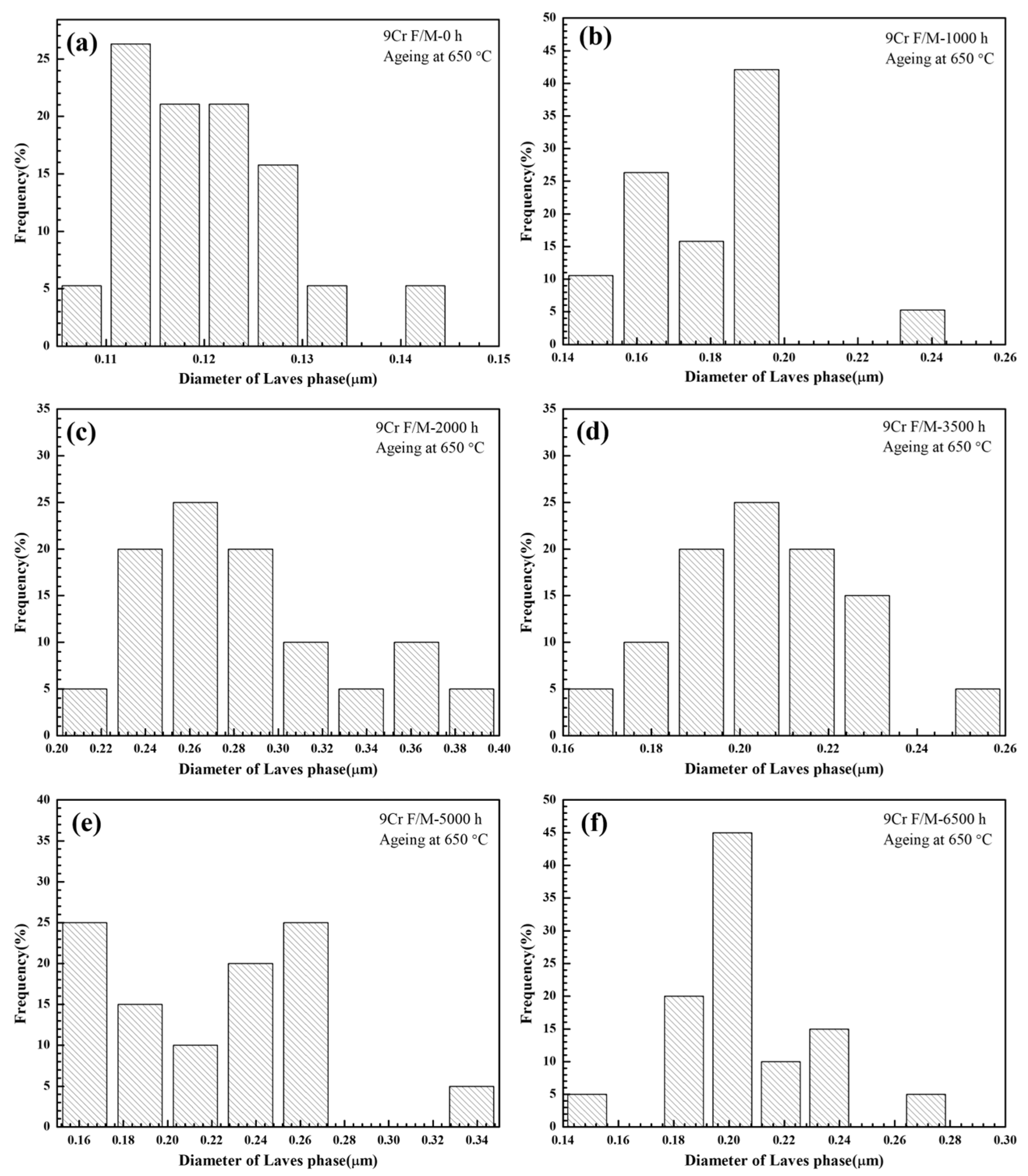
3.1. Characterization of the Laves Phase
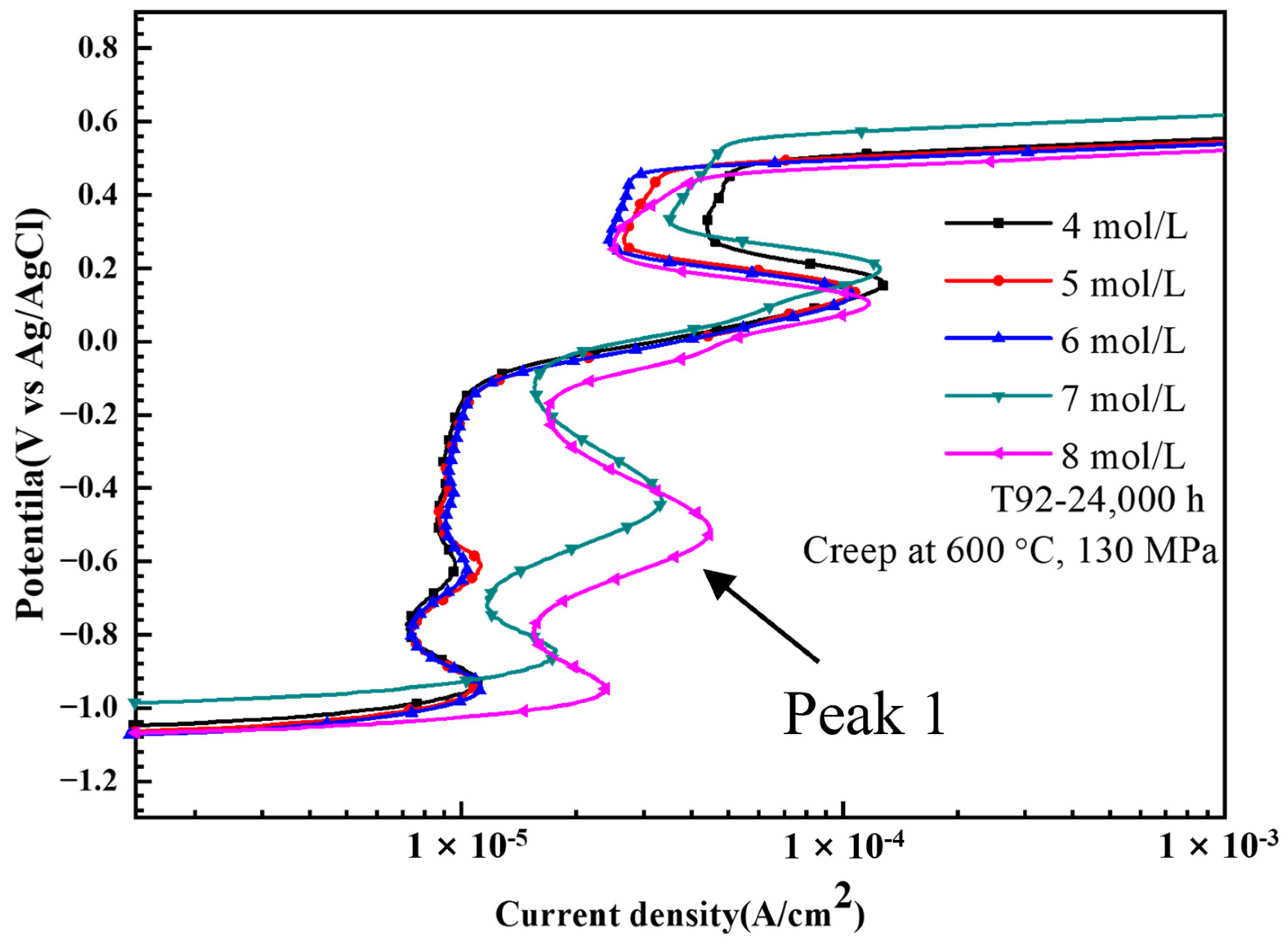
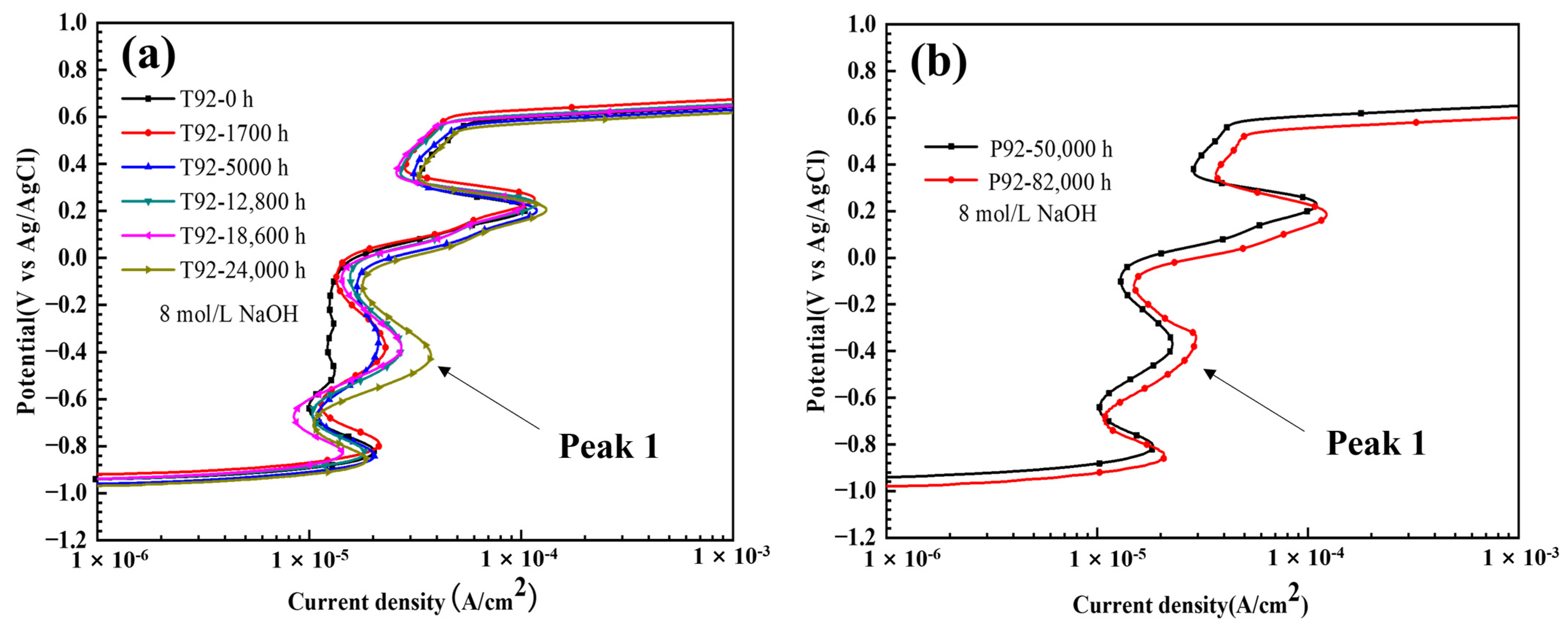
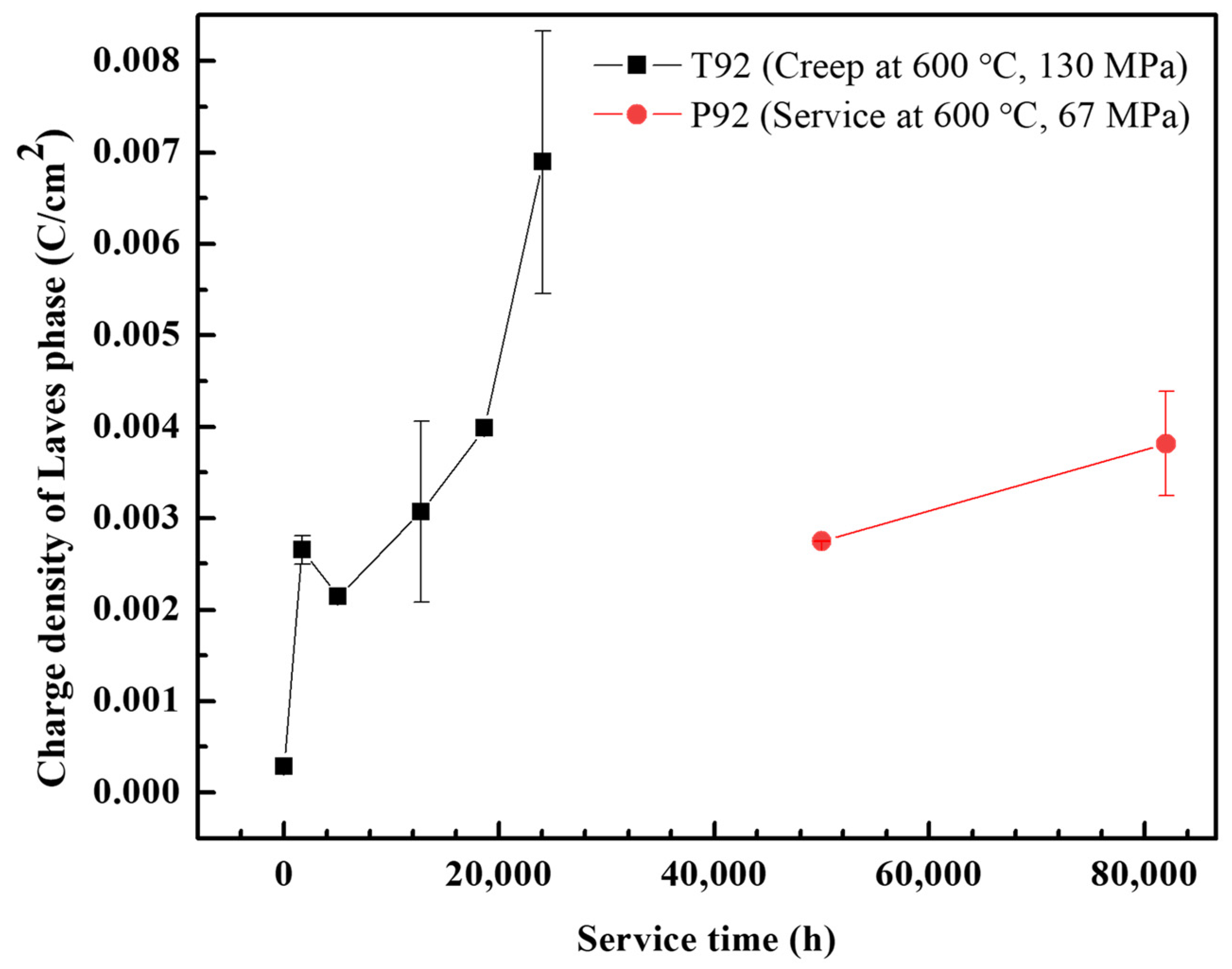
3.2. Quantitative Analysis of Laves Phase Amount by Electrochemical Method
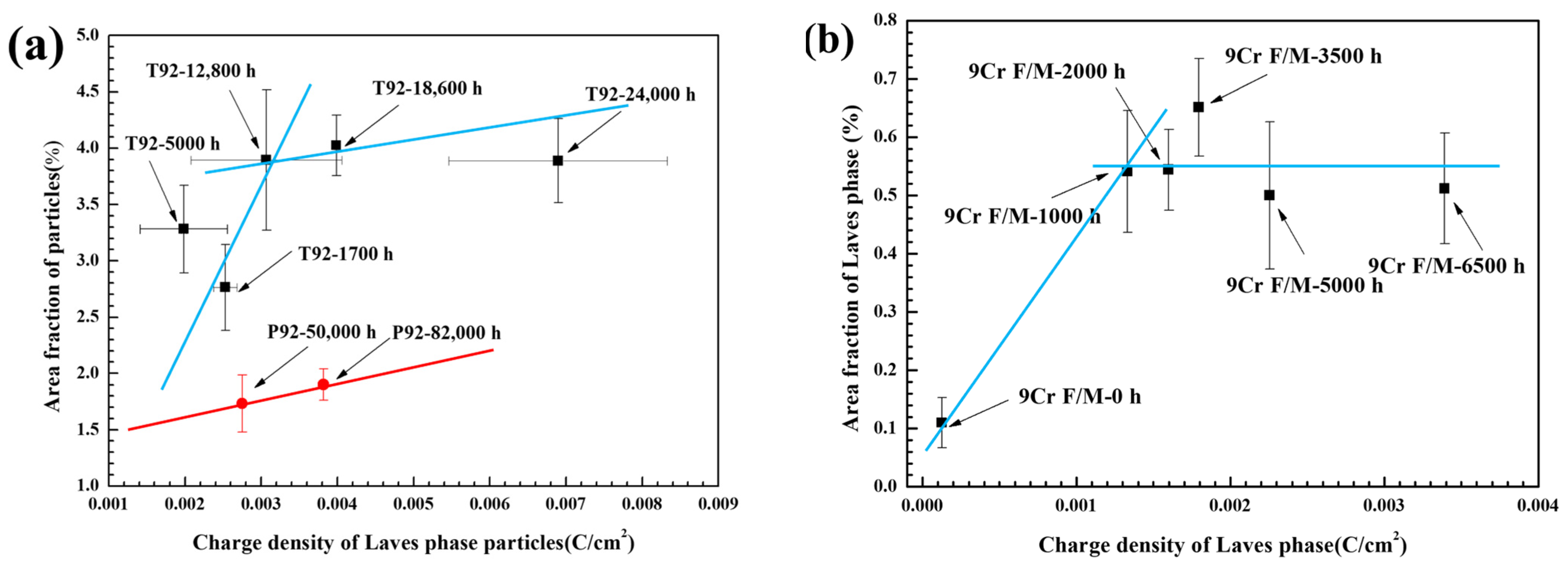
3.3. Correlation between Electrochemical and SEM Data
4. Discussion
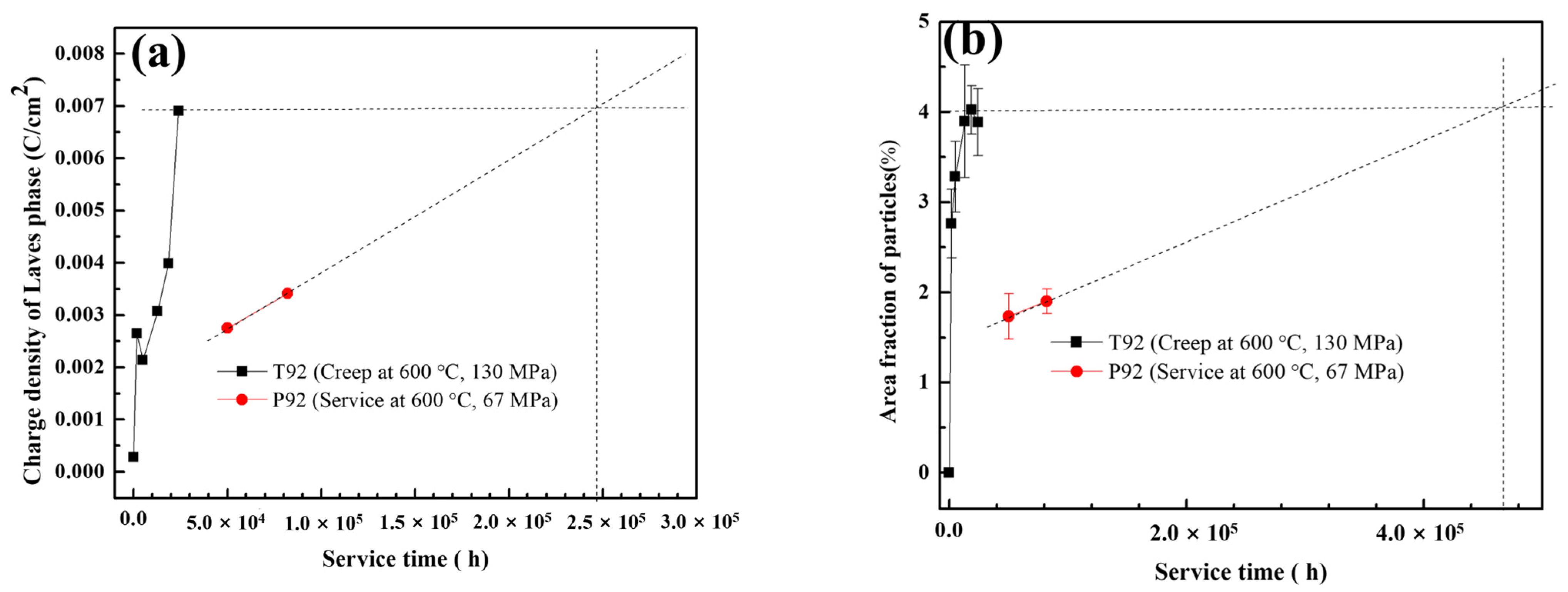
4.1. Creep Life Prediction Based on Laves Phase Content
4.2. Advantages of the Electrochemical Method
5. Conclusions
- (1)
- Both T92/P92 samples after creep and 9Cr F/M samples after thermal ageing will precipitate Laves phase particles, and the content of the Laves phase increases with the service time. However, the content of the Laves phase in 9Cr F/M steel (0.54%) is much lower than that in T/P92 steels (4.0%).
- (2)
- An electrochemical method was used to detect and quantify the Laves phase in T92/P92 steels and 9Cr F/M steel, and the Laves phase in all materials was found to be preferentially dissolved in a strong alkaline solution. The relationship between the area fraction of the Laves phase and the charge density corresponding to the dissolution peak of the Laves phase in all materials shows a similar two-stage linear relationship, which is related to the diameter change of Laves phase particles during creep. The results also demonstrate the general applicability of electrochemical methods for the quantification of Laves phase particles in 9Cr martensitic steel.
- (3)
- An extrapolation method to predict the residual creep life is proposed based on the correlation between the Laves phase content and creep time. It is found that the creep life of P92 can be predicted more accurately by the Laves phase content obtained by the dissolved charge density than by the area fraction. This may be related to the fact that the dissolved charge density of the Laves phase, relative to the area fraction, also takes into account the particle size information. This method has the potential to evaluate the service state of materials and predict the long-term creep life in the industrial field.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Di Gianfrancesco, A.; Vipraio, S.T.; Venditti, D. Long Term Microstructural Evolution of 9–12%Cr Steel Grades for Steam Power Generation Plants. Procedia Eng. 2013, 55, 27–35. [Google Scholar] [CrossRef]
- Hald, J. Microstructure and long-term creep properties of 9–12% Cr steels. Int. J. Press. Vessel. Pip. 2008, 85, 30–37. [Google Scholar] [CrossRef]
- Korcakova, L.J.H.; Somers, M.A.J. Quantification of Laves phase particle size in 9CrW steel. Mater. Charact. 2001, 47, 111–117. [Google Scholar] [CrossRef]
- Dimmler, G.; Weinert, P.; Kozeschnik, E.; Cerjak, H. Quantification of the Laves phase in advanced 9–12% Cr steels using a standard SEM. Mater. Charact. 2003, 51, 341–352. [Google Scholar] [CrossRef]
- Isik, M.I.; Kostka, A.; Eggeler, G. On the nucleation of Laves phase particles during high-temperature exposure and creep of tempered martensite ferritic steels. Acta Mater. 2014, 81, 230–240. [Google Scholar] [CrossRef]
- Hu, P.; Yan, W.; Sha, W.; Wang, W.; Guo, Z.; Shan, Y.; Yang, K. Study on Laves phase in an advanced heat-resistant steel. Front. Mater. Sci. China 2009, 3, 434–441. [Google Scholar] [CrossRef]
- Stein, F.; Leineweber, A. Laves phases: A review of their functional and structural applications and an improved fundamental understanding of stability and properties. J. Mater. Sci. 2020, 56, 5321–5427. [Google Scholar] [CrossRef]
- Junek, M.; Svobodova, M.; Horvath, J.; Mara, V. The Effect of Long-Term Ageing on Microstructural Properties and Laves Phase Precipitation of Welded P91 and P92 Steels. Steel Res. Int. 2021, 93, 2100311. [Google Scholar] [CrossRef]
- Abe, F. Creep rates and strengthening mechanisms in tungsten-strengthened 9Cr steels. Mater. Sci. Eng. A 2001, 319–321, 770–773. [Google Scholar] [CrossRef]
- Abe, F. Effect of Fine Precipitation and Subsequent Coarsening of Fe2W Laves Phase on the Creep Defotmation Behavior of Tempered Martensitic 9Cr-W Steels. Mater. Sci. Eng. A 2005, 36, 321–332. [Google Scholar]
- Wang, Z.; Xu, J.; Takeda, Y.; Shoji, T.; Watanabe, Y.; Yamashita, M.; Izumi, S. An electrochemical method for detection and quantification of Laves phase in 12Cr martensitic stainless steel. Corros. Sci. 2018, 135, 215–221. [Google Scholar] [CrossRef]
- Wang, Z.; Takeda, Y.; Zhong, X.; Shoji, T.; Xu, J.; Watanabe, Y.; Yamashita, M.; Izumi, S. Mechanistic Understanding of the Dissolution Behavior of the Precipitates in 12Cr Martensitic Steel during Potentiodynamic Polarization in Strong Alkaline Solutions. J. Electrochem. Soc. 2020, 167, 141501. [Google Scholar] [CrossRef]
- Wu, Y.; Yuan, S.; Yan, J.; Liu, J.; Nong, J.; Guo, Q.; Xu, J. Dissolution Behaviour of Laves Phase in P92 High Alloy Steel in Alkaline Solutions. J. Electrochem. Soc. 2021, 168, 031505. [Google Scholar] [CrossRef]
- Schneider, A.; Inden, G. Simulation of the kinetics of precipitation reactions in ferritic steels. Acta Mater. 2005, 53, 519–531. [Google Scholar] [CrossRef]
- Panait, C.G.; Bendick, W.; Fuchsmann, A.; Gourgues-Lorenzon, A.F.; Besson, J. Study of the microstructure of the Grade 91 steel after more than 100,000 h of creep exposure at 600 °C. Int. J. Press. Vessel. Pip. 2010, 87, 326–335. [Google Scholar] [CrossRef]
- Prasad, S.; Rajkumar, V.B.; Hari Kumar, K.C. Numerical simulation of precipitate evolution in ferritic–martensitic power plant steels. Calphad 2012, 36, 1–7. [Google Scholar] [CrossRef]
- Xu, Y.; Nie, Y.; Wang, M.; Li, W.; Jin, X. The effect of microstructure evolution on the mechanical properties of martensite ferritic steel during long-term aging. Acta Mater. 2017, 131, 110–122. [Google Scholar] [CrossRef]
- Golpayegani, A.; Andrén, H.-O.; Danielsen, H.; Hald, J. A study on Z-phase nucleation in martensitic chromium steels. Mater. Sci. Eng. A 2008, 489, 310–318. [Google Scholar] [CrossRef]
- Cipolla, L.; Danielsen, H.K.; Venditti, D.; Di Nunzio, P.E.; Hald, J.; Somers, M.A.J. Conversion of MX nitrides to Z-phase in a martensitic 12% Cr steel. Acta Mater. 2010, 58, 669–679. [Google Scholar] [CrossRef]
- Hald, J. Metallurgy and creep properties of new 9–12%Cr steels. Steel Res. 1996, 67, 369–374. [Google Scholar] [CrossRef]
- Lee, J.S.; Armaki, H.G.; Maruyama, K.; Muraki, T.; Asahi, H. Causes of breakdown of creep strength in 9Cr–1.8W–0.5Mo–VNb steel. Mater. Sci. Eng. A 2006, 428, 270–275. [Google Scholar] [CrossRef]
- Aghajani, A.; Richter, F.; Somsen, C.; Fries, S.G.; Steinbach, I.; Eggeler, G. On the formation and growth of Mo-rich Laves phase particles during long-term creep of a 12% chromium tempered martensite ferritic steel. Scr. Mater. 2009, 61, 1068–1071. [Google Scholar] [CrossRef]
- Maddi, L.; Deshmukh, G.S.; Ballal, A.R.; Peshwe, D.R.; Paretkar, R.K.; Laha, K.; Mathew, M.D. Effect of Laves phase on the creep rupture properties of P92 steel. Mater. Sci. Eng. A 2016, 668, 215–223. [Google Scholar] [CrossRef]
- Samuel, E.I.; Choudhary, B.K.; Palaparti, D.P.R.; Mathew, M.D. Creep Deformation and Rupture Behaviour of P92 Steel at 923K. Procedia Eng. 2013, 55, 64–69. [Google Scholar] [CrossRef]
- Orr, R.L.; Sherby, O.D.; Dorn, J.E. CORRELATIONS OF RUPTURE DATA FOR METALS AT ELEVATED TEMPERATURES. Available online: https://www.osti.gov/servlets/purl/4425999 (accessed on 12 November 2023).
- Bråthe, L. Estimation of kachanov parameters and extrapolation from isothermal creep rupture data. Int. J. Mech. Sci. 1978, 20, 617–624. [Google Scholar] [CrossRef]
- Evans, R.W.; Wilshire, B. Creep of Metals and Alloys; IMM North American Publishing Center: Brookfield, VT, USA, 1985. [Google Scholar]




| Material | Fe | Cr | W | Mo | C | Si | Mn | V | Nb |
|---|---|---|---|---|---|---|---|---|---|
| T92/P92 | Bal. | 8.56 | 1.92 | 0.39 | 0.12 | 0.18 | 0.38 | 0.19 | 0.07 |
| 9Cr F/M | Bal. | 8.50 | 1.46 | — | 0.23 | 1.81 | 0.60 | 0.20 | 0.10 |
| No. | Status | Time (h) | Creep Life Loss (%) |
|---|---|---|---|
| 1 | as-received | 0 | 0 |
| 2 | creep interrupt sample | 1700 | 7 |
| 3 | creep interrupt sample | 5000 | 20 |
| 4 | creep interrupt sample | 12,800 | 53 |
| 5 | creep interrupt sample | 18,600 | 77 |
| 6 | creep fracture sample | 24,000 | 100 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yuan, S.; Li, J.; Guo, Q.; Tang, L.; Xu, J.; Zhou, R.; Zhang, H. Electrochemical Characterization Method of Laves Phase in 9Cr Martensitic Heat-Resistant Steel and Creep Life Prediction. Crystals 2023, 13, 1600. https://doi.org/10.3390/cryst13111600
Yuan S, Li J, Guo Q, Tang L, Xu J, Zhou R, Zhang H. Electrochemical Characterization Method of Laves Phase in 9Cr Martensitic Heat-Resistant Steel and Creep Life Prediction. Crystals. 2023; 13(11):1600. https://doi.org/10.3390/cryst13111600
Chicago/Turabian StyleYuan, Sui, Ji Li, Qi Guo, Liying Tang, Jian Xu, Rongcan Zhou, and Hongjun Zhang. 2023. "Electrochemical Characterization Method of Laves Phase in 9Cr Martensitic Heat-Resistant Steel and Creep Life Prediction" Crystals 13, no. 11: 1600. https://doi.org/10.3390/cryst13111600
APA StyleYuan, S., Li, J., Guo, Q., Tang, L., Xu, J., Zhou, R., & Zhang, H. (2023). Electrochemical Characterization Method of Laves Phase in 9Cr Martensitic Heat-Resistant Steel and Creep Life Prediction. Crystals, 13(11), 1600. https://doi.org/10.3390/cryst13111600







