In Situ Growth of Mg-Fe Layered Double Hydroxides (LDH) Film on Titanium Dental Implant Substrates for pH Regulation in Oral Environments
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. LDH Synthesis
2.3. Surface Characterization
2.4. pH Measurement
3. Results and Discussion
3.1. Mechanism for the Growth of LDH Films
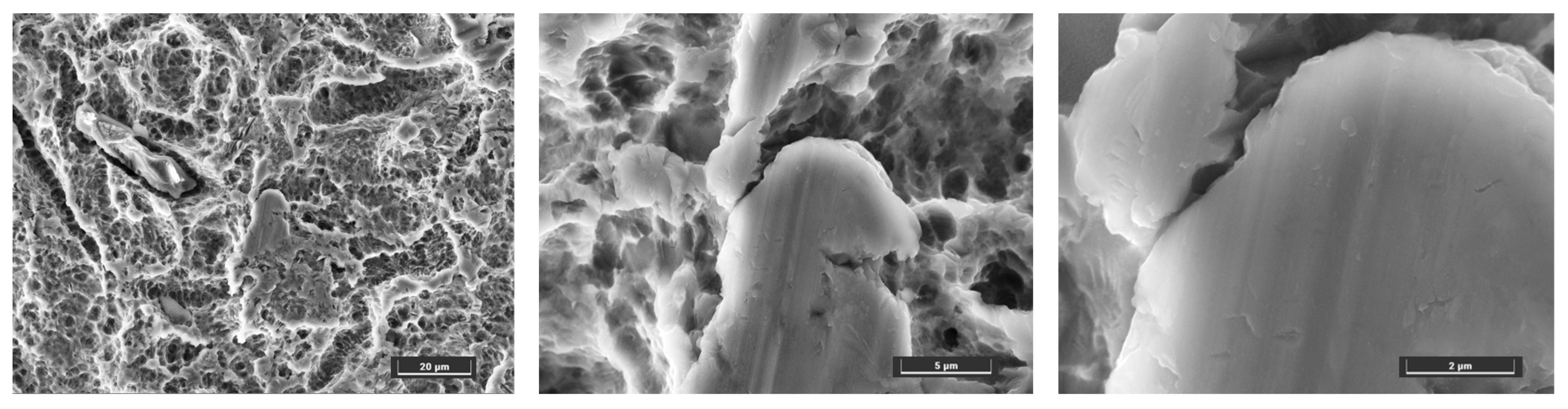
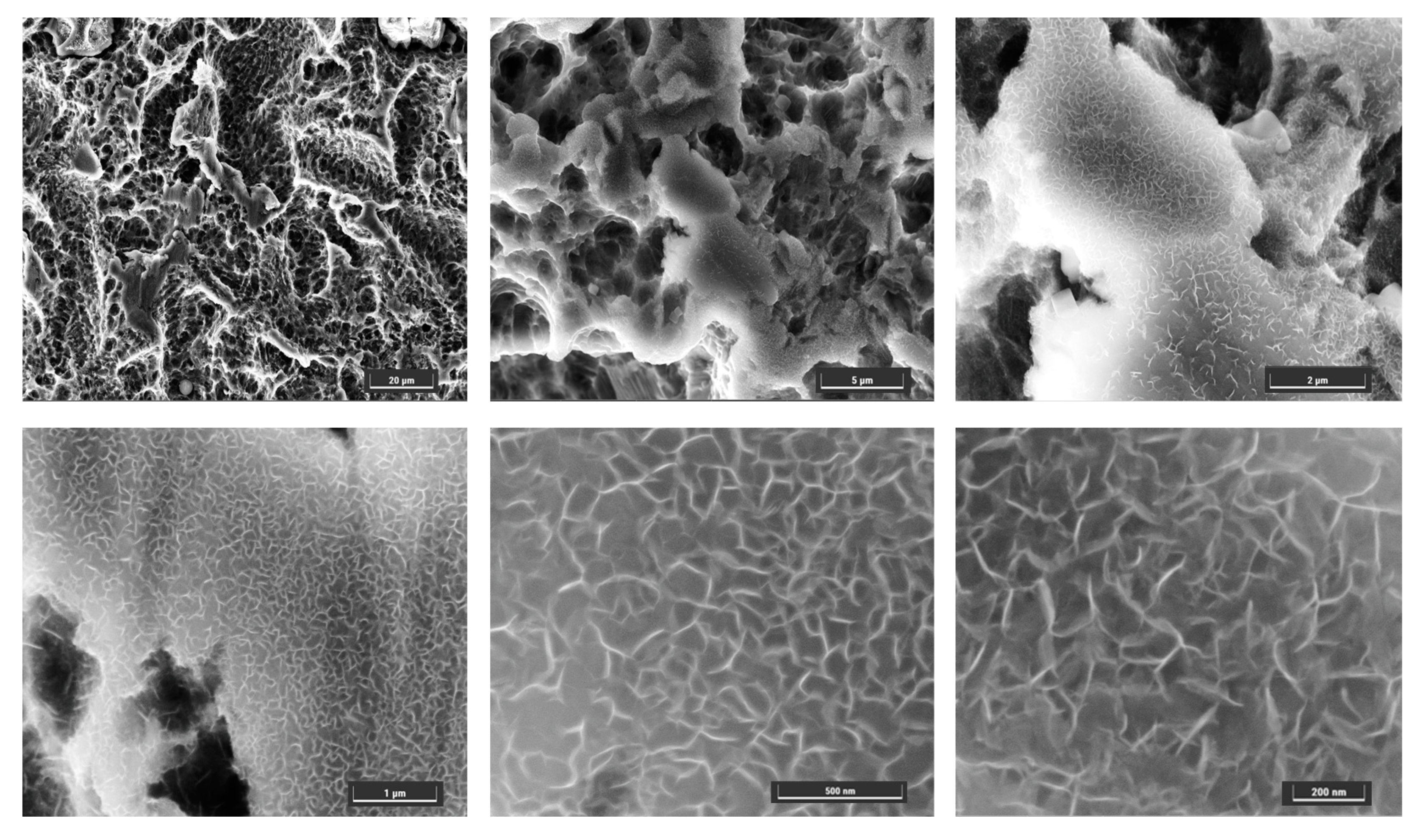
3.2. Surface Characterization
3.3. pH Measurement
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Geetha, M.; Singh, A.K.; Asokamani, R.; Gogia, A.K. Ti Based Biomaterials, the Ultimate Choice for Orthopaedic Implants—A Review. Prog. Mater. Sci. 2009, 54, 397–425. [Google Scholar] [CrossRef]
- Nicholson, J.W. Titanium Alloys for Dental Implants: A Review. Prosthesis 2020, 2, 100–116. [Google Scholar] [CrossRef]
- Li, Q.; Wang, D.; Qiu, J.; Peng, F.; Liu, X. Regulating the Local PH Level of Titanium: Via Mg-Fe Layered Double Hydroxides Films for Enhanced Osteogenesis. Biomater. Sci. 2018, 6, 1227–1237. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Yin, Y.; Jian, L.; Han, X.; Zhao, X.; Wang, D. Enhanced Bactericidal Effect of Calcinated Mg–Fe Layered Double Hydroxide Films Driven by the Fenton Reaction. Int. J. Mol. Sci. 2023, 24, 272. [Google Scholar] [CrossRef]
- Kligman, S.; Ren, Z.; Chung, C.H.; Perillo, M.A.; Chang, Y.C.; Koo, H.; Zheng, Z.; Li, C. The Impact of Dental Implant Surface Modifications on Osseointegration and Biofilm Formation. J. Clin. Med. 2021, 10, 1641. [Google Scholar] [CrossRef] [PubMed]
- Chouirfa, H.; Bouloussa, H.; Migonney, V.; Falentin-Daudré, C. Review of Titanium Surface Modification Techniques and Coatings for Antibacterial Applications. Acta Biomater. 2019, 83, 37–54. [Google Scholar] [CrossRef]
- Shen, Y.; Liu, W.; Wen, C.; Pan, H.; Wang, T.; Darvell, B.W.; Lu, W.W.; Huang, W. Bone Regeneration: Importance of Local PH—Strontium-Doped Borosilicate Scaffold. J. Mater. Chem. 2012, 22, 8662–8670. [Google Scholar] [CrossRef]
- Tan, J.; Wang, D.; Cao, H.; Qiao, Y.; Zhu, H.; Liu, X. Effect of Local Alkaline Microenvironment on the Behaviors of Bacteria and Osteogenic Cells. ACS Appl. Mater. Interfaces 2018, 10, 42018–42029. [Google Scholar] [CrossRef]
- Liu, W.; Wang, T.; Yang, C.; Darvell, B.W.; Wu, J.; Lin, K.; Chang, J.; Pan, H.; Lu, W.W. Alkaline Biodegradable Implants for Osteoporotic Bone Defects—Importance of Microenvironment PH. Osteoporos. Int. 2016, 27, 93–104. [Google Scholar] [CrossRef]
- Wennerberg, A.; Jimbo, R.; Stübinger, S.; Obrecht, M.; Dard, M.; Berner, S. Nanostructures and hydrophilicity influence osseointegration—A biomechanical study in the rabbit tibia. Clin. Oral. Implant. Res. 2014, 25, 1041–1050. [Google Scholar] [CrossRef]
- Yamamura, K.; Miura, T.; Kou, I.; Muramatsu, T.; Furusawa, M.; Yoshinari, M. Influence of various superhydrophilic treatments of titanium on the initial attachment, proliferation, and differentiation of osteoblast-like cells. Dent. Mater. J. 2015, 34, 120–127. [Google Scholar] [CrossRef] [PubMed]
- Delgado-Ruiz, R.; Romanos, G. Potential Causes of Titanium Particle and Ion Release in Implant Dentistry: A Systematic Review. Int. J. Mol. Sci. 2018, 19, 3585. [Google Scholar] [CrossRef]
- Gianfreda, F.; Antonacci, D.; Raffone, C.; Muzzi, M.; Pistilli, V.; Bollero, P. Microscopic Characterization of Bioactivate Implant Surfaces: Increasing Wettability Using Salts and Dry Technology. Materials 2021, 14, 2608. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Cheng, S.; Tan, J.; Xie, J.; Zhang, Y.; Chen, S.; Du, H.; Qian, S.; Qiao, Y.; Peng, F.; et al. Black Mn-Containing Layered Double Hydroxide Coated Magnesium Alloy for Osteosarcoma Therapy, Bacteria Killing, and Bone Regeneration. Bioact. Mater. 2022, 17, 394–405. [Google Scholar] [CrossRef]
- Daniel, S.; Thomas, S. Layered double hydroxides: Fundamentals to applications. In Layered Double Hydroxide Polymer NanoComposites; Woodhead Publishing: Sawston, UK, 2020; pp. 1–76. ISBN 9780081019030. [Google Scholar]
- Yu, S.; Choi, G.; Choy, J.-H. Multifunctional Layered Double Hydroxides for Drug Delivery and Imaging. Nanomaterials 2023, 13, 1102. [Google Scholar] [CrossRef]
- Al Alawi, A.M.; Majoni, S.W.; Falhammar, H. Magnesium and Human Health: Perspectives and Research Directions. Int. J. Endocrinol. 2018, 2018, 9041694. [Google Scholar] [CrossRef] [PubMed]
- Abbaspour, N.; Hurrell, R.; Kelishadi, R. Review on Iron and Its Importance for Human Health. J. Res. Med. Sci. 2014, 19, 164–174. [Google Scholar]
- Cao, Y.; Zheng, D.; Zhang, F.; Pan, J.; Lin, C. Layered Double Hydroxide (LDH) for Multi-Functionalized Corrosion Protection of Metals: A Review. J. Mater. Sci. Technol. 2022, 102, 232–263. [Google Scholar] [CrossRef]
- Izbudak, B.; Cecen, B.; Anaya, I.; Miri, A.K.; Bal-Ozturk, A.; Karaoz, E. Layered Double Hydroxide-Based Nanocomposite Scaffolds in Tissue Engineering Applications. RSC Adv. 2021, 11, 30237–30252. [Google Scholar] [CrossRef]
- Rojas, R.; Mosconi, G.; Zanin, J.P.; Gil, G.A. Layered Double Hydroxide Applications in Biomedical Implants. Appl. Clay Sci. 2022, 224, 106514. [Google Scholar] [CrossRef]
- Ding, X.; Wu, L.; Chen, J.; Zhang, G.; Xie, Z.; Sun, D.; Jiang, B.; Atrens, A.; Pan, F. Enhanced Protective Nanoparticle-Modified MgAl-LDHs Coatings on Titanium Alloy. Surf. Coat. Technol. 2020, 404, 126449. [Google Scholar] [CrossRef]
- Gianfreda, F.; Raffone, C.; Antonacci, D.; Mussano, F.; Genova, T.; Chinigò, G.; Canullo, L.; Bollero, P. Early Biological Response of an Ultra-Hydrophilic Implant Surface Activated by Salts and Dry Technology: An In-Vitro Study. Appl. Sci. 2021, 11, 6120. [Google Scholar] [CrossRef]
- Othman, M.R.; Helwani, Z.; Martunus, W.; Fernando, J.N. Synthetic hydrotalcites from different routes and their application as catalysts and gas adsorbents: A review. Appl. Organo-Met. Chem. 2009, 23, 335. [Google Scholar] [CrossRef]
- Crepaldi, E.L.; Pavan, P.C.; Valim, J.B. Comparative study of the coprecipitation methods for the preparation of layered double hydroxides. Braz. Chem. Soc. 2000, 11, 64. [Google Scholar] [CrossRef]
- Silva, C.G.; Bouizi, Y.; Fornes, V.; Garcia, H. Layered double hydroxides as highly efficient photocatalysts for visible light oxygen generation from water. JACS 2009, 131, 13833. [Google Scholar] [CrossRef] [PubMed]
- Scarpellini, D.; Leonardi, C.; Mattoccia, A.; Giamberardino, L.D.; Medaglia, P.G.; Mantini, G.; Gatta, F.; Giovine, E.; Foglietti, V.; Falconi, C.; et al. Solution-grown Zn/Al layered double hydroxide nanoplatelets onto Al thin films: Fine control of position and lateral thickness. J. Nanomater. 2015, 16, 178. [Google Scholar] [CrossRef]
- Shan, R.R.; Yan, L.G.; Yang, Y.M.; Yang, K.; Yu, S.J.; Yu, H.Q.; Zhu, B.C.; Du, B. Highly efficient removal of three red dyes by adsorption onto Mg-Al-layered double hydroxide. J. Ind. Eng. Chem. 2015, 21, 561–568. [Google Scholar] [CrossRef]
- Li, Y.; Narducci, R.; Varone, A.; Kaciulis, S.; Bolli, E.; Pizzoferrato, R. Zn–Al Layered Double Hydroxides Synthesized on Aluminum Foams for Fluoride Removal from Water. Processes 2021, 9, 2109. [Google Scholar] [CrossRef]
- Moses, O.; Bengazi, F.; Ferri, M.; Gianfreda, F.; Velez, J.U.; Botticelli, D.; Canullo, L. Bioactivated Implant Surfaces Placed in Healed Sites or Extraction Sockets: A Preliminary Experimental Study in Dogs. Int. J. Oral Maxillofac. Implants 2022, 37, 963–970. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, J.; Wu, R.; Wei, J. Construction of functional surfaces for dental implants to enhance osseointegration. Front. Bioeng. Biotechnol. 2023, 11, 1320307. [Google Scholar] [CrossRef]
- Hori, N.; Att, W.; Ueno, T.; Sato, N.; Yamada, M.; Saruwatari, L.; Suzuki, T.; Ogawa, T. Age-dependent Degradation of the Protein Adsorption Capacity of Titanium. J. Dent. Res. 2009, 88, 663–667. [Google Scholar] [CrossRef]
- Albrektsson, T.; Johansson, C. Osteoinduction, osteoconduction and osseointegration. Eur. Spine J. 2001, 10, S96–S101. [Google Scholar]
- Oh, J.M.; Park, D.H.; Choi, S.J.; Choy, J.H. LDH nanocontainers as bio-reservoirs and drug delivery carriers. Recent Pat. Nanotechnol. 2012, 6, 200–217. [Google Scholar] [CrossRef] [PubMed]
- Buser, D.; Broggini, N.; Wieland, M.; Schenk, R.K.; Denzer, A.J.; Cochran, D.L.; Hoffmann, B.; Lussi, A.; Steinemann, S.G. Enhanced bone apposition to a chemically modified SLA titanium surface. J. Dent. Res. 2004, 83, 529–533. [Google Scholar] [CrossRef] [PubMed]
- Schupbach, P.; Glauser, R.; Rocci, A.; Martignoni, M.; Sennerby, L.; Lundgren, A.; Gottlow, J. The human bone-oxidized titanium implant interface: A light microscopic, scanning electron microscopic, back-scatter scanning electron microscopic, and energy-dispersive X-ray study of clinically retrieved dental implants. Clin. Implant. Dent. Relat. Res. 2005, 7, S36–S43. [Google Scholar] [CrossRef] [PubMed]




| Composition | Ti-6A1-4V |
|---|---|
| Titanium | 90% |
| Aluminum | 6% |
| Vanadium | 4% |
| UTS (MPa) | 900 |
| Yields | |
| Strength (MPa) | 850 |
| Elongation at failure (%) | 10 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Gianfreda, F.; Danesi, C.; Bollero, P.; Ermini, A.; Pizzoferrato, R.; Nicolai, E. In Situ Growth of Mg-Fe Layered Double Hydroxides (LDH) Film on Titanium Dental Implant Substrates for pH Regulation in Oral Environments. Crystals 2023, 13, 1636. https://doi.org/10.3390/cryst13121636
Li Y, Gianfreda F, Danesi C, Bollero P, Ermini A, Pizzoferrato R, Nicolai E. In Situ Growth of Mg-Fe Layered Double Hydroxides (LDH) Film on Titanium Dental Implant Substrates for pH Regulation in Oral Environments. Crystals. 2023; 13(12):1636. https://doi.org/10.3390/cryst13121636
Chicago/Turabian StyleLi, Yuliu, Francesco Gianfreda, Carlotta Danesi, Patrizio Bollero, Anita Ermini, Roberto Pizzoferrato, and Eleonora Nicolai. 2023. "In Situ Growth of Mg-Fe Layered Double Hydroxides (LDH) Film on Titanium Dental Implant Substrates for pH Regulation in Oral Environments" Crystals 13, no. 12: 1636. https://doi.org/10.3390/cryst13121636







