Relevant Parameters for the Mechanochemical Synthesis of Bimetallic Supported Catalysts
Abstract
:1. Introduction
2. Choice of Precursors
- Thermal stability;
- Hygroscopic tendencies;
- Surface area, especially for metallic powders.
2.1. Thermal Stability
2.2. Hygroscopic Behavior of Precursor Salts
2.3. Surface Area
2.4. Other Parameters
- Intrinsic volatility of the precursor salt might cause metal loss;
- Different treatment atmospheres or support oxides affect volatility;
- Heavy hydration of precursor salts leads to inhomogeneous and/or irreproducible results;
- When using metallic nanopowders, high surface area usually promotes chemical interaction;
- Hardness values could play an additional role, facilitating the milling of soft materials.
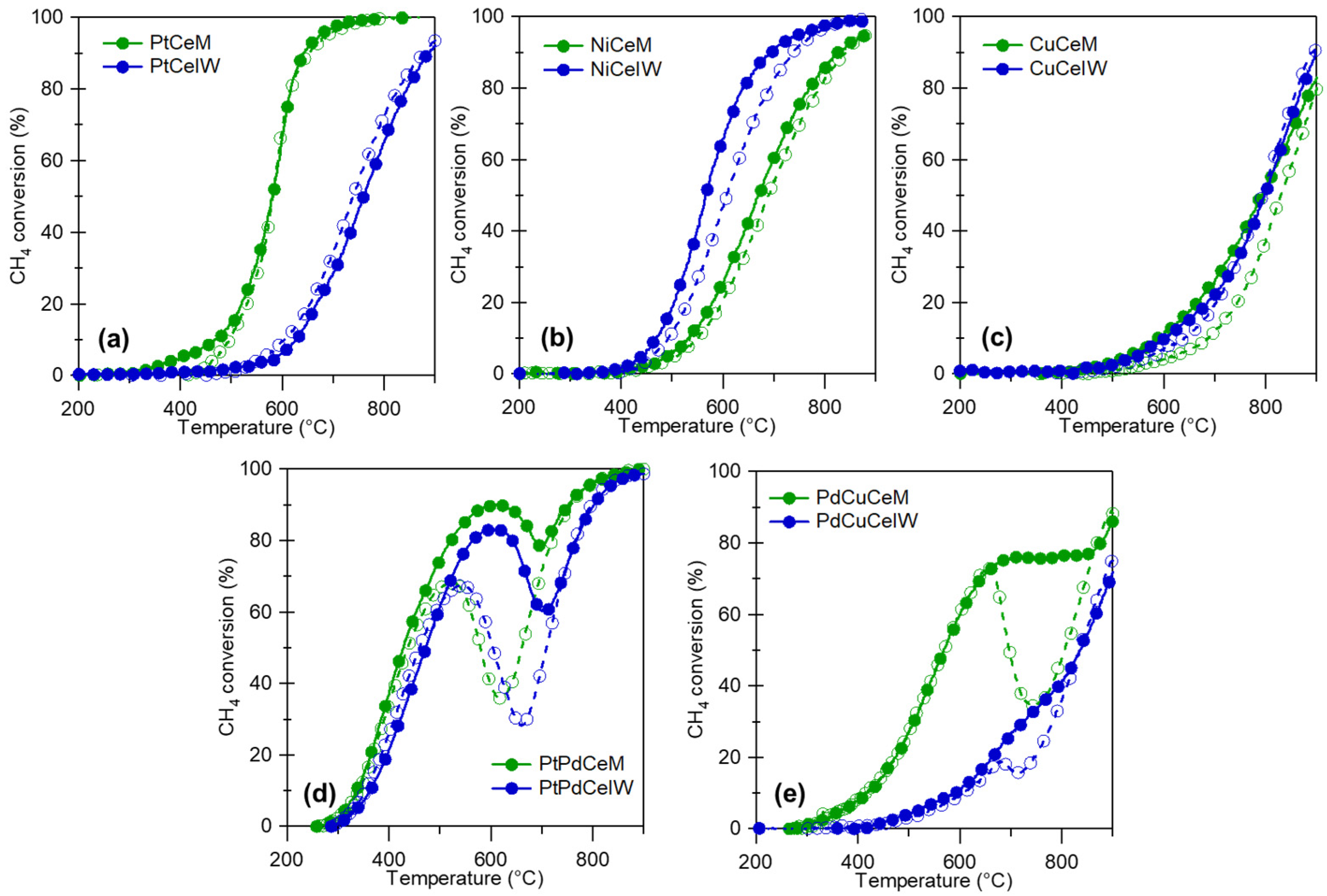
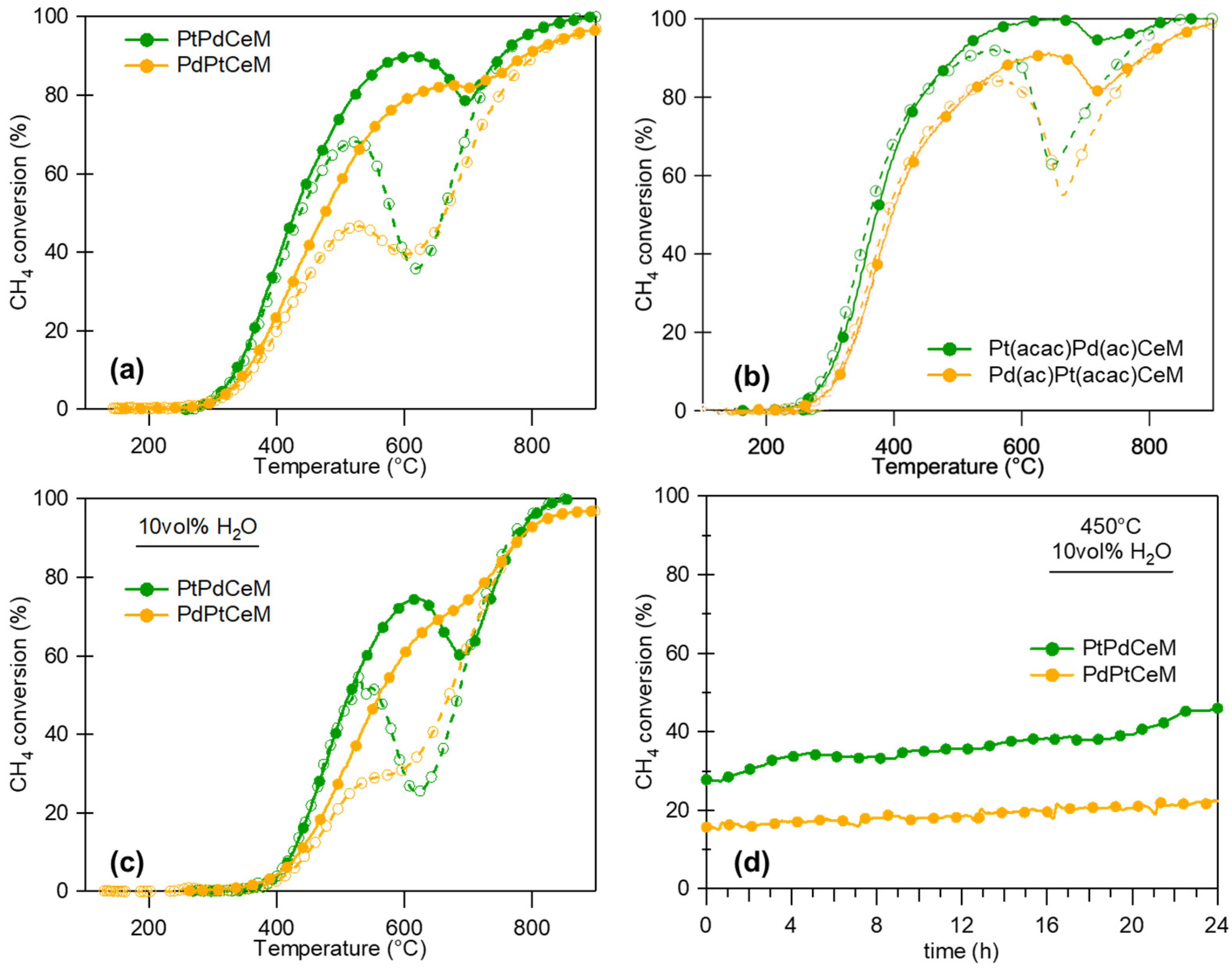
3. Order of Mixing
- The sequential vs. simultaneous milling of metals over the support might affect the formation of bimetallic nanoparticles;
- By affecting either their alloying and/or their dispersion;
- The reciprocal affinity between metals, or between each metal and the support, should be considered;
- Following Le Chatelier’s principle, too strong and too weak an affinity will likely lead to unsatisfactory results.
4. DOE Approach
5. Comparison with Wet Methods
6. Conclusions
7. Patents
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lin, I.J.; Nadiv, S. Review of the Phase Transformation and Synthesis of Inorganic Solids Obtained by Mechanical Treatment (Mechanochemical Reactions). Mater. Sci. Eng. 1979, 39, 193–209. [Google Scholar] [CrossRef]
- Fasman, A.B.; Mikhailenko, S.D.; Kalinina, O.T.; Ivanov, E.Y.; Golubkova, G.V. Synthesis and Regeneration of Raney Catalysts by Mechanochemical Methods. In Preparation of Catalysts V; Poncelet, G., Jacobs, P.A., Grange, P., Delmon, B., Eds.; Elsevier Science Publishers B.V.: Amsterdam, The Netherlands, 1991; pp. 591–600. ISBN 2013206534. [Google Scholar]
- Alves, A.M.; Rosenthal, R.; Teixeira Da Silva, V.L.S. Production of Bimetallic Carbides by Mechanical Alloying for Catalysis. Mater. Sci. Forum 1999, 299–300, 121–125. [Google Scholar] [CrossRef]
- Denis, M.C.; Lalande, G.; Guay, D.; Dodelet, J.P.; Schulz, R. High Energy Ball-Milled Pt and Pt-Ru Catalysts for Polymer Electrolyte Fuel Cells and Their Tolerance to CO. J. Appl. Electrochem. 1999, 29, 951–960. [Google Scholar] [CrossRef]
- Lucariello, M.; Penazzi, N.; Arca, E.; Mulas, G.; Enzo, S. A Structure Investigation of Pt-Co Bimetallic Catalysts Fabricated by Mechanical Alloying. Mater. Chem. Phys. 2009, 114, 227–234. [Google Scholar] [CrossRef]
- Coutts, J.L.; Devor, R.W.; Aitken, B.; Hampton, M.D.; Quinn, J.W.; Clausen, C.A.; Geiger, C.L. The Use of Mechanical Alloying for the Preparation of Palladized Magnesium Bimetallic Particles for the Remediation of PCBs. J. Hazard. Mater. 2011, 192, 1380–1387. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.S.; Yang, N.; Ni, S.Q.; Natarajan, V.; Ahmad, H.A.; Xu, S.; Fang, X.; Zhan, J. One-Pot Synthesis of Highly Active Ni/Fe Nano-Bimetal by Simultaneous Ball Milling and in Situ Chemical Deposition. RSC Adv. 2018, 8, 26469–26475. [Google Scholar] [CrossRef]
- Peera, S.G.; Liu, C. Unconventional and Scalable Synthesis of Non-Precious Metal Electrocatalysts for Practical Proton Exchange Membrane and Alkaline Fuel Cells: A Solid-State Co-Ordination Synthesis Approach. Coord. Chem. Rev. 2022, 463, 214554. [Google Scholar] [CrossRef]
- Kosimov, A.; Alimbekova, A.; Assafrei, J.-M.; Yusibova, G.; Aruväli, J.; Käärik, M.; Leis, J.; Paiste, P.; Ahmadi, M.; Roohi, K.; et al. Template-Assisted Mechanosynthesis Leading to Benchmark Energy Efficiency and Sustainability in the Production of Bifunctional Fe–N–C Electrocatalysts. ACS Sustain. Chem. Eng. 2023, 11, 10825–10834. [Google Scholar] [CrossRef]
- Samriti; Tyagi, R.; Ruzimuradov, O.; Prakash, J. Fabrication Methods and Mechanisms for Designing Highly-Efficient Photocatalysts for Energy and Environmental Applications. Mater. Chem. Phys. 2023, 307, 128108. [Google Scholar] [CrossRef]
- De Bellis, J.; Petersen, H.; Ternieden, J.; Pfänder, N.; Weidenthaler, C.; Schüth, F. Direct Dry Synthesis of Supported Bimetallic Catalysts: A Study on Comminution and Alloying of Metal Nanoparticles. Angew. Chem.-Int. Ed. 2022, 61, e202208016. [Google Scholar] [CrossRef] [PubMed]
- Guczi, L.; Takács, L.; Stefler, G.; Koppány, Z.; Borkó, L. Re-Co/Al2O3 Bimetallic Catalysts Prepared by Mechanical Treatment: CO Hydrogenation and CH4 Conversion. Catal. Today 2002, 77, 237–243. [Google Scholar] [CrossRef]
- Mussio, A.; Danielis, M.; Divins, N.J.; Llorca, J.; Colussi, S.; Trovarelli, A. Structural Evolution of Bimetallic PtPd/CeO2 Methane Oxidation Catalysts Prepared by Dry Milling. ACS Appl. Mater. Interfaces 2021, 13, 31614–31623. [Google Scholar] [CrossRef]
- De Bellis, J.; Felderhoff, M.; Schüth, F. Mechanochemical Synthesis of Supported Bimetallic Catalysts. Chem. Mater. 2021, 33, 2037–2045. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, S.; Yuan, Z.; Chen, H.; Fan, X. Mechanochemical Synthesis of RuCo/MgTiO3 Catalysts for Nonthermal Plasma-Assisted Ammonia Synthesis. Ind. Eng. Chem. Res. 2022, 61, 14199–14210. [Google Scholar] [CrossRef]
- Ralphs, K.; Collins, G.; Manyar, H.; James, S.L.; Hardacre, C. Selective Hydrogenation of Stearic Acid Using Mechanochemically Prepared Titania-Supported Pt and Pt-Re Bimetallic Catalysts. ACS Sustain. Chem. Eng. 2022, 10, 6934–6941. [Google Scholar] [CrossRef]
- da Silva, R.T.P.; Córdoba De Torresi, S.I.; de Oliveira, P.F.M. Mechanochemical Strategies for the Preparation of SiO2-Supported AgAu Nanoalloy Catalysts. Front. Chem. 2022, 10, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Kley, K.S.; De Bellis, J.; Schüth, F. Selective Hydrogenation of Highly Concentrated Acetylene Streams over Mechanochemically Synthesized PdAg Supported Catalysts. Catal. Sci. Technol. 2022, 13, 119–131. [Google Scholar] [CrossRef]
- Fazlikeshteli, S.; Vendrell, X.; Llorca, J. Catalytic Partial Oxidation of Methane over Bimetallic Ru–Ni Supported on CeO2 for Syngas Production. Int. J. Hydrogen Energy 2023, in press. [Google Scholar] [CrossRef]
- Gunnarson, A.; De Bellis, J.; Imhof, T.; Pfänder, N.; Ledendecker, M.; Schüth, F. Facile Solid-State Synthesis of Supported PtNi and PtCo Bimetallic Nanoparticles for the Oxygen Reduction Reaction. Chem. Mater. 2023, 35, 2006–2015. [Google Scholar] [CrossRef]
- Braga, A.; Armengol-Profitós, M.; Pascua-Solé, L.; Vendrell, X.; Soler, L.; Serrano, I.; Villar-Garcia, I.J.; Pérez-Dieste, V.; Divins, N.J.; Llorca, J. Bimetallic NiFe Nanoparticles Supported on CeO2 as Catalysts for Methane Steam Reforming. ACS Appl. Nano Mater. 2023, 6, 7173–7185. [Google Scholar] [CrossRef]
- Wu, T.; Dang, Q.; Wu, Y.; Lei, T.; Yu, J. Catalytic Hydropyrolysis of Biomass over NiMo Bimetallic Carbon-Based Catalysts. J. Environ. Chem. Eng. 2023, 11, 110024. [Google Scholar] [CrossRef]
- Mukherjee, P.; Patil, I.; Kakade, B.; Kumar Das, S.; Sahu, A.K.; Swami, A. Methodical Designing of Pt3−xCo0.5+yNi0.5+y/C (x = 0, 1, 2; y = 0, 0.5, 1) Particles Using a Single-Step Solid State Chemistry Method as Efficient Cathode Catalyst in H2-O2 Fuel Cells. Catal. Today 2023, 423, 113963. [Google Scholar] [CrossRef]
- Dumitrescu, M.A.; Lucariello, M.; Arca, E.; Manzoli, M.; Francia, C.; Ambrosio, E.P. Nanostructured Bimetallic Alloys Prepared via Mechanochemical Synthesis as PEMFC Electrocatalysts for Automotive Applications. J. Appl. Electrochem. 2009, 39, 2115–2121. [Google Scholar] [CrossRef]
- Divins, N.J.; Braga, A.; Vendrell, X.; Serrano, I.; Garcia, X.; Soler, L.; Lucentini, I.; Danielis, M.; Mussio, A.; Colussi, S.; et al. Investigation of the Evolution of Pd-Pt Supported on Ceria for Dry and Wet Methane Oxidation. Nat. Commun. 2022, 13, 5080. [Google Scholar] [CrossRef] [PubMed]
- Munnik, P.; de Jongh, P.E.; de Jong, K.P. Recent Developments in the Synthesis of Supported Catalysts. Chem. Rev. 2015, 115, 6687–6718. [Google Scholar] [CrossRef] [PubMed]
- Hierso, J.-C.; Feurer, R.; Kalck, P. Platinum, Palladium and Rhodium Complexes as Volatile Precursors for Depositing Materials. Coord. Chem. Rev. 1998, 178–180, 1811–1834. [Google Scholar] [CrossRef]
- Stern, K.H. High Temperature Properties and Decomposition of Inorganic Salts Part 3, Nitrates and Nitrites. J. Phys. Chem. Ref. Data 1972, 1, 747–772. [Google Scholar] [CrossRef]
- Gallagher, P.K.; Gross, M.E. The Thermal Decomposition of Palladium Acetate. J. Therm. Anal. 1986, 31, 1231–1241. [Google Scholar] [CrossRef]
- Yuvaraj, S.; Fan-Yuan, L.; Tsong-Huei, C.; Chuin-Tih, Y. Thermal Decomposition of Metal Nitrates in Air and Hydrogen Environments. J. Phys. Chem. B 2003, 107, 1044–1047. [Google Scholar] [CrossRef]
- Jones, J.; Xiong, H.; DeLaRiva, A.T.; Peterson, E.J.; Pham, H.; Challa, S.R.; Qi, G.; Oh, S.; Wiebenga, M.H.; Pereira Hernandez, X.I.; et al. Thermally Stable Single-Atom Platinum-on-Ceria Catalysts via Atom Trapping. Science 2016, 353, 150–154. [Google Scholar] [CrossRef]
- Mercado-Zúñiga, C.; Vargas-García, J.R.; Hernández-Pérez, M.A.; Figueroa-Torres, M.Z.; Cervantes-Sodi, F.; Torres-Martínez, L.M. Synthesis of Highly Dispersed Platinum Particles on Carbon Nanotubes by an in Situ Vapor-Phase Method. J. Alloys Compd. 2014, 615, S538–S541. [Google Scholar] [CrossRef]
- Danielis, M.; Colussi, S.; de Leitenburg, C.; Soler, L.; Llorca, J.; Trovarelli, A. Outstanding Methane Oxidation Performance of Palladium-Embedded Ceria Catalysts Prepared by a One-Step Dry Ball-Milling Method. Angew. Chem. Int. Ed. 2018, 57, 10212–10216. [Google Scholar] [CrossRef] [PubMed]
- Danielis, M.; Colussi, S.; de Leitenburg, C.; Trovarelli, A. The Role of Palladium Salt Precursors in Pd-PdO/CeO2 Catalysts Prepared by Dry Milling for Methane Oxidation. Catal. Commun. 2020, 135, 105899. [Google Scholar] [CrossRef]
- Pascoli, C. Bimetallic Methane Oxidation Catalysts Prepared by Dry Methods: Precursors Effect; Università degli Studi di Udine: Udine, Italy, 2017. [Google Scholar]
- Křenek, T.; Kovářík, T.; Pola, M.; Jakubec, I.; Bezdička, P.; Bastl, Z.; Pokorná, D.; Urbanová, M.; Galíková, A.; Pola, J. Enhancement of Thermal Stability of Silver(I) Acetylacetonate by Platinum(II) Acetylacetonate. Thermochim. Acta 2013, 554, 1–7. [Google Scholar] [CrossRef]
- Sun, H.; Wang, Y.; Xu, S.; Osman, A.I.; Stenning, G.; Han, J.; Sun, S.; Rooney, D.; Williams, P.T.; Wang, F.; et al. Understanding the Interaction between Active Sites and Sorbents during the Integrated Carbon Capture and Utilization Process. Fuel 2021, 286, 119308. [Google Scholar] [CrossRef]
- Arellano-Treviño, M.A.; Kanani, N.; Jeong-Potter, C.W.; Farrauto, R.J. Bimetallic Catalysts for CO2 Capture and Hydrogenation at Simulated Flue Gas Conditions. Chem. Eng. J. 2019, 375, 121953. [Google Scholar] [CrossRef]
- Mussio, A. Bimetallic Catalysts Prepared by Mechanical Milling for Natural Gas Vehicles Applications; Università degli Studi di Udine: Udine, Italy, 2021. [Google Scholar]
- Mongiat, L. Bimetallic Catalysts for Natural Gas Fueled Vehicles Methane Abatement; Università degli Studi di Udine: Udine, Italy, 2015. [Google Scholar]
- Shatov, A.V.; Ponomarev, S.S.; Firstov, S.A. 1.09-Hardness and Deformation of Hardmetals at Room Temperature. In Comprehensive Hard Materials; Sarin, V.K., Ed.; Elsevier: Oxford, UK, 2014; pp. 267–299. ISBN 978-0-08-096528-4. [Google Scholar]
- Bradt, R.C. Ceramic Crystals and Polycrystals, Hardness Of. In Encyclopedia of Materials: Science and Technology; Buschow, K.H.J., Cahn, R.W., Flemings, M.C., Ilschner, B., Kramer, E.J., Mahajan, S., Veyssière, P., Eds.; Elsevier: Oxford, UK, 2001; pp. 1045–1051. ISBN 978-0-08-043152-9. [Google Scholar]
- Wu, Q.; Miao, W.; Zhang, Y.; Gao, H.; Hui, D. Mechanical Properties of Nanomaterials: A Review. Nanotechnol. Rev. 2020, 9, 259–273. [Google Scholar] [CrossRef]
- Bardini, L.; Pappacena, A.; Dominguez-Escalante, M.; Llorca, J.; Boaro, M.; Trovarelli, A. Structural and Electrocatalytic Properties of Molten Core Sn@SnOx Nanoparticles on Ceria. Appl. Catal. B Environ. 2016, 197, 254–261. [Google Scholar] [CrossRef]
- Feng, X.; Gong, X.; Liu, D.; Li, Y.; Jiang, Y.; Zhang, Y. Bayesian Optimization-Guided Discovery of High-Performance Methane Combustion Catalysts Based on Multi-Component PtPd@CeZrOx Core–Shell Nanospheres. Angew. Chem. Int. Ed. 2023, 62, e202313068. [Google Scholar] [CrossRef]
- Hajji, H.; Nasr, S.; Millot, N.; Salem, E.B. Study of the Effect of Milling Parameters on Mechanosynthesis of Hydroxyfluorapatite Using the Taguchi Method. Powder Technol. 2019, 356, 566–580. [Google Scholar] [CrossRef]
- Leardi, R. Experimental Design in Chemistry: A Tutorial. Anal. Chim. Acta 2009, 652, 161–172. [Google Scholar] [CrossRef]
- Czitrom, V. Teacher’s Corner: One-Factor-at-a-Time Versus Designed Experiments. Am. Stat. 1999, 53, 126–131. [Google Scholar]
- Brereton, R.G. Chemometrics: Data Analysis for the Laboratory and Chemical Plant; John Wiley & Sons: Hoboken, NJ, USA, 2016; ISBN 0-471-48977-8. [Google Scholar]
- Box, G.E.P.; Hunter, J.S.; Hunter, W.G. Statistics for Experimenters: Design, Innovation, and Discovery; Wiley, Ed.; Wiley: Hoboken, NJ, USA, 2005; ISBN 978-0-471-71813-0. [Google Scholar]
- Yu, W.; Porosoff, M.D.; Chen, J.G. Review of Pt-Based Bimetallic Catalysis: From Model Surfaces to Supported Catalysts. Chem. Rev. 2012, 112, 5780–5817. [Google Scholar] [CrossRef]
- Miyata, T.; Li, D.; Shiraga, M.; Shishido, T.; Oumi, Y.; Sano, T.; Takehira, K. Promoting Effect of Rh, Pd and Pt Noble Metals to the Ni/Mg(Al)O Catalysts for the DSS-like Operation in CH4 Steam Reforming. Appl. Catal. A Gen. 2006, 310, 97–104. [Google Scholar] [CrossRef]
- Jaiswar, V.K.; Katheria, S.; Deo, G.; Kunzru, D. Effect of Pt Doping on Activity and Stability of Ni/MgAl2O4 Catalyst for Steam Reforming of Methane at Ambient and High Pressure Condition. Int. J. Hydrogen Energy 2017, 42, 18968–18976. [Google Scholar] [CrossRef]
- Schilling, C.; Hofmann, A.; Hess, C.; Ganduglia-Pirovano, M.V. Raman Spectra of Polycrystalline CeO2: A Density Functional Theory Study. J. Phys. Chem. C 2017, 121, 20834–20849. [Google Scholar] [CrossRef]
- Liu, Y.M.; Wang, L.C.; Chen, M.; Xu, J.; Cao, Y.; He, H.Y.; Fan, K.N. Highly Selective Ce-Ni-O Catalysts for Efficient Low Temperature Oxidative Dehydrogenation of Propane. Catal. Lett. 2009, 130, 350–354. [Google Scholar] [CrossRef]
- Fantozzi, N.; Volle, J.-N.; Porcheddu, A.; Virieux, D.; García, F.; Colacino, E. Green Metrics in Mechanochemistry. Chem. Soc. Rev. 2023, 52, 6680–6714. [Google Scholar] [CrossRef]
- Ardila-Fierro, K.J.; Hernández, J.G. Sustainability Assessment of Mechanochemistry by Using the Twelve Principles of Green Chemistry. ChemSusChem 2021, 14, 2145–2162. [Google Scholar] [CrossRef]
- Felderhoff, S.R. Michael On the Theory and Recent Developments in “Batch Mechanochemical Synthesis–Scale-Up”. In Mechanochemistry and Emerging Technologies for Sustainable Chemical Manufacturing; CRC Press: Boca Raton, FL, USA, 2023; ISBN 978-1-00-317818-7. [Google Scholar]
- Santhanam, P.R.; Dreizin, E.L. Predicting Conditions for Scaled-up Manufacturing of Materials Prepared by Ball Milling. Powder Technol. 2012, 221, 403–411. [Google Scholar] [CrossRef]
- Mio, H.; Kano, J.; Saito, F. Scale-up Method of Planetary Ball Mill. Chem. Eng. Sci. 2004, 59, 5909–5916. [Google Scholar] [CrossRef]
- He, X.; Deng, Y.; Zhang, Y.; He, Q.; Xiao, D.; Peng, M.; Zhao, Y.; Zhang, H.; Luo, R.; Gan, T.; et al. Mechanochemical Kilogram-Scale Synthesis of Noble Metal Single-Atom Catalysts. Cell Rep. Phys. Sci. 2020, 1, 100004. [Google Scholar] [CrossRef]
- Acevedo-Córdoba, L.F.; Vargas-Montañez, O.J.; Velasco-Rozo, E.A.; Mora-Vergara, I.D.; de León, J.N.D.; Pérez-Martínez, D.; Morales-Valencia, E.M.; Baldovino-Medrano, V.G. Mechanochemical Approach for the Preparation of Technical Catalysts. Catal. Today 2023, 114474, in press. [Google Scholar] [CrossRef]

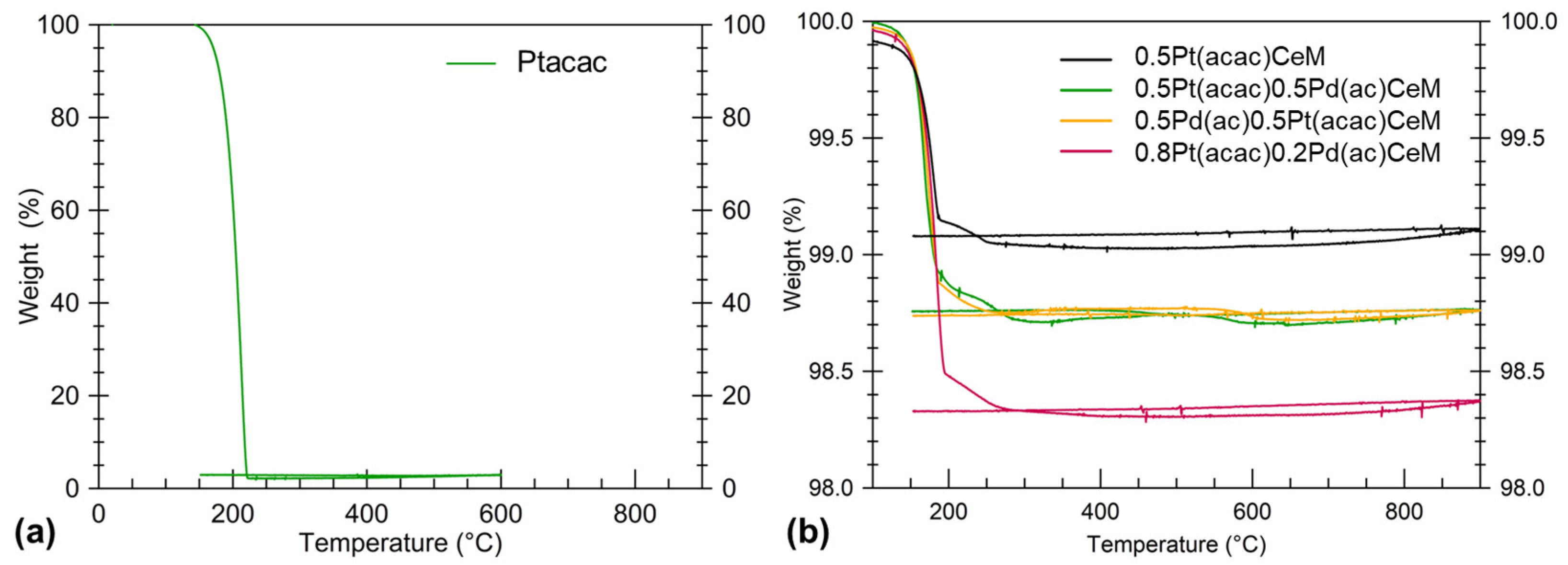

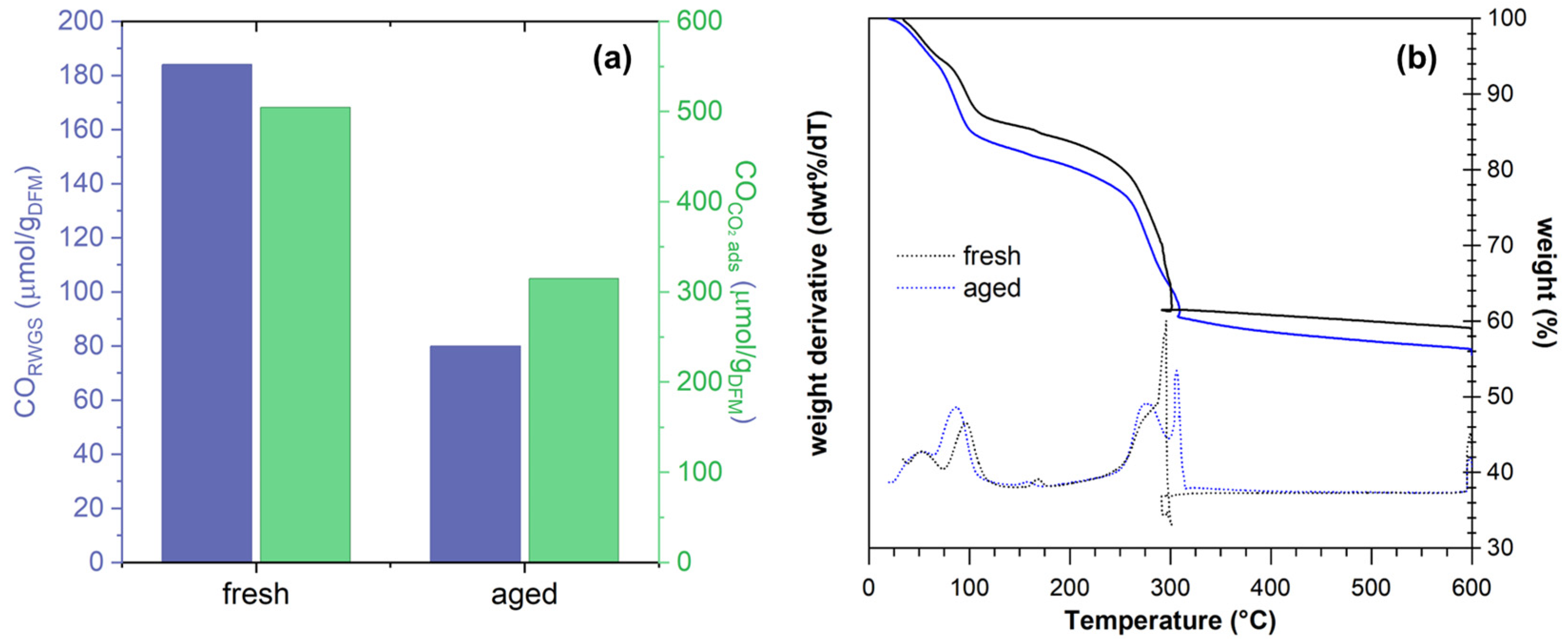

| Sample | %wtmax | %wtmin | %wtmeas | %Ptnom | %PtTGA | %PtICP |
|---|---|---|---|---|---|---|
| 0.5Pt(acac)CeM | 99.5 | 99.0 | 99.1 | 0.5 | 0.10 | n.a. |
| 0.5Pt(acac)0.5Pd(ac)CeM | 98.9 | 98.5 | 98.7 | 0.5 | 0.25 | 0.20 |
| 0.5Pd(ac)0.5Pt(acac)CeM | 98.9 | 98.5 | 98.7 | 0.5 | 0.25 | n.a. |
| 0.8Pt(acac)0.2Pd(ac)CeM | 99.0 | 98.2 | 98.3 | 0.8 | 0.11 | 0.12 |
| Sample | Provider | Particle Size | BET S.A. (m2/g) 1 |
|---|---|---|---|
| Ru black | Strem Chemicals 2 | n.a. | 51 |
| Pd black | Sigma-Aldrich 3 | 10 μm | 40 |
| Pt black | Sigma-Aldrich | <20 μm | 33 |
| Ni nanopowder | Sigma-Aldrich | <20 μm | <1 |
| Cu | Sigma-Aldrich | <425 μm | 6 |
| PdO | Sigma-Aldrich | <10 μm | 74 |
| Sample | Frequency (Hz) | Milling Time (min) | BPR |
|---|---|---|---|
| 1-PtNiCe(−−+) | 15 | 5 | 20 |
| 2-PtNiCe(−+−) | 15 | 45 | 5 |
| 3-PtNiCe(+−−) | 50 | 5 | 5 |
| 4-PtNiCe(000) | 32.5 | 25 | 12.5 |
| 5-PtNiCe(+++) | 50 | 45 | 20 |
| Mechanochemical Synthesis | Wet Synthesis | |
|---|---|---|
| Use of solvents | no | necessary |
| Thermal treatments | maybe | necessary |
| Order of metal addition | relevant | maybe relevant |
| Choice of precursors | relevant | relevant |
| Contamination from synthesis media | possible | unlikely |
| Versatility | limited * | yes |
| Scalability | yes | yes |
| Industrial readiness level | In progress | Full application |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Danielis, M.; Braga, A.; Divins, N.J.; Llorca, J.; Trovarelli, A.; Colussi, S. Relevant Parameters for the Mechanochemical Synthesis of Bimetallic Supported Catalysts. Crystals 2023, 13, 1685. https://doi.org/10.3390/cryst13121685
Danielis M, Braga A, Divins NJ, Llorca J, Trovarelli A, Colussi S. Relevant Parameters for the Mechanochemical Synthesis of Bimetallic Supported Catalysts. Crystals. 2023; 13(12):1685. https://doi.org/10.3390/cryst13121685
Chicago/Turabian StyleDanielis, Maila, Andrea Braga, Núria J. Divins, Jordi Llorca, Alessandro Trovarelli, and Sara Colussi. 2023. "Relevant Parameters for the Mechanochemical Synthesis of Bimetallic Supported Catalysts" Crystals 13, no. 12: 1685. https://doi.org/10.3390/cryst13121685





