Study of the Bonding Characteristics at β-Ga2O3(201)/4H-SiC(0001) Interfaces from First Principles and Experiment
Abstract
:1. Introduction
2. Experimental and Calculation Methodology
2.1. Calculation Methodology
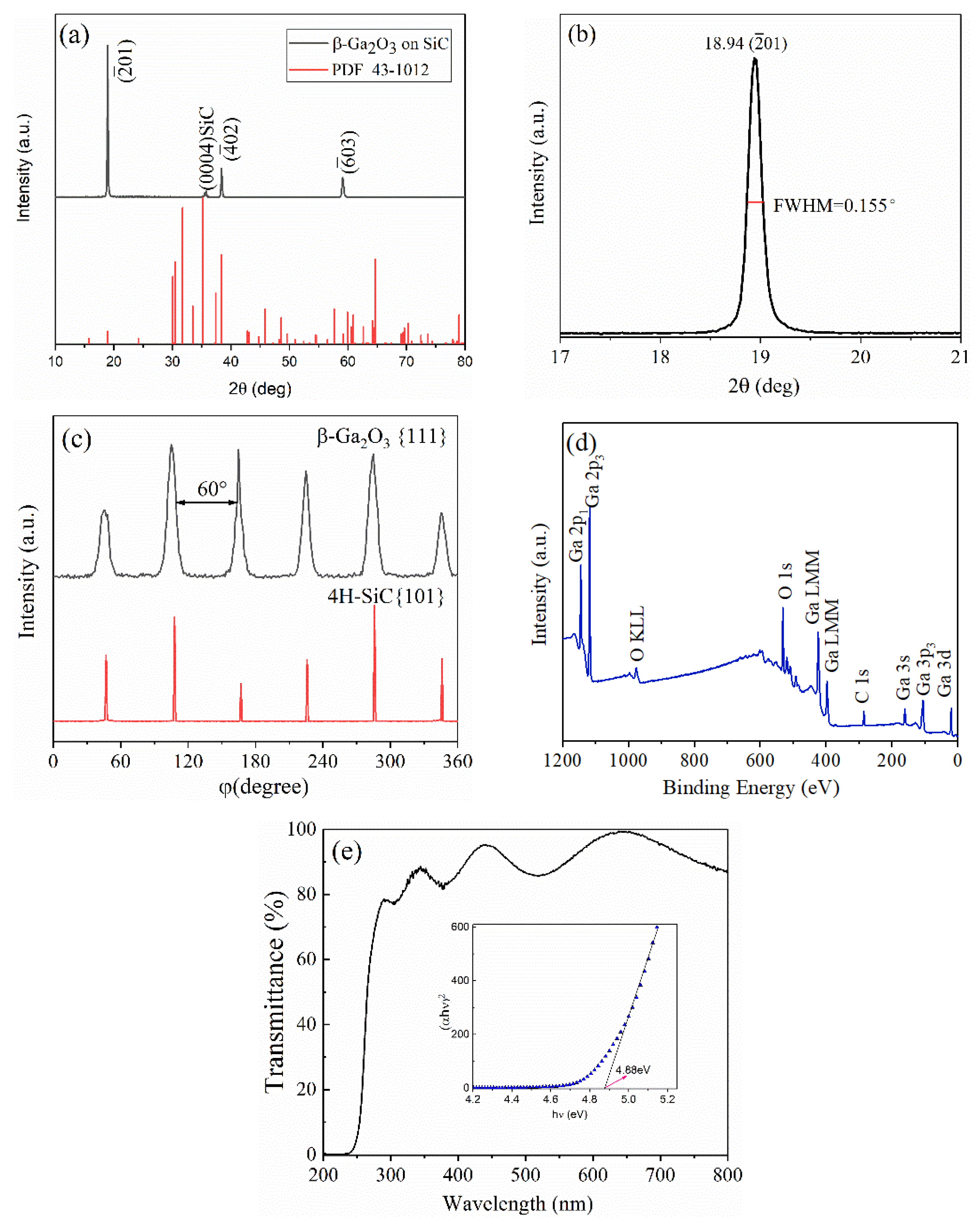
2.2. Experimental
3. Results and Discussion
3.1. Computational Medels
3.2. Work of Adhesion
3.3. Bonding Characteristics
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wu, C.; Wu, F.M.; Ma, C.Q.; Li, S.; Liu, A.P.; Yang, X.; Chen, Y.C.; Wang, J.B.; Guo, D.Y. A general strategy to ultrasensitive Ga2O3 based self-powered solar-blind photodetectors. Mater. Today Phys. 2022, 23, 100643. [Google Scholar] [CrossRef]
- Wu, C.; Guo, D.Y.; Zhang, L.Y.; Li, P.G.; Zhang, F.B.; Tan, C.K.; Wang, S.L.; Liu, A.P.; Wu, F.M.; Tang, W.H. Systematic investigation of the growth kinetics of β-Ga2O3 epilayer by plasma enhanced chemical vapor deposition. Appl. Phys. Lett. 2020, 116, 072102. [Google Scholar] [CrossRef]
- Cao, J.; Chen, L.; Chen, X.; Zhu, Y.; Dong, J.; Wang, B.; He, M.; Wang, X. Performance Improvement of Amorphous Ga2O3 /P-Si Deep Ultraviolet Photodetector by Oxygen Plasma Treatment. Crystals 2021, 11, 1248. [Google Scholar] [CrossRef]
- Yu, Y.T.; Xiang, X.Q.; Zhou, X.Z.; Zhou, K.; Xu, G.W.; Zhao, X.L.; Long, S.B. Device topological thermal management of β-Ga2O3 Schottky barrier diodes. Chin. Phys. B 2021, 30, 067302. [Google Scholar] [CrossRef]
- Yuan, C.; Zhang, Y.; Montgomery, R.; Kim, S.; Shi, J.; Mauze, A.; Itoh, T.; Speck, J.S.; Graham, S. Modeling and analysis for thermal management in gallium oxide field-effect transistors. J. Appl. Phys. 2020, 127, 154502. [Google Scholar] [CrossRef] [Green Version]
- Xiao, J.B.; Yao, J.P. First Principle Calculation of Electronic Structure and Stabilities of TiC/Mg Composites Interfaces. IOP Conf. Ser. Mater. Sci. Eng. 2020, 774, 012096. [Google Scholar] [CrossRef]
- Lv, W.; Yan, L.; Pang, X.; Yang, H.; Qiao, L.; Su, Y.; Gao, K. Study of the stability of α-Fe/MnS interfaces from first principles and experiment. Appl. Surf. Sci. 2020, 501, 144017. [Google Scholar] [CrossRef]
- Qu, Y.; Wu, Z.; Ai, M.; Guo, D.; An, Y.; Yang, H.; Li, L.; Tang, W. Enhanced Ga2O3/SiC ultraviolet photodetector with graphene top electrodes. J. Alloy. Compd. 2016, 680, 247–251. [Google Scholar] [CrossRef]
- Byun, D.W.; Lee, Y.J.; Oh, J.M.; Schweitz, M.A.; Koo, S.M. Morphological and electrical properties of β-Ga2O3 /4H-SiC heterojunction diodes. Electron. Mater. Lett. 2021, 17, 479–484. [Google Scholar] [CrossRef]
- Xu, W.; You, T.; Wang, Y.; Shen, Z.; Liu, K.; Zhang, L.; Sun, H.; Qian, R.; An, Z.; Mu, F.; et al. Efficient thermal dissipation in wafer-scale heterogeneous integration of single-crystalline β- Ga2O3 thin film on SiC. Fundam. Res. 2021, 1, 691–696. [Google Scholar] [CrossRef]
- Nakagomi, S.; Momo, T.; Takahashi, S.; Kokubun, Y. Deep ultraviolet photodiodes based on β-Ga2O3 /SiC heterojunction. Appl. Phys. Lett. 2013, 103, 072105. [Google Scholar] [CrossRef] [Green Version]
- Yu, J.; Nie, Z.; Dong, L.; Yuan, L.; Li, D.; Huang, Y.; Zhang, L.; Zhang, Y.; Jia, R. Influence of annealing temperature on structure and photoelectrical performance of β-Ga2O3 /4H-SiC heterojunction photodetectors. J. Alloy. Compd. 2019, 798, 458–466. [Google Scholar] [CrossRef]
- Nepal, N.; Katzer, D.S.; Downey, B.P.; Wheeler, V.D.; Nyakiti, L.O.; Storm, D.F.; Hardy, M.T.; Freitas, J.A.; Jin, E.N.; Vaca, D.; et al. Heteroepitaxial growth of β-Ga2O3 films on SiC via molecular beam epitaxy. J. Vac. Sci. Technol. A Vac. Surf. Film. 2020, 38, 063406. [Google Scholar] [CrossRef]
- Yu, M.; Wang, H.; Wei, W.; Peng, B.; Yuan, L.; Hu, J.; Zhang, Y.; Jia, R. Analysis of electronic structure and properties of Ga2O3 /CuAlO2 heterojunction. Appl. Surf. Sci. 2021, 568, 150826. [Google Scholar] [CrossRef]
- He, X.; Hu, J.; Zhang, Z.; Liu, W.; Song, K.; Meng, J. Study on the interface electronic properties of AlN(0001)/ β- Ga2O3100). Surf. Interfaces 2022, 28, 101585. [Google Scholar] [CrossRef]
- Xu, P.; Gui, X.; Zhang, X.; Zhang, M.; Liu, G.; Guo, Q.; Qiao, G. Wetting and interfacial behavior of Al–Ti/4H–SiC system: A combined study of experiment and DFT simulation. Ceram. Int. 2021, 47, 32545–32553. [Google Scholar] [CrossRef]
- Wang, L.; Dhar, S.; Feldman, L.C.; Kuroda, M.A. Nitrogen-Induced Changes in the Electronic and Structural Properties of 4H-SiC (0001)/SiO2 Interfaces. Phys. Status Solidi (B) 2022, 259, 2100224. [Google Scholar] [CrossRef]
- Liu, B.B.; Yang, J.F. Mg on adhesion of Al (111)/3C-SiC (111) interfaces from first principles study. J. Alloy. Compd. 2019, 791, 530–539. [Google Scholar] [CrossRef]
- Zacherle, T.; Schmidt, P.C.; Martin, M. Ab initio calculations on the defect structure of beta- Ga2O3. Phys, Rev. B 2013, 87, 23520. [Google Scholar]
- Geller, S. Crystal Structure of β-Ga2O3. J. Chem. Phys. 1960, 33, 676–684. [Google Scholar] [CrossRef]
- Su, J.; Guo, R.; Lin, Z.; Zhang, S.; Zhang, J.; Chang, J.; Hao, Y. Unusual electronic and optical properties of two-dimensional Ga2O3 predicted by density functional theory, J. Phys. Chem. C. 2018, 122, 24592–24599. [Google Scholar] [CrossRef]
- Park, C.; Cheong, B.-H.; Lee, K.-H.; Chang, K.J. Structural and electronic properties of cubic, 2 H, 4 H, and 6 H SiC. Phys. Rev. B 1994, 49, 4485–4493. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, M.; Peng, P.; Gao, H.; Wang, J.; Sun, B. Preferred orientation at the Al/graphene interface: First-principles calculations and experimental observation. J. Alloy. Compd. 2022, 900, 163304. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, W.; Fang, S.; Dai, B.; Zhu, J. The interface characteristics of TiN (100)/MgO (100) multilayer on oxidized Si (100) substrate via first-principle calculations and experimental investigation. Mol. Simul. 2021, 47, 552–559. [Google Scholar] [CrossRef]
- Liu, X.; Dong, H.; Lv, X.; Bai, C.; Hu, N.; Wen, L.; Yang, Z. The adhesion, stability, and electronic structure of γ-TiAl/VN interface: A first-principle study. Appl. Phys. A 2018, 124, 531. [Google Scholar] [CrossRef]
- Zhu, N.; Ma, K.; Xue, X.; Su, J. The formation and role of the SiO2 oxidation layer in the 4H-SiC/β-Ga2O3 interface. Appl. Surf. Sci. 2022, 581, 151956. [Google Scholar] [CrossRef]
- Wu, Z.; Pang, M.; Zhan, Y.; Shu, S.; Xiong, L.; Li, Z. The bonding characteristics of the Cu (111)/WC (0001) interface: An insight from first-principle calculations. Vacuum 2021, 191, 110218. [Google Scholar] [CrossRef]
- Yuan, H.; Su, J.; Guo, R.; Tian, K.; Lin, Z.; Zhang, J.; Chang, J.; Hao, Y. Contact barriers modulation of graphene/β-Ga2O3 interface for high-performance Ga2O3 devices. Appl. Surf. Sci. 2020, 527, 146740. [Google Scholar] [CrossRef]
- Jia, B.; Hu, Y.; Guan, X.; Hao, J.; Yan, B.; Zu, Y.; Liu, G.; Zhang, Q.; Peng, G.D.; Lu, P. Atomic structures and electronic properties of different interface types at Al/c-SiO2 interfaces. Appl. Surf. Sci. 2022, 578, 151932. [Google Scholar] [CrossRef]
- Yang, X.; Du, X.; Liu, J.; Chen, R.; Wang, D.; Le, Y.; Zhu, H.; Feng, B.; Ma, J.; Xiao, H. Effects of porosity on the structural and optoelectronic properties of Er-doped Ga2O3 epitaxial films on etched epi-GaN/sapphire substrates. Ceram. Int. 2021, 47, 9597–9605. [Google Scholar] [CrossRef]
- Joshi, G.; Chauhan, Y.S.; Verma, A. Temperature dependence of β-Ga2O3 heteroepitaxy on c-plane sapphire using low pressure chemical vapor deposition. J. Alloy. Compd. 2021, 883, 160799. [Google Scholar] [CrossRef]
- Cao, Q.; He, L.; Xiao, H.; Feng, X.; Lv, Y.; Ma, J. β- Ga2O3 epitaxial films deposited on epi-GaN/sapphire (0001) substrates by MOCVD. Mater. Sci. Semicond. Process. 2018, 77, 58–63. [Google Scholar] [CrossRef]
- Lv, Y.; Ma, J.; Mi, W.; Luan, C.; Zhu, Z.; Xiao, H. Characterization of β- Ga2O3 thin films on sapphire (0001) using metal-organic chemical vapor deposition technique. Vacuum 2012, 86, 1850–1854. [Google Scholar] [CrossRef]
- Zhang, T.; Li, Y.; Zhang, Y.; Feng, Q.; Ning, J.; Zhang, C.; Zhang, J.; Hao, Y. Investigation of β- Ga2O3 thin films grown on epi-GaN/sapphire (0001) substrates by low pressure MOCVD. J. Alloy. Compd. 2021, 859, 157810. [Google Scholar] [CrossRef]
- Tian, R.; Pan, M.; Sai, Q.; Zhang, L.; Qi, H.; Mohamed, H.F. Crucial Role of Oxygen Vacancies in Scintillation and Optical Properties of Undoped and Al-Doped β- Ga2O3 Single Crystals. Crystals 2022, 12, 429. [Google Scholar] [CrossRef]

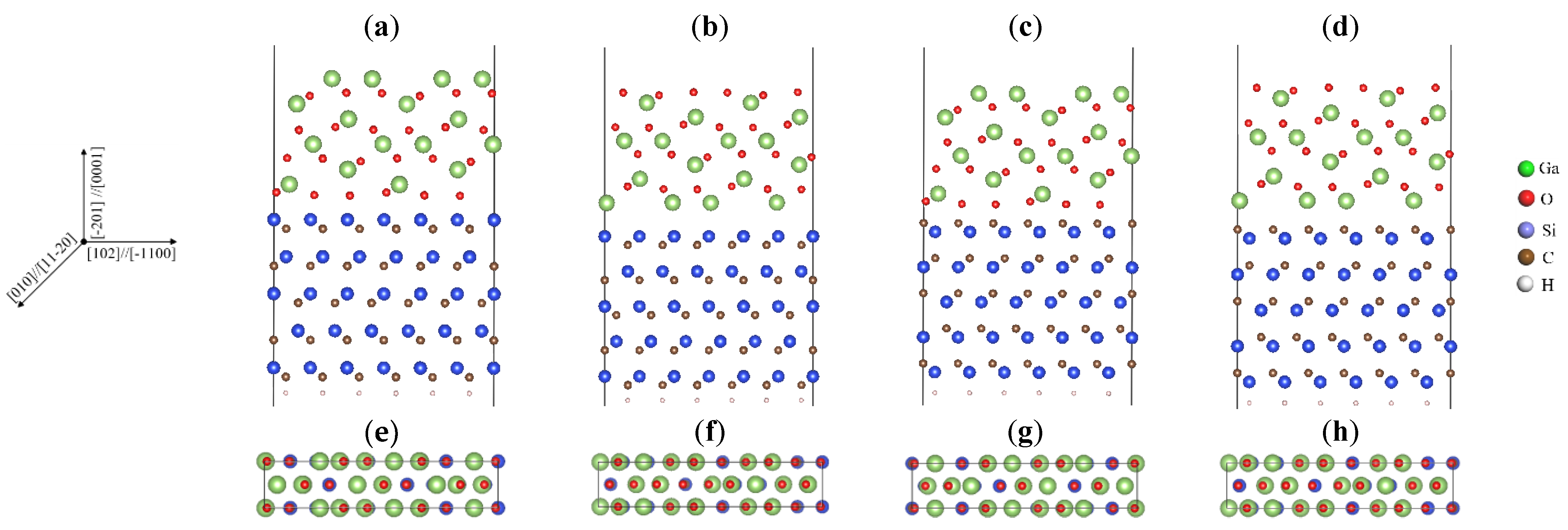


| Inteface | Energy/eV | Interface Spacing (Å) | Unrelaxed Bond Length (Å) | Relaxed Bond Length (Å) | Wad/(J/m2) |
|---|---|---|---|---|---|
| Si-O | −707.72901323 | 1.7 | 1.80 | 1.66 | 8.5 |
| Si-Ga | −686.20897765 | 2.3 | 2.31 | 2.44 | 3.1 |
| C-O | −691.84100188 | 1.5 | 1.60 | 1.47 | 4.3 |
| C-Ga | −684.10880119 | 1.9 | 1.91 | 1.95 | 3.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, B.; Hu, J.; Meng, J.; He, X.; Wang, X.; Pu, H. Study of the Bonding Characteristics at β-Ga2O3(201)/4H-SiC(0001) Interfaces from First Principles and Experiment. Crystals 2023, 13, 160. https://doi.org/10.3390/cryst13020160
Xu B, Hu J, Meng J, He X, Wang X, Pu H. Study of the Bonding Characteristics at β-Ga2O3(201)/4H-SiC(0001) Interfaces from First Principles and Experiment. Crystals. 2023; 13(2):160. https://doi.org/10.3390/cryst13020160
Chicago/Turabian StyleXu, Bei, Jichao Hu, Jiaqi Meng, Xiaomin He, Xi Wang, and Hongbin Pu. 2023. "Study of the Bonding Characteristics at β-Ga2O3(201)/4H-SiC(0001) Interfaces from First Principles and Experiment" Crystals 13, no. 2: 160. https://doi.org/10.3390/cryst13020160





