Towards Binder Jetting and Sintering of AZ91 Magnesium Powder
Abstract
:1. Introduction
2. Materials and Methods
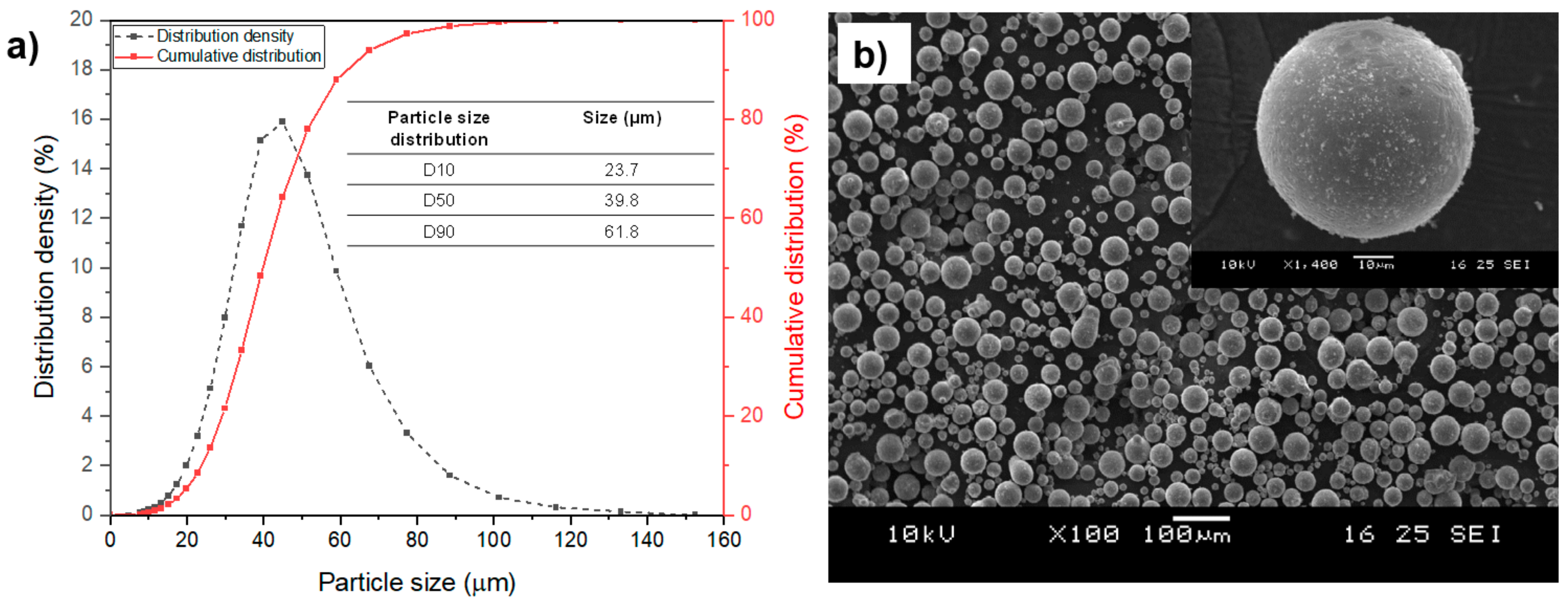
2.1. Raw Powder
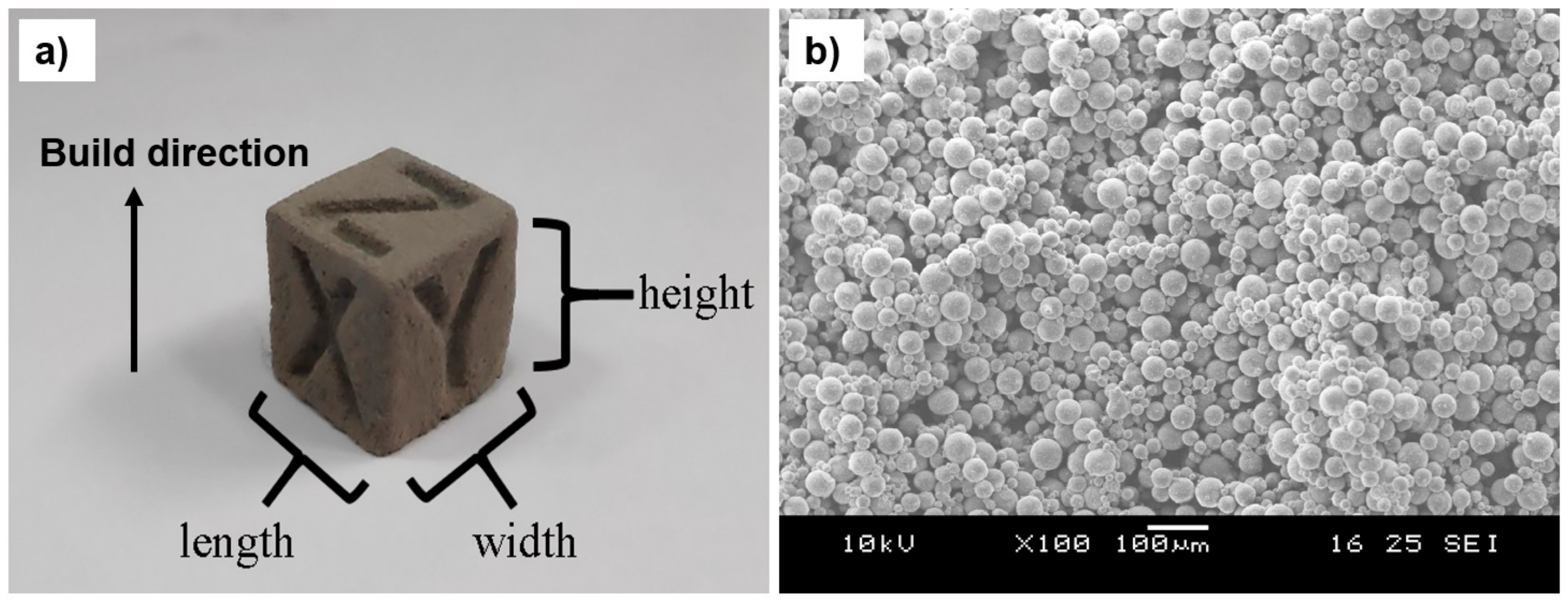
2.2. Binder Jetting
2.3. Sintering
2.4. Characterization Methods
3. Results and Discussion
3.1. Binder Jetting of Green AZ91D Objects
3.1.1. Determination of an Ink Saturation Level and Its Effect on Dimensional Accuracy
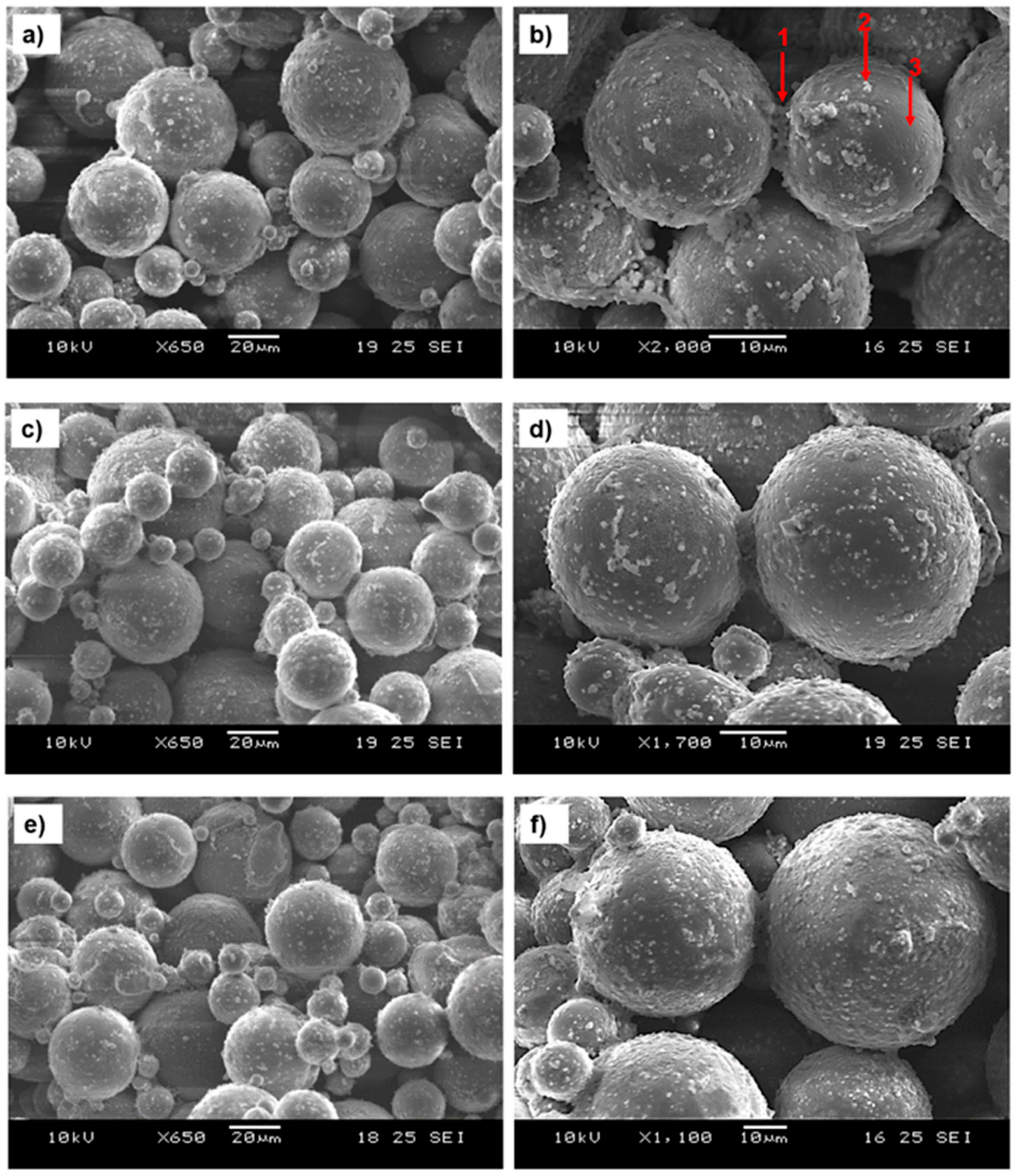
3.1.2. Influence of Ink Saturation Levels on Interactions with AZ91 Powder
3.2. Sintering of Green AZ91D Objects
- (1)
- Characteristics of raw powder: elemental composition, particle size and distribution, morphology, flowability, and impurity concentration, especially with respect to oxygen content.
- (2)
- Binder jetting process parameters: powder–ink interactions, layer thickness, ink saturation level, and density of as-printed green samples.
- (3)
- Sintering profiles: residue, sintering furnace, temperature, duration, and atmospheres.
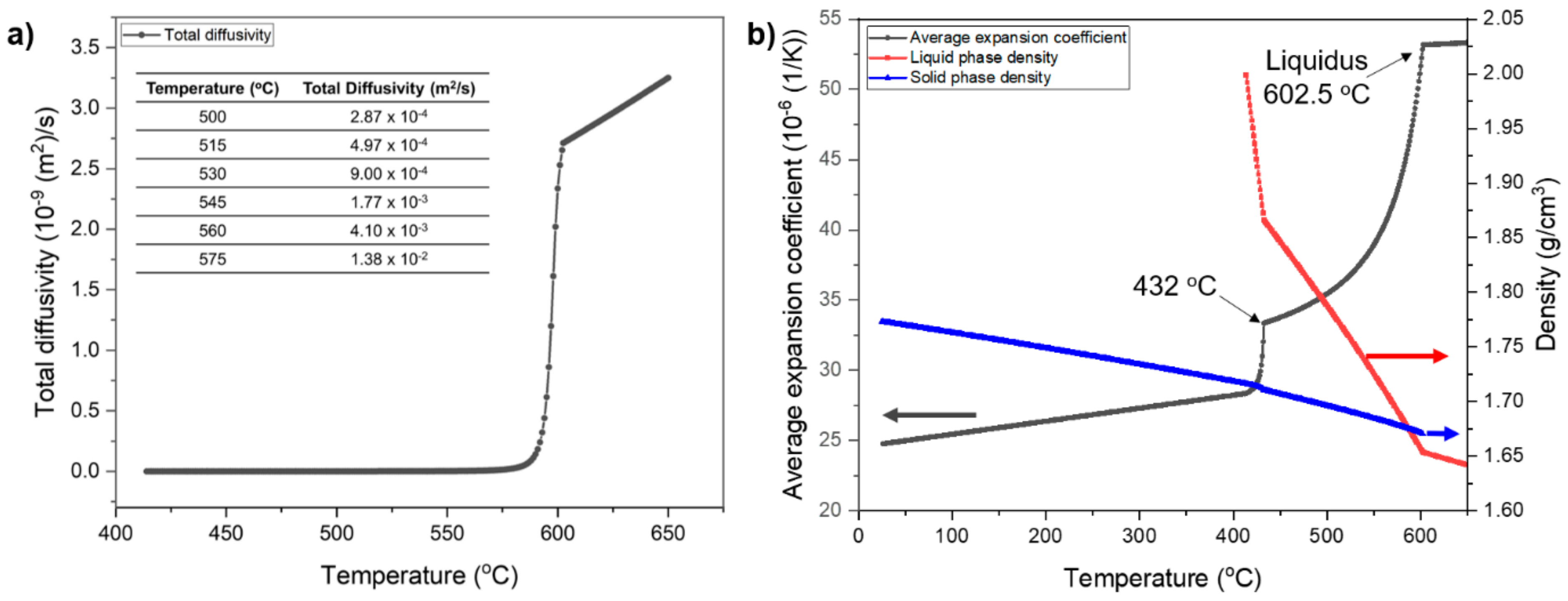
3.2.1. Determination of Sintering Temperature Range
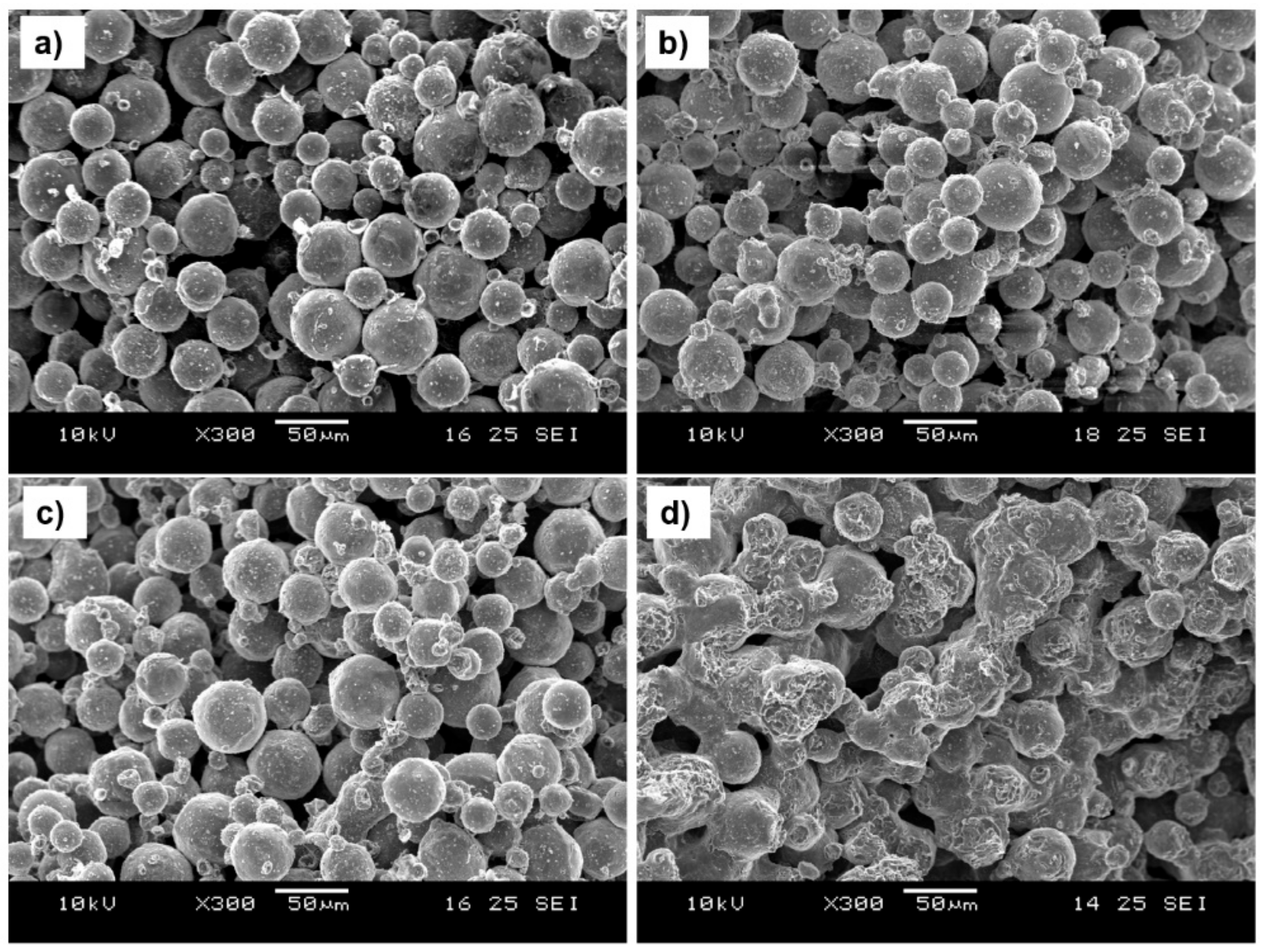
3.2.2. Influence of Sintering Temperatures on Microstructure
3.2.3. Influence of Sintering Temperatures on Macrostructure
3.2.4. Influence of Sintering Temperatures on Density
3.2.5. Influence of Sintering Time on Density
4. Conclusions
- (1)
- An ink saturation level higher than the minimum threshold value (i.e., 29.3% SL) was needed for the adequate vertical and lateral permeation of ink and the formation of fully evolved solid interparticle bridges between AZ91D particles. The green cubes printed with 90% and 110% SLs had great dimensional accuracy and mechanical integrity.
- (2)
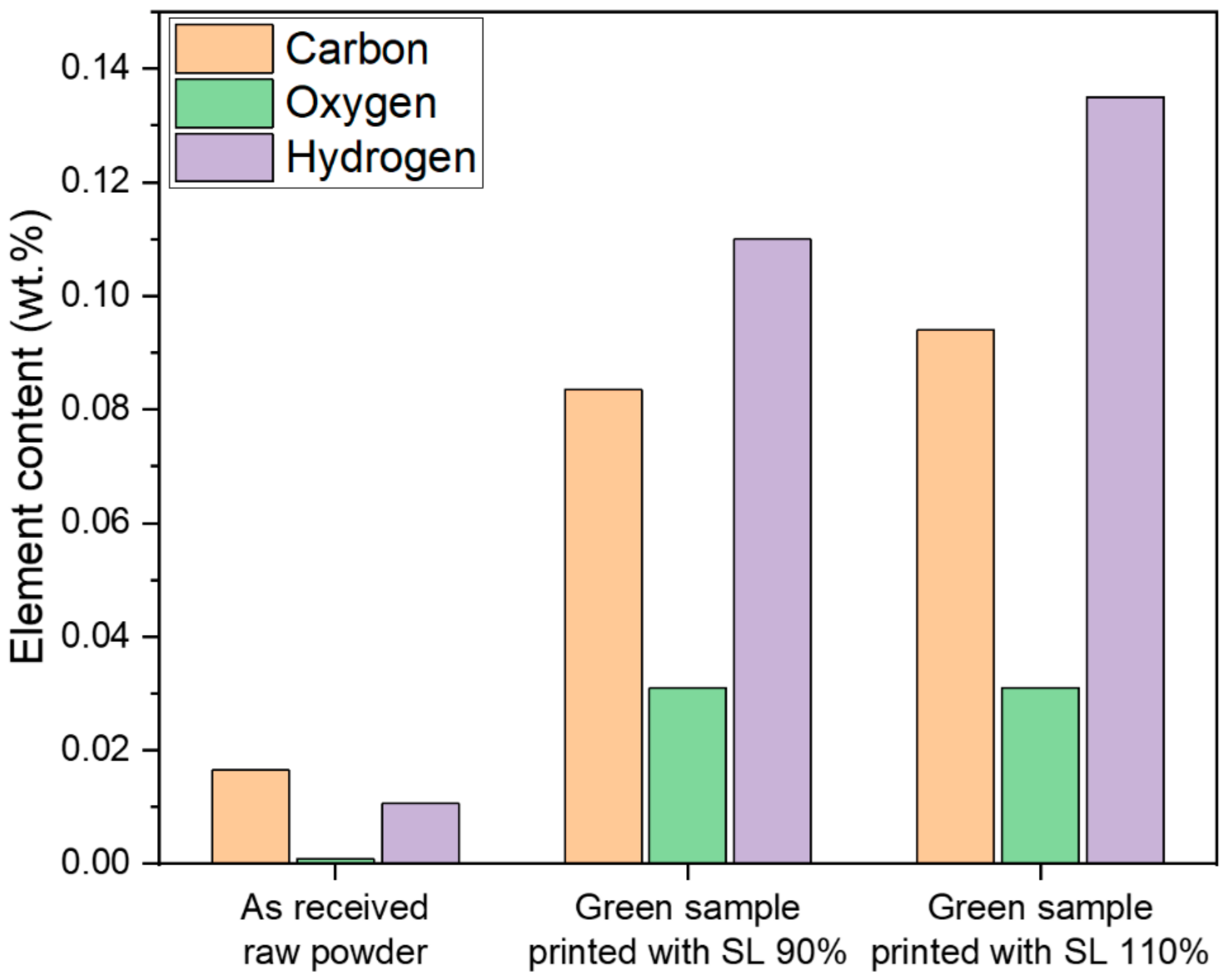
- The dimensionless number of bridges per powder particle and the results of elemental analyses demonstrated that a lower ink saturation level minimized the compositional change in as-printed AZ91 objects. The green objects printed with a 90% SL showed a marginal compositional shift in O, H, and C compared with the raw AZ91D powder.
- (3)
- Increasing the sintering temperature from 530 °C to 575 °C increased the nominal liquid fraction during the sintering of as-printed AZ91 objects from 30.5 vol. % to 54.5 vol. %. Because the swelling occurred at temperatures >545 °C, the actual liquid content in the sinter neck regions at a higher sintering temperature remained the same (i.e., 30.5 vol. % to 35.8 vol. %).
- (4)
- With increasing sintering temperatures and time, the density of as-sintered samples increased slightly compared with those of the as-printed condition (i.e., 1.5% to 5.1%).
- (5)
- The sintering densification rate of binder jet-printed Mg powder was governed by the swelling phenomenon and the packing density of as-printed green objects.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gusieva, K.; Davies, C.H.J.; Scully, J.R.; Birbilis, N. Corrosion of magnesium alloys: The role of alloying. Int. Mater. Rev. 2015, 60, 169–194. [Google Scholar] [CrossRef]
- Weiler, J.P. Exploring the concept of castability in magnesium die-casting alloys. J. Magnes. Alloys 2021, 9, 102–111. [Google Scholar] [CrossRef]
- Ezhilmaran, V.; Suya Prem Anand, P.; Kannan, S.; Sivashanmugam, N.; Jayakrishna, K.; Kalusuraman, G. Review of bioresorbable AZ91, AZ31 and Mg–Zn–Ca implants and their manufacturing methods. Mater. Sci. Technol. 2022, 1–25. [Google Scholar] [CrossRef]
- Zeng, Z.; Salehi, M.; Kopp, A.; Xu, S.; Esmaily, M.; Birbilis, N. Recent progress and perspectives in additive manufacturing of magnesium alloys. J. Magnes. Alloys 2022, 10, 1511–1541. [Google Scholar] [CrossRef]
- Schmid, D.; Renza, J.; Zaeh, M.F.; Glasschroeder, J. Process Influences on Laser-beam Melting of the Magnesium Alloy AZ91. Phys. Procedia 2016, 83, 927–936. [Google Scholar] [CrossRef]
- Li, X.; Fang, X.; Wang, S.; Wang, S.; Zha, M.; Huang, K. Selective laser melted AZ91D magnesium alloy with superior balance of strength and ductility. J. Magnes. Alloys 2022. [Google Scholar] [CrossRef]
- Bi, J.; Shen, J.; Hu, S.; Zhen, Y.; Yin, F.; Bu, X. Microstructure and mechanical properties of AZ91 Mg alloy fabricated by cold metal transfer additive manufacturing. Mater. Lett. 2020, 276, 128185. [Google Scholar] [CrossRef]
- Vishwanath, A.S.; Rane, K.; Schaper, J.; Strano, M.; Casati, R. Rapid production of AZ91 Mg alloy by extrusion based additive manufacturing process. Powder Metall. 2021, 64, 370–377. [Google Scholar] [CrossRef]
- Su, C.; Wang, J.; Li, H.; You, Z.; Li, J. Binder-jetting additive manufacturing of Mg alloy densified by two-step sintering process. J. Manuf. Process. 2021, 72, 71–79. [Google Scholar] [CrossRef]
- Mostafaei, A.; Elliott, A.; Barnes, J.; Li, F.; Tan, W.; Cramer, C.; Nandwana, P.; Chmielus, M. Binder jet 3D printing—Process parameters, materials, properties, modeling, and challenges. Prog. Mater. Sci. 2021, 119, 100707. [Google Scholar] [CrossRef]
- Ziaee, M.; Crane, N. Binder jetting: A review of process, materials, and methods. Addit. Manuf. 2019, 28, 781–801. [Google Scholar] [CrossRef]
- Salehi, M.; Maleksaeedi, S.; Nai, S.M.L.; Meenashisundaram, G.K.; Goh, M.H.; Gupta, M. A paradigm shift towards compositionally zero-sum binderless 3D printing of magnesium alloys via capillary-mediated bridging. Acta Mater. 2019, 165, 294–306. [Google Scholar] [CrossRef]
- Salehi, M.; Maleksaeedi, S.; Nai, M.L.S.; Gupta, M. Towards additive manufacturing of magnesium alloys through integration of binderless 3D printing and rapid microwave sintering. Addit. Manuf. 2019, 29, 100790. [Google Scholar] [CrossRef]
- Salehi, M.; Seet, H.L.; Gupta, M.; Farnoush, H.; Maleksaeedi, S.; Nai, M.L.S. Rapid densification of additive manufactured magnesium alloys via microwave sintering. Addit. Manuf. 2021, 37, 101655. [Google Scholar] [CrossRef]
- Salehi, M.; Maleksaeedi, S.; Sapari, M.A.B.; Nai, M.L.S.; Meenashisundaram, G.K.; Gupta, M. Additive manufacturing of magnesium–zinc–zirconium (ZK) alloys via capillary-mediated binderless three-dimensional printing. Mater. Des. 2019, 169, 107683. [Google Scholar] [CrossRef]
- Kuah, K.X.; Salehi, M.; Huang, Z.; Zhang, S.X.; Seet, H.L.; Nai, M.L.S.; Blackwood, D.J. Surface Modification with Phosphate and Hydroxyapatite of Porous Magnesium Scaffolds Fabricated by Binder Jet Additive Manufacturing. Crystals 2022, 12, 1850. [Google Scholar] [CrossRef]
- Kuah, K.X.; Salehi, M.; Ong, W.K.; Seet, H.L.; Nai, M.L.S.; Wijesinghe, S.; Blackwood, D.J. Insights into the influence of oxide inclusions on corrosion performance of additive manufactured magnesium alloys. npj Mater. Degrad. 2022, 6, 36. [Google Scholar] [CrossRef]
- Kuah, K.X.; Blackwood, D.J.; Ong, W.K.; Salehi, M.; Seet, H.L.; Nai, M.L.S.; Wijesinghe, S. Analysis of the corrosion performance of binder jet additive manufactured magnesium alloys for biomedical applications. J. Magnes. Alloys 2022, 10, 1296–1310. [Google Scholar] [CrossRef]
- Scheel, M.; Seemann, R.; Brinkmann, M.; Di Michiel, M.; Sheppard, A.; Breidenbach, B.; Herminghaus, S. Morphological clues to wet granular pile stability. Nat. Mater. 2008, 7, 189. [Google Scholar] [CrossRef]
- Kohonen, M.M.; Geromichalos, D.; Scheel, M.; Schier, C.; Herminghaus, S. On capillary bridges in wet granular materials. Phys. A Stat. Mech. Its Appl. 2004, 339, 7–15. [Google Scholar] [CrossRef]
- Salehi, M.; Gupta, M.; Maleksaeedi, S.; Nai, M.L.S. Inkjet Based 3D Additive Manufacturing of Metals; Materials Research Forum LLC: Millersville, PA, USA, 2018. [Google Scholar]
- Lu, K.; Hiser, M.; Wu, W. Effect of particle size on three dimensional printed mesh structures. Powder Technol. 2009, 192, 178–183. [Google Scholar] [CrossRef]
- Zhou, Z.; Buchanan, F.; Mitchell, C.; Dunne, N. Printability of calcium phosphate: Calcium sulfate powders for the application of tissue engineered bone scaffolds using the 3D printing technique. Mater. Sci. Eng. C 2014, 38, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Miyanaji, H.; Zhang, S.; Lassell, A.; Zandinejad, A.; Yang, L. Process Development of Porcelain Ceramic Material with Binder Jetting Process for Dental Applications. JOM 2016, 68, 831–841. [Google Scholar] [CrossRef]
- Dong, J.; Li, Y.; Lin, P.; Leeflang, M.A.; van Asperen, S.; Yu, K.; Tümer, N.; Norder, B.; Zadpoor, A.A.; Zhou, J. Solvent-cast 3D printing of magnesium scaffolds. Acta Biomater. 2020, 114, 497–514. [Google Scholar] [CrossRef]
- Salehi, M.; Maleksaeedi, S.; Farnoush, H.; Nai, M.L.S.; Meenashisundaram, G.K.; Gupta, M. An investigation into interaction between magnesium powder and Ar gas: Implications for selective laser melting of magnesium. Powder Technol. 2018, 333, 252–261. [Google Scholar] [CrossRef]
- German, R.M. Sintering: From Empirical Observations to Scientific Principles; Elsevier Science & Technology Books: Amsterdam, The Netherlands, 2014. [Google Scholar]
- Fujii, H.; Izutani, S.; Matsumoto, T.; Kiguchi, S.; Nogi, K. Evaluation of unusual change in contact angle between MgO and molten magnesium. Mater. Sci. Eng. A 2006, 417, 99–103. [Google Scholar] [CrossRef]
- Liu, Y.; Tandon, R.; German, R.M. Modeling of supersolidus liquid phase sintering: I. Capillary force. Metall. Mater. Trans. A 1995, 26, 2415–2422. [Google Scholar] [CrossRef]
- Liu, J.; Cardamone, A.L.; German, R.M. Estimation of capillary pressure in liquid phase sintering. Powder Metall. 2001, 44, 317–324. [Google Scholar] [CrossRef]



| Saturation Level (%) | Length (mm) | Width (mm) | Height (mm) |
|---|---|---|---|
| 70 | 14.73 ± 0.21 | 14.88 ± 0.22 | 14.40 ± 0.29 |
| 90 | 15.09 ± 0.16 | 15.12 ± 0.11 | 15.14 ± 0.04 |
| 110 | 15.28 ± 0.08 | 15.43 ± 0.20 | 15.09 ± 0.13 |
| 130 | 15.46 ± 0.14 | 15.47 ± 0.26 | 15.18 ± 0.10 |
| Specimen Condition | Density (g/cm3) | |
|---|---|---|
| Pycnometer density of AZ91 powder | 1.8872 ± 0.0009 | 100 |
| Apparent density of AZ91 powder | 0.9413 ± 0.0833 | 49.9 |
| Tap density of AZ91 powder | 1.195 ± 0.016 | 63.3 |
| Pycnometer density of as-printed green object | 2.0277 ± 0.0009 | 100 |
| As-printed green object | 1.0108 ± 0.0241 | 49.8 |
| As-printed green object after sintering at 530 °C for 5 h | 0.969 ± 0.007 | 51.3 |
| As-printed green object after sintering at 545 °C for 5 h | 1.000 ± 0.007 | 53.0 |
| As-printed green object after sintering at 560 °C for 5 h | 1.005 ± 0.005 | 53.3 |
| As-printed green object after sintering at 575 °C for 5 h | 1.036 ± 0.023 | 54.9 |
| As-printed green object after sintering at 530 °C for 50 h | 1.030 ± 0.005 | 54.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salehi, M.; Kuah, K.X.; Ho, J.H.; Zhang, S.X.; Seet, H.L.; Nai, M.L.S. Towards Binder Jetting and Sintering of AZ91 Magnesium Powder. Crystals 2023, 13, 286. https://doi.org/10.3390/cryst13020286
Salehi M, Kuah KX, Ho JH, Zhang SX, Seet HL, Nai MLS. Towards Binder Jetting and Sintering of AZ91 Magnesium Powder. Crystals. 2023; 13(2):286. https://doi.org/10.3390/cryst13020286
Chicago/Turabian StyleSalehi, Mojtaba, Kai Xiang Kuah, Jia Hern Ho, Su Xia Zhang, Hang Li Seet, and Mui Ling Sharon Nai. 2023. "Towards Binder Jetting and Sintering of AZ91 Magnesium Powder" Crystals 13, no. 2: 286. https://doi.org/10.3390/cryst13020286





