Abstract
Magnesium (Mg) and its alloys are considered an ideal material for aerospace, medical, energy, and automotive purposes, because of their low density and high specific strength. Researchers are interested in AZ alloys because of their superior flow characteristics. This review makes an effort to summarise the numerous processing methods that have been adapted for use with AZXX alloy. One of the main obstacles to Mg alloys being used in their intended context is the difficulty of processing Mg and its alloys. Curiously, the homogenization process is often used in tandem with extrusion and rolling. It also gives an insight into the microstructure, mechanical (hardness, tensile, impact, fatigue, and creep), and electrochemical corrosion properties of AZXX alloys. The improvement of AZXX alloy can be attributed to the grain boundary strengthening and the second phase strengthening mechanisms. The effects of Al content and phases on properties are extensively discussed. This article summarises what has recently happened with AZXX wrought Mg alloy and offers some predictions for its future.
1. Introduction
Demand for lighter products has been reported in numerous sectors, including aviation, healthcare, energy, transportation, construction, chemicals, and the 3C (Computer, Communication, and Consumer Electronics) sector. There is a lot of interest in magnesium (Mg) alloys because of their high strength-to-weight ratio and low weight [1,2]. At 1.74–2.0 g/cm3, magnesium is 33% lighter than aluminium and 77% lighter than steel [3]. Despite its low weight, magnesium is not widely used in aerospace and automotive applications for a number of reasons. These include magnesium’s poor cold workability and strength, toughness, and creep resistance at elevated temperature; its high chemical activity at room and elevated temperature; and its relatively low ductility [4]. Magnesium is essential for the body’s proper functioning, serving essential functions like muscle support and energy production. However, due to a low Pilling-Bedworth ratio of 0.81 and the extremely negative standard potential of −2.37 V, Mg-based alloys have a high corrosion rate and degrade completely in the bio-fluid. To combat these drawbacks while still maintaining their mechanical strength and biocompatibility, Mg-based bio-composites and alloys were created [5]. Magnesium alloys have a high corrosion rate, leading to premature loss of mechanical integrity, which limits their clinical use in load-bearing sections [6]. Natural bone has a density between 1.8 and 2.1 g/cm3, which is also similar to Mg’s density. Pure magnesium has an elastic modulus of 45 GPa, which is nearly equal to the modulus of human bone (40 to 57 GPa). Magnesium, with its similar elastic modulus to water, could be used to prevent stress shielding and bone resorption in hard tissue engineering applications. Therefore, it has the potential to be a material for the development of biodegradable orthopaedic implants [7]. Hydrogen is safely stored in metal hydrides due to its exothermic oxidation in the atmosphere, and scientists have been working for years to adapt Mg for this purpose.
Studies show that at 400 °C, pure Mg can store up to 7.8% hydrogen [8,9,10]. Magnesium can also be used as an anode in a metal-air battery, with oxygen from the air serving as the cathode. However, Mg’s self-corrosive properties limit the metal’s applications. Mg (anode) performance is limited because Mg(OH)2 readily forms on the surface when dissolved in an electrolyte with a pH between 0 and 10.5. Because of this, Mg is always used in conjunction with an alloying element to enhance its properties [11]. Aluminium is by far the most important metal, and it was one of the first alloying components for magnesium. Almost all structural uses involve the Mg-Al alloy system (mainly AZ91 and AM60). In practice, Al-based Mg alloys are often alloyed with trace amounts of zinc to increase their strength and corrosion resistance. Conventional primary processing techniques (casting and forming) rely heavily on the AZXX alloying system [12], which features Al concentrations from 3 to 9% wt and Zn content from less than 1% wt. Corrosion rates in aqueous chloride solutions were decreased in Mg alloys from the AZ-series and AM-series when the alloys were treated with rare earth elements (REE). According to the findings, the effectiveness against atmospheric corrosion can also be improved through alloying with Zinc (Zn) and Calcium (Ca) dissolved in a Mg-matrix. Zn is dispersed throughout the Mg matrix, forming a compact surface layer that improves the alloy’s resistance to degradation. Addition of Ca to an alloy improves Mg’s resistance to corrosion. Because of this, it is clear that the properties and behaviour of Magnesium are modified due to the presence of the alloying elements [13]. There is room to improve the application-specific properties of Mg and its alloys, despite their identification as potential materials for a wide range of engineering and biomedical applications [14]. The primary barrier to Mg’s use in its final applications is the difficulty of processing Mg and its alloys [1]. According to the literature [14,15], Mg alloys in the AZ series are known to have desirable properties and are used for a variety of purposes. The purpose of this article is to investigate the function/importance of AZ series Mg alloy through a systematic review of the literature that establishes the relationship between mechanical characteristics and microstructure. It also summarizes the relationship between the microstructure and phases of the alloy with corrosion behaviour. Additionally, it emphasizes the importance of the Al alloying with Mg. This article summarizes what has recently happened with AZXX wrought Mg alloy and offers some predictions for its future.
2. Processing of AZXX Mg Alloy
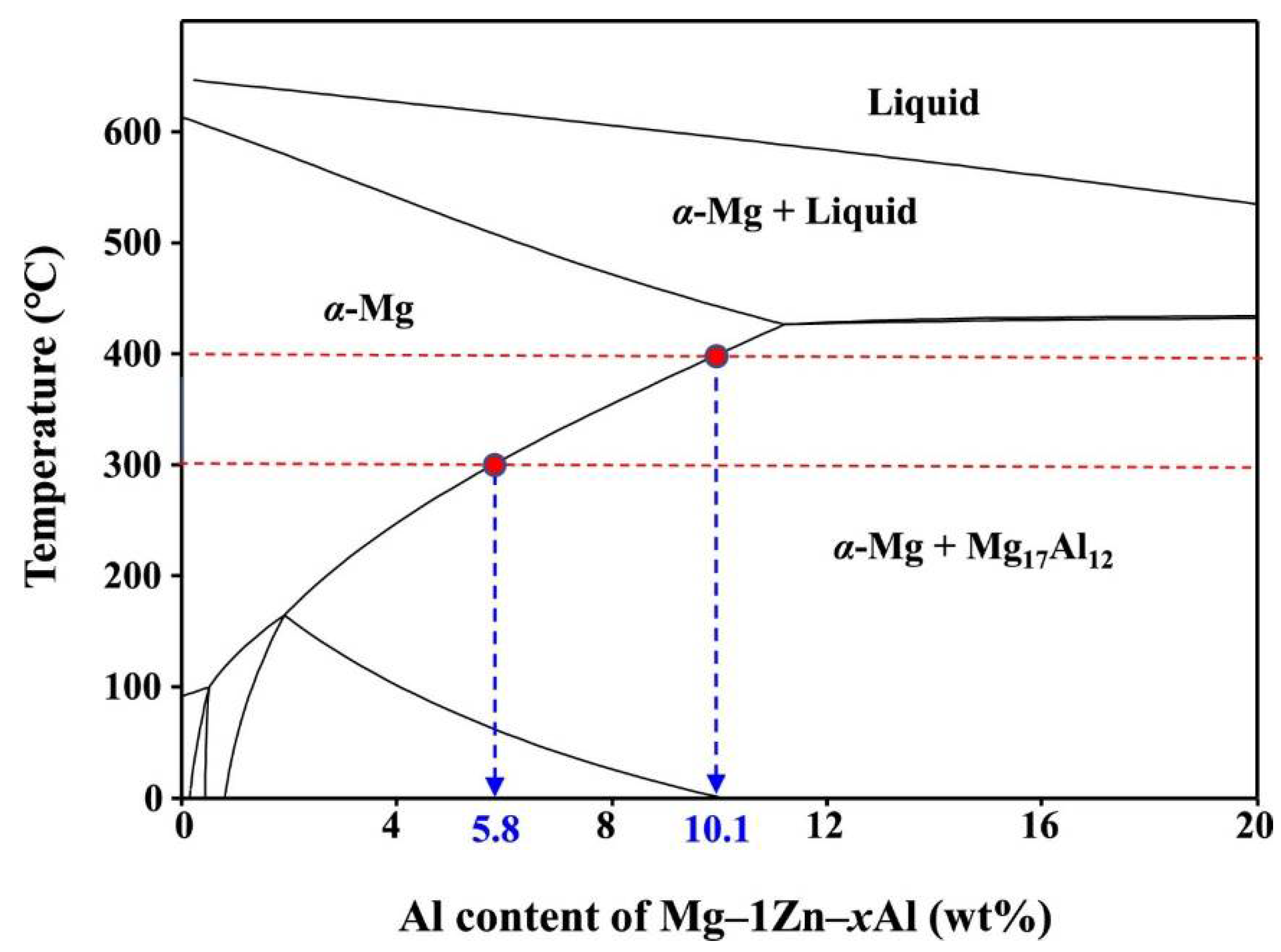
Casting and extruding are typically the first steps in the production process when making Mg alloy. There have been successful squeeze castings of AZ91, as well as rheo and thixocastings of AZ91, AZ71, and AZ80. Table 1 [16] displays the results of solutionizing at 400 °C for 24 h to improve the characteristics of the examined alloy. AZ61 alloys were extruded at a speed of 4 m/min and a temperature of 300 °C [17]. Forward extrusion involves separating the top and bottom halves and homogenising them at 380 °C for 10 h [18]. Thermomechanical processing, in the form of two or three passes of hot rolling, was used to achieve a fine-grained microstructure that is resistant to superplastic deformation [18]. Direct hydraulic extrusion at 300 and 400 °C with an extrusion ratio of 25 has been used to create AZ91 after solid solutionizing (homogenization) at 420 °C [19]. The melting point of the alloying element is largely determined by the temperature at which the material is extruded or deformed. The phase diagram of Mg–1Zn–xAl (x = 1–20 wt%) (shown in Figure 1) demonstrates the temperature-dependent decrease in solubility.
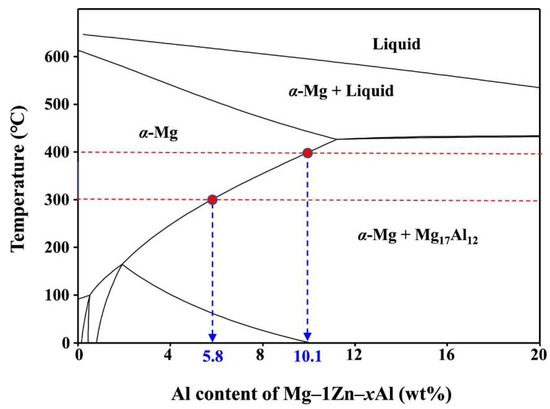
Figure 1.
Phase diagram of Mg–1Zn–xAl (PANDAT Software) [19].
Rolling mills are designed so that the friction between the rolls and the workpiece drives the material forward as it is rolled [17]. Compressive stress results in a thinner, finer grain structure in the work material, which is the direct result of the stress. Based on the recrystallization temperature (TR), bulk forming can be divided into two distinct categories. Hot working (above TR) and cold working (below TR) describe the two processes, respectively [20]. The initial press speed of 10 mm/s was used to facilitate the multidirectional forging, which was controlled along two orthogonal directions to ensure workability. Quickly dipping the samples into water helps freeze the microstructure. This proved the deformation-caused lengthening along the z axis. The efficiency of the forging process can be determined from the freezing microstructure of the Mg alloy.
Graphite powder was used as a high-temperature lubricant, which tends to prevent sticking of working material at high temperatures, to achieve reasonable homogenous deformation. Before forging, a resistive furnace was used to heat the homogenised samples and two anvils to 360 °C for half an hour. During forging, the samples were not annealed. After the final pass, these forgings were cooled in water. The AZ80 was made stronger through age hardening at 175 °C and subsequent forging [21]. High pressure die casting (HPDC) has recently been applied to the production of Mg alloy systems of Mg, Al, Zn, and Mn in an effort to achieve superior mechanical properties without compromising the cost of processing. Because the mechanical properties of various processed materials are listed in Table 1, it is easy to see how the physical phenomenon or processing parameters of the alloy affects the microstructure and the phases in the alloy [22]. The depositing process is called wire additive manufacturing, and it employs extruded AZ80M alloy with a diameter of 1.6 mm. There is micro-cracking in the inter-transition layer and inhomogeneity in the grain structure in AZ80M that was produced using additive manufacturing. The horizontal direction also has greater tensile strength than the vertical [23]. Different properties along the horizontal and vertical directions, as well as their significance depending on the product’s intended use, are present in additively made parts. Rolling and extrusion, two common bulk deformation processes, are frequently used in the processing of AZXX Mg alloys [24]. The refined grains were observed on the outer surface, but they became coarser towards the centre. On other hand, the casting process leads to uniform grain size throughout the specimen with the coarser grains. Refined grains, homogeneity, and hardness of AZXX Mg alloy cannot be achieved by a single processing technique. However, it is possible through the combination of processing techniques, along with a heat treatment process (solid solutionizing, and age hardening) [17].


Table 1.
Processing of AZXX Mg alloy and its corresponding mechanical properties.
Table 1.
Processing of AZXX Mg alloy and its corresponding mechanical properties.
| S. No. | Material | Process | Solutionizing—Temp. (C); Time (Hours); Quenching Medium | T6 Temp. (C); Time (Hours) | Hardness (HV) | YS (MPa) | UTS (MPa) | Ductility (%) | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| 1 | AZ80 | Casting | - | - | - | 100 | 190 | 6 | [25] |
| 420; 4; water | - | 110 | 270 | 17 | |||||
| 420; 8; water | - | 100 | 260 | 18 | |||||
| 420; 10; water | - | 90 | 265 | 16 | |||||
| 420; 16; water | - | 85 | 270 | 15 | |||||
| 420; 4; water | 200; 10 | - | 150 | 280 | 7 | ||||
| 420; 8; water | 200; 10 | - | 140 | 260 | 12 | ||||
| 420; 10; water | 200; 10 | - | 150 | 290 | 9 | ||||
| 420; 16; water | 200; 10 | - | 130 | 280 | 7 | ||||
| 2 | AZ80 | Extrusion | - | - | 69.1 | 201 | 312 | 10.1 | [26] |
| 400; 10; water | 66.6 | 185 | 305 | 10.3 | |||||
| 400; 10; water | 200 | 92 | 227 | 334 | 4 | ||||
| 4 | AZ80 | Casting | - | - | 61.4 | - | - | - | [27] |
| 360; 1; water | 170; 2 | 62.5 | - | - | - | ||||
| 360; 1; water | 170; 4 | 61.7 | - | - | - | ||||
| 360; 1; water | 170; 6 | 60.8 | - | - | - | ||||
| 360; 1; water | 170; 8 | 53 | - | - | - | ||||
| 5 | AZ80 | Casting | - | - | 61.2 | - | - | - | [28] |
| 420; 10; water | 64 | - | - | - | |||||
| 420; 10; water | 200; 10 | 83.5 | - | - | - | ||||
| 380; 10; air cooling + 420 C; 10; water | - | 63.7 | - | - | - | ||||
| 380; 10; air cooling + 420 C; 10; water | 200; 10 | 86 | - | - | - | ||||
| 6 | AZ80 | casting | - | - | 68 | - | - | - | [29] |
| 250; 6 | - | 69 | - | - | - | ||||
| 250; 12 | - | 73 | - | - | - | ||||
| 350; 6 | - | 67.5 | - | - | - | ||||
| 350; 12 | - | 70 | - | - | - | ||||
| 450; 6 | - | 66 | - | - | - | ||||
| 450; 12 | - | 66 | - | - | - | ||||
| 7 | AZ80 | wire arc additive manufacturing | - | - | - | 146 | 308.7 | [23] | |
| 8 | AZ80 | Multitemperature Multidirectional Forging + Extrusion | 400; 24 | - | - | 292 | 420 | - | [30] |
| 9 | AZ80 | Casting | - | 73.1–84.4 | 138–153.7 | - | [31] | ||
| 10 | AZ80 | Horizontal Flat Die Extrusion | 64–72 | 287 | 162 | - | [32] | ||
| AZ80 | Thixocasting | - | - | - | 102 | 187 | 3.5 | [16] | |
| 400; 24; water | - | - | 92 | 224 | 7.7 | ||||
| AZ91 | Squeeze casting | - | - | - | 104 | 183 | 4.5 | ||
| Rheocasting | - | - | - | 105 | 171 | 3.4 | |||
| 400; 24; water | - | - | 94 | 241 | 8.3 | ||||
| AZ71 | - | - | - | 98 | 185 | 4.7 | |||
| 400; 24; water | - | - | 88 | 262 | 11.2 | ||||
| 11 | AZ21 | Gravity Die Casting | 53.9 ± 2.1 | 89 | 113 | 3.4 | [33] | ||
| 12 | AZ31 | Hot rolled | 147 | - | 260 | 22.5 | [34] | ||
| AZ61 | Hot rolled | - | - | 260 | 22.5 | [34] | |||
| 13 | AZ31 | Extrusion | - | 191.8 | 268.1 | 23.9 | [35] | ||
| 14 | AZ31 | Extrusion | 450; 3; water | - | 162 | - | - | [36] | |
| AZ61 | 420; 6; water | - | 55.4 | 198 | - | - | |||
| AZ91 | 400; 10; water | - | 62.7 | 227 | - | - | |||
| AZ91 | 400; 10; water | 210; 15 | 81.6 | 271 | - | - | |||
| 15 | AZ91D | HPDC | 188 | 255 | 3.0 | [37] | |||
| 16 | AZ91 | HPDC | 150 | 240 | 3.0 | [38] |
3. Microstructural Analysis
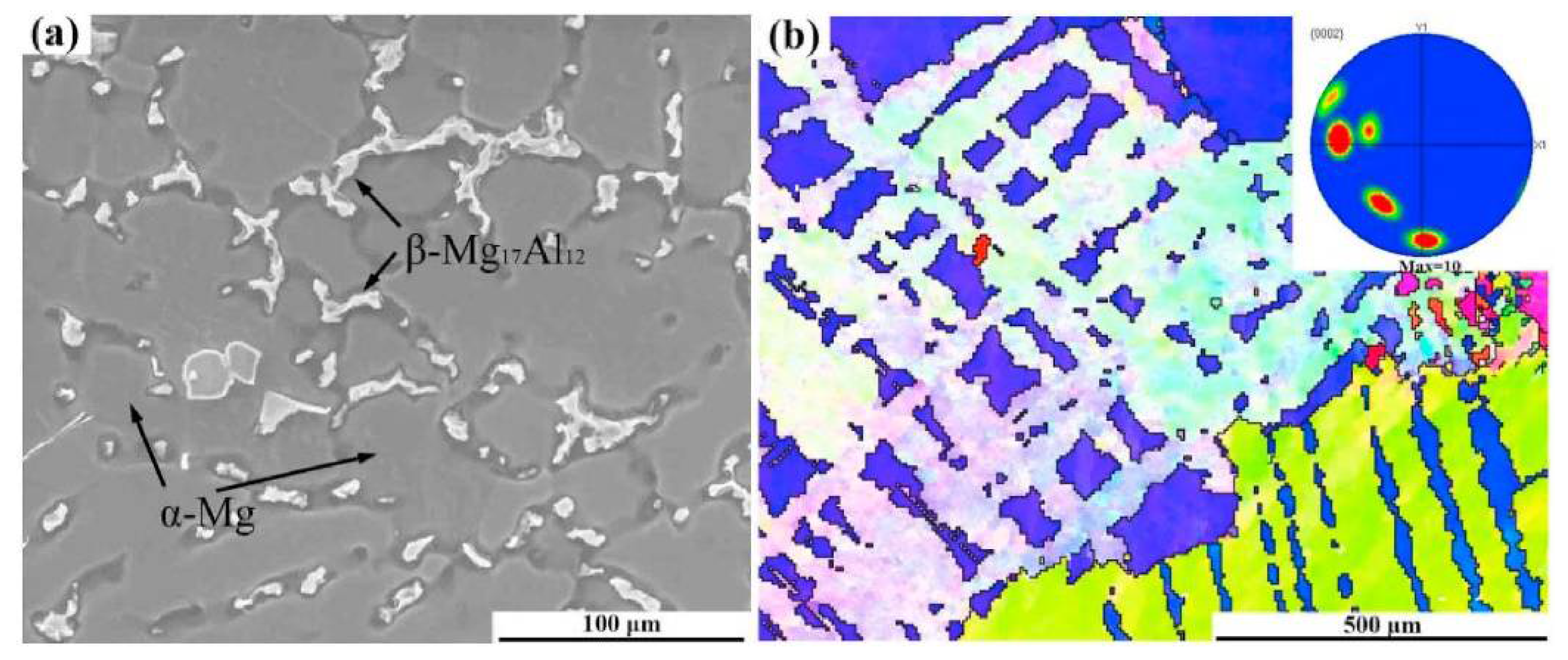
Casting, extruding, rolling, homogenising, solutionizing, age hardening, and other processing methods all have a significant impact on the microstructure of an alloy. In Figure 2a, a secondary electron micrograph of the as-cast AZ61 alloy is displayed. This micrograph reveals the presence of second phases of α-Mg, which are located in the grain boundaries. The inverse pole figure of the corresponding micrograph is shown in Figure 2b [18,39].
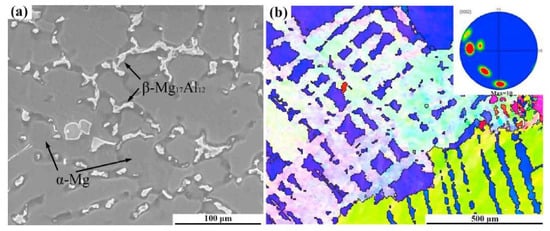
Figure 2.
As-cast AZ61 Magnesium alloy (a) SE micrograph (b) inverse pole figure [39].
It is the α-Mg phase and the secondary β-Mg17Al12 phase that AZ31 is designed to house. The twin-roll cast (TRC) has lower accumulation of the second phase on grain boundaries than conventionally cast (CC) AZ31 [40]. When comparing CC AZ31 and TRC AZ31, you can see that the grain size of the former is 300 µm while the latter is 200 µm. There is also more deformation near the surface during the TRC than in the middle of the strip [41]. The surface grain size is reduced to 50 µm as a result. After 10 h of ageing at 450 °C, the grains of cast AZ31 were seen to have become slightly coarser. The secondary β-Al12Mg17 phase in the cast AZ31 partially dissolves with age and almost no β-phase particles were observed within the grains. Morphology reveals that alloy microstructures are significantly impacted by partial remelting temperatures [42]. After rolling, grain sizes were reduced for all materials, and it was noticed that this was also correlated with a decrease in rolling temperature. In particular, they found that the material’s yield strength had increased during the treatment [43]. Dendritic structure is drastically reduced after homogenization [44].
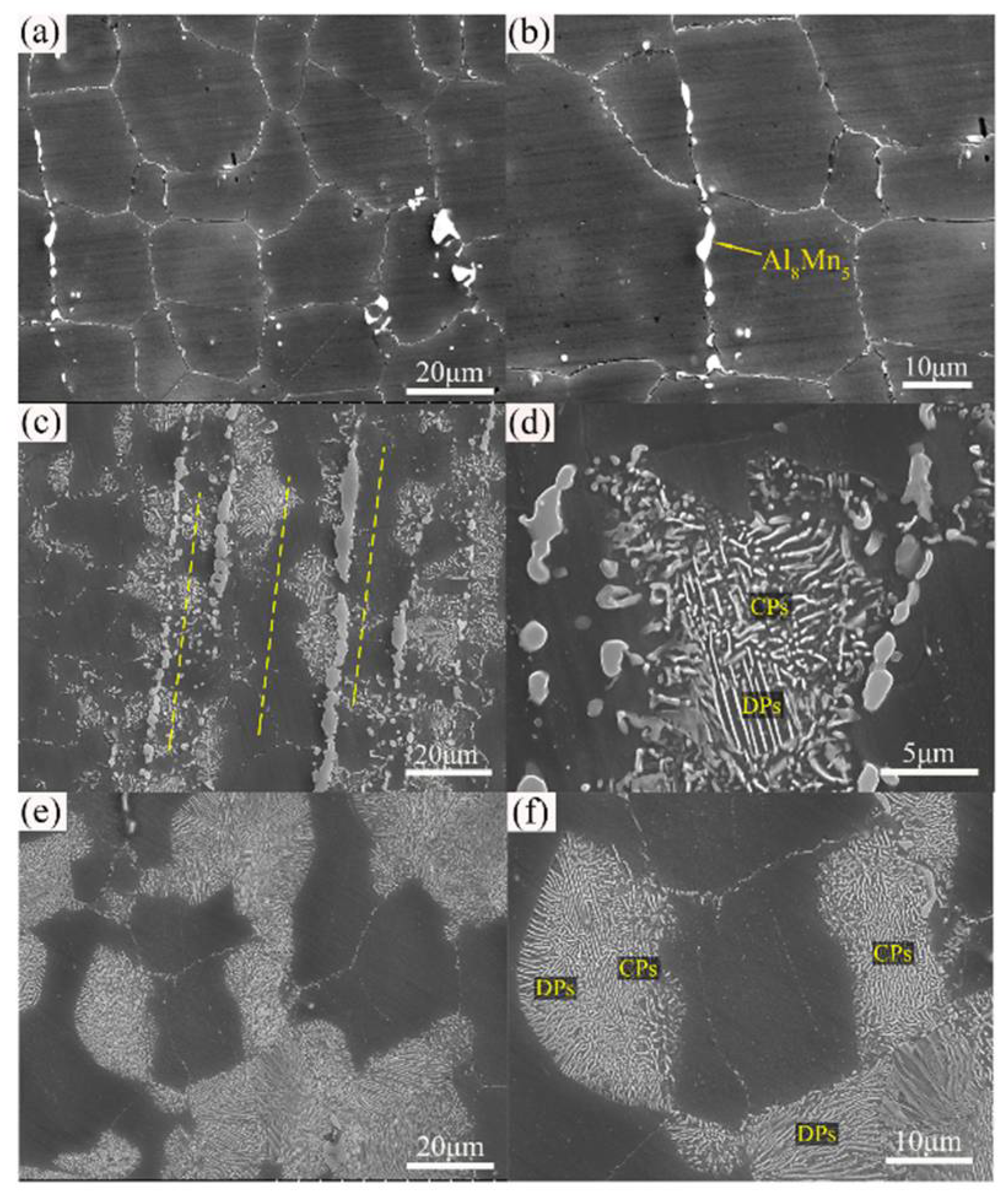
Figure 3 shows the effect of heat treatment (T4, T5, and T6) on microstructure of the extruded AZ80 [45]. After 12 h of ageing, precipitates formed along grain boundaries and grew into grains in AZ80, where the typical grain size of the as-cast alloy is 80–100 m [46]. At 36 h, DP’s characteristic behaviour was nearly over. As compared to its age-hardened for 12 h counterpart, the T6–12 h sample demonstrates superior sedimentation behaviour in the fine grain region. After this, β-Mg17Al12 phase precipitation is more evident in both large and small grains. As the ageing time was increased to 36 h, the area proportion of precipitated regions steadily increased in both aged alloys. As a result of this, aged T6 samples have relatively low hardness values [47]. The AZXX alloys are composed primarily of a matrix of α-Mg with β-Mg17Al12 along the grain boundaries. The AZXX non-equilibrium solidification process produces the intermetallic β-Mg17Al12 phase. The second β-Mg17Al12, which is separated from the α-Mg solid solution up on cooling, forms a lamellar microstructure around the eutectic phase. Mg17Al12 particles tend to cluster near grain boundaries, but most of those particles were dispersed [48]. The inverse pole figure of Mg, AZ31, and AZ91 at various deformation stages is depicted in Figure 4.
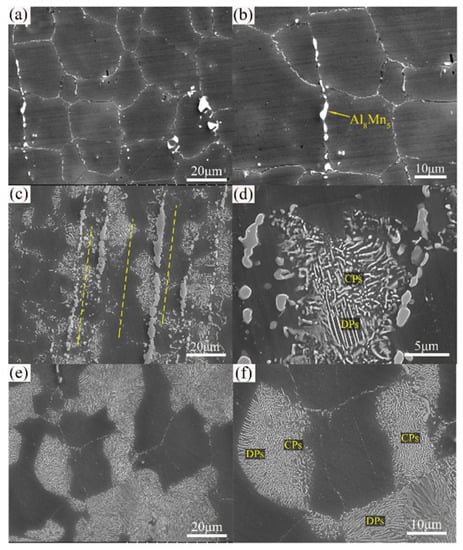
Figure 3.
Microstructure of extruded AZ80 Mg alloy after heat treatment (a,b) T4, (c,d) T5, and (e,f) T6. CP, continuous precipitate; DP, discontinuous precipitate [45].
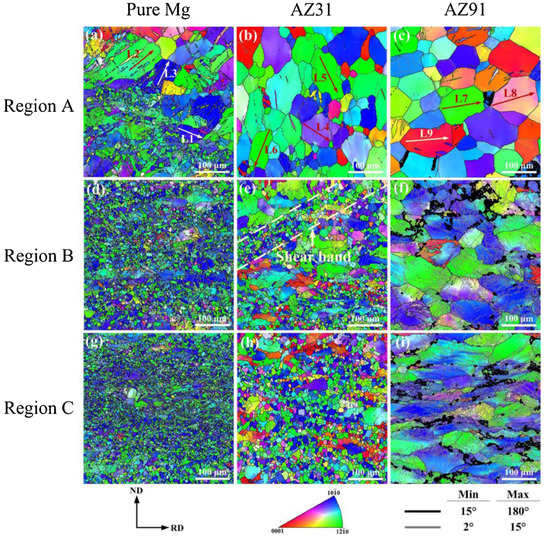
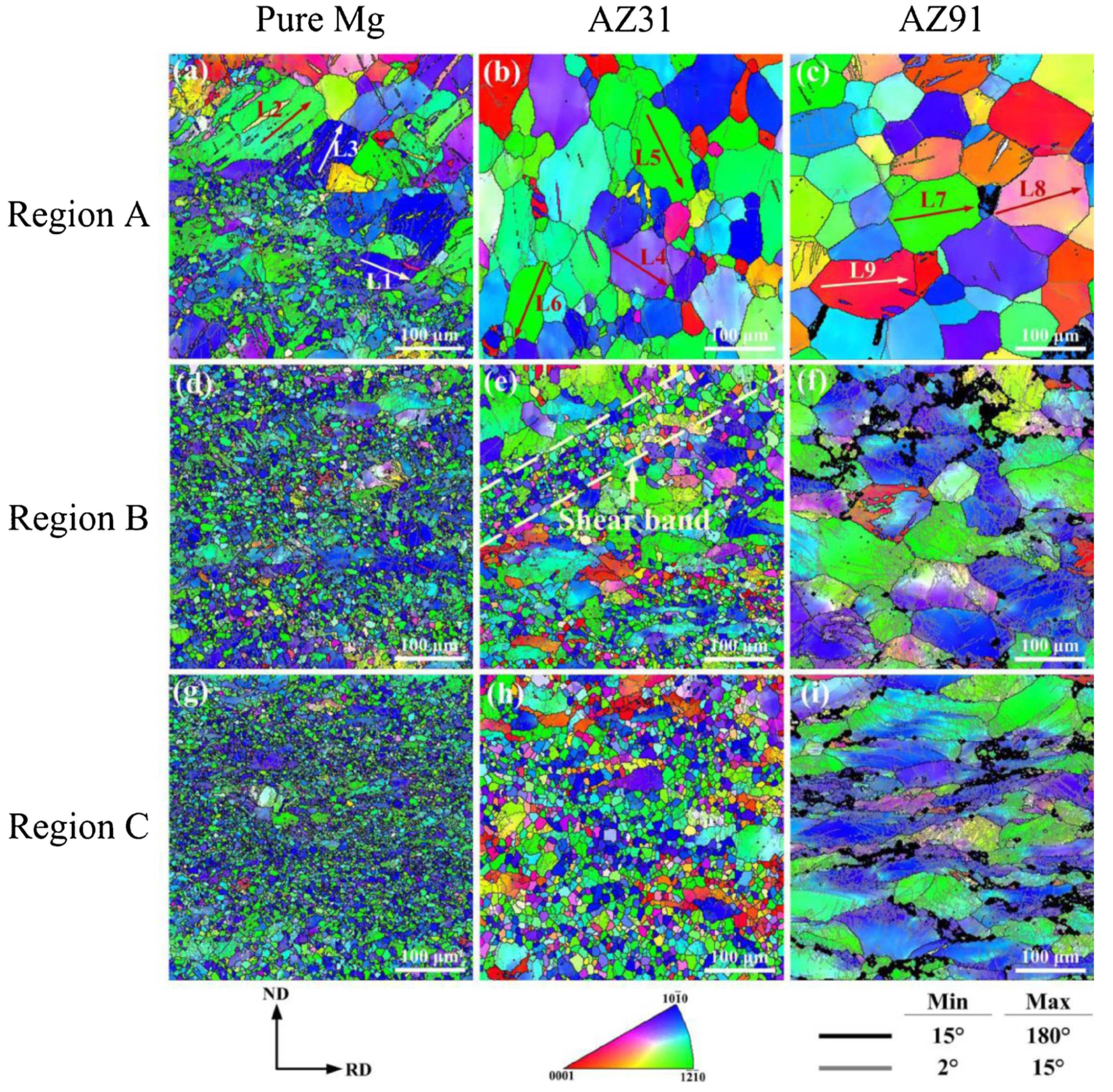
Figure 4.
Electron Back Scattered Diffraction’s Inverse pole figure maps of rolled Mg, AZ31 and AZ91 alloys at different deformation stages: (a–c) Region A—initial (d–f) Region B—medium, (g–i) Region C—final [49].
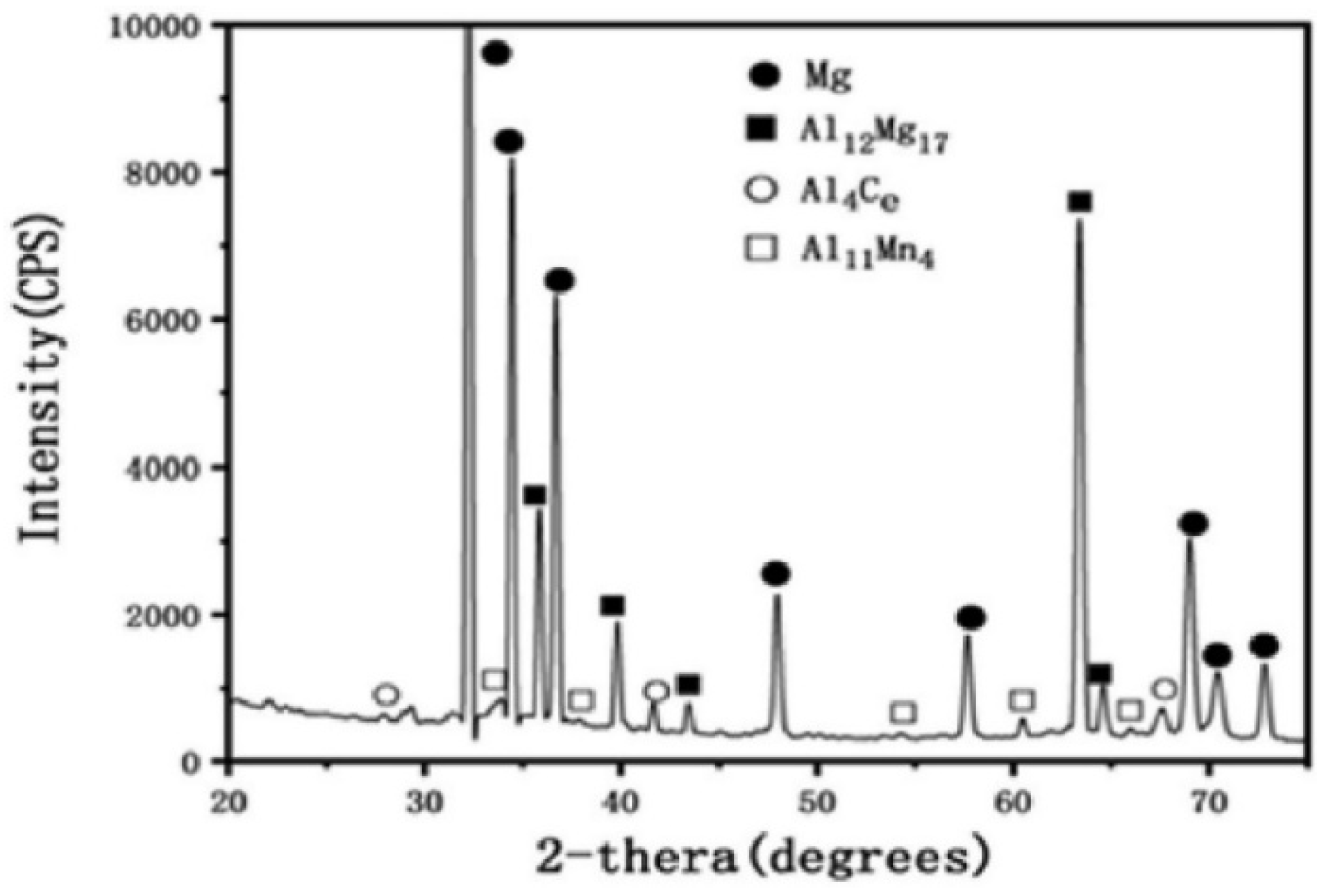
Studies have shown that as extrusion temperature is lowered, fewer dynamic recrystallized (DRX) grains are produced [50,51]. Strain-induced precipitates are affected by the high effective strain at the interface between the extruder and along the sample, which decreases gradually toward the centre [52,53]. As strain increases, the lamellar Mg17Al12 phases surrounding grain boundaries become thinner and narrower, and the percentage of dynamically recrystallized grains in the sample’s volume rises. DRX typically initiates at gradients near grain boundaries because these regions are rich in potential nucleation sites [54]. As strain increases, the defect density and significant distortion of the lattice increase, leading to the formation of tiny crystal particles at the nucleation sites. Hot deformation causes a reduction in the amount of lamellar β-Mg17Al12 phases and an increase in the number of fine grains [48] by breaking the coarse and dendritic microstructures of the Mg and β-Mg17Al12 phases, elongating the grains, and dissolving the majority of the β-Mg17Al12 phases into the Mg matrix. The coarse grain boundaries were clearly seen, and the average grain size was measured to be 40 m. Figure 5 shows an XRD pattern for the extruded alloy, which reveals the presence of three intermetallic phases with distinct shapes: Mg17Al12 (lamellar), Al4Ce (block), and Al11Mn4. The microstructures of the extruded magnesium alloy showed non-uniform grain structure with fine and coarse grains after being in solution at 370 °C for 12 h [55]. The benefits of using finer grains are many times greater than the costs associated with using coarser grains [56]. Alloying elements, such as rare earth elements (REE), are frequently used in AZXX alloy to increase its strength. Since Al has a higher electronegativity than Mg, the REE readily forms intermetallic phases with Al (for example, Al2Nd) instead of Mg [57,58,59] In Mg alloys like AZ31, AZ61, AZ80, and AZ91, the presence of the lamellar-shaped Mg17Al12 phase has been observed [59].
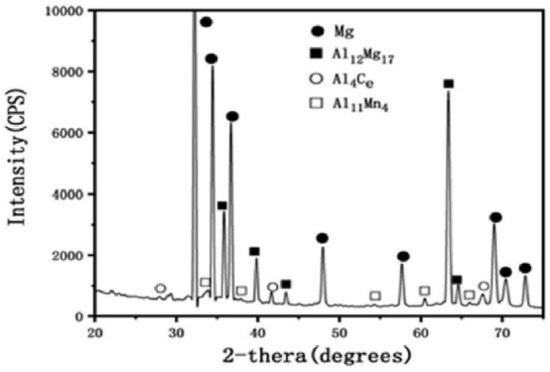
Figure 5.
XRD results of rare elements based AZXX alloy [55].
4. Mechanical Properties
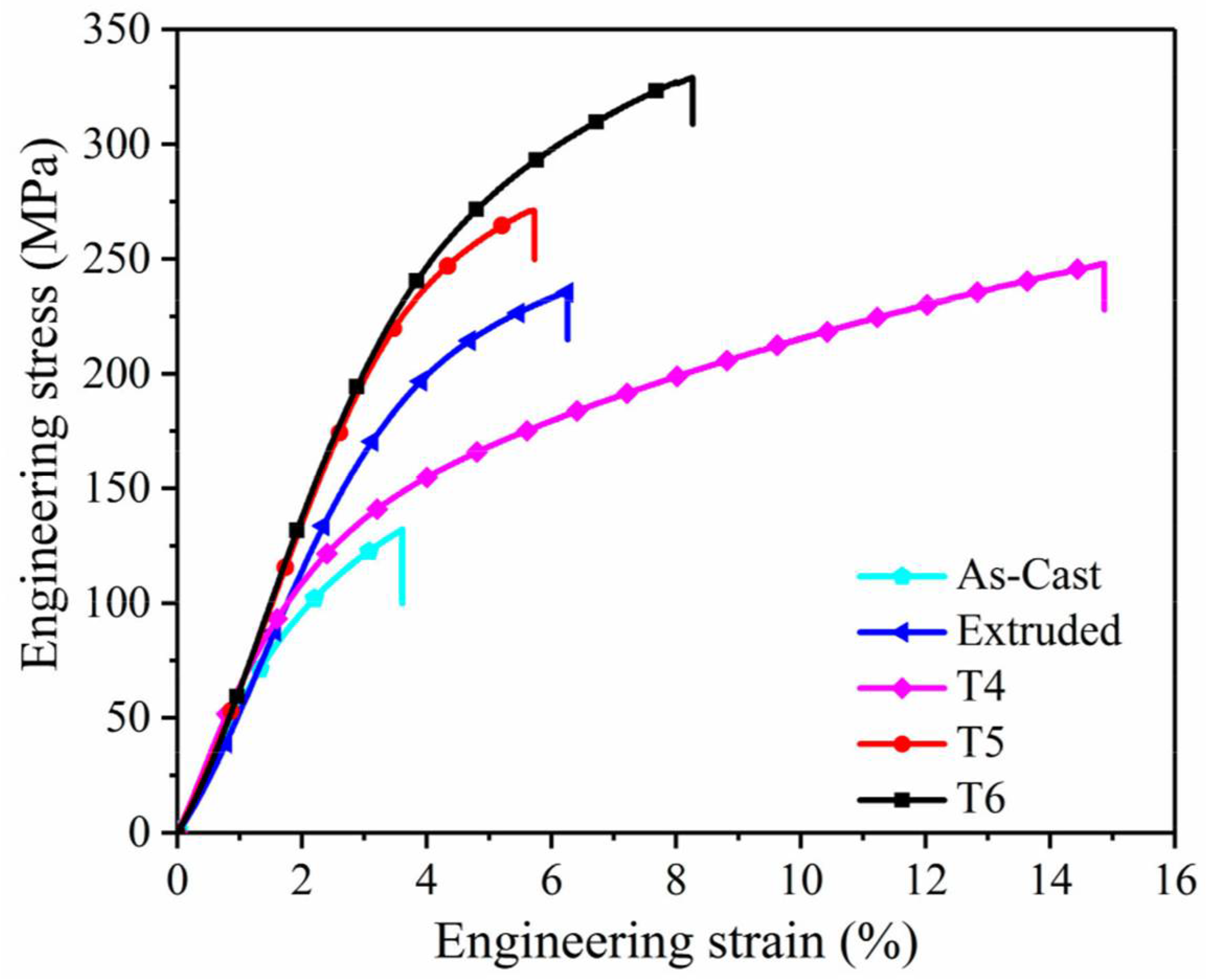
The Vickers microhardness test revealed that the hardness of Mg AZ80 alloy was constant at its core, but ranged from 64 HV to 66 HV over its surface. Microhardness in the surface layer is highly heterogeneous in the width direction. It is hardest at the plate’s outermost edge and gradually softens inward [32]. The findings also corroborate the hypothesis that hardness improves with smaller grain size [32,60,61]. The tensile properties of AZ31 were found to be significantly impacted by the material’s microstructure. The homogenization process did not significantly alter or improve the mechanical properties. Specifically, if the temperature at which the material ages is raised, the yield strength will fall, and vice versa [62]. In general, the yield strength and tensile strength of AZXX increase with the weight percentage of Al content, while the castability and weldability rapidly decrease [63]. The ductility improves dramatically across the board for twin rolling deformation temperatures. The dislocation substructure is removed, and homogeneous fine-grained structures form as a result, leading to more consistent deformation. Due to this, the homogenised material’s yield strength decreases over time [44]. The mechanical properties of AZXX are modified by the presence of brittle secondary phases [64]. Figure 6 shows the effect of processing technique on tensile properties of the AZ80 alloy.
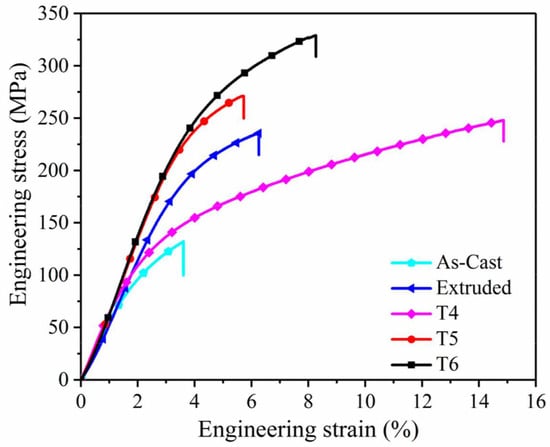
Figure 6.
Stress vs strain graph of AZ80 alloy with different processing techniques [45].
As the AZ61 alloy is heat treated, the intermetallic particles dissolve, which leads to the formation of Al in higher concentrations on the Mg matrix, that in return induces solid solution strengthening [65]. In AZ91 alloy, it is observed that the alloys with increasing content of Sc have enhanced tensile properties as a result where Al is not segregated along the grain boundaries in the Mg matrix. It also pins down motion of dislocations causing dispersion hardening [66]. In most cases, the cracks are initiated at the intermetallic phases. These intermetallic phases are the main cause of failure, where the distribution and shape of the intermetallic phases play a significant role in altering the strength of the alloy [45].
The LCF (Low-Cycle Fatigue) lifetime of the AZ91 alloy at a given strain amplitude grows longer as the extrusion temperature rises. Since LCF failure results from the loss of fracture ductility due to cyclic deformation, the elongation of a metallic material is directly related to its LCF resistance. According to the research, the tensile elongation of AZ91 extruded at 400 °C is significantly lower than that of AZ91 extruded at 300 °C. The former has longer LCFs than the latter do.
Regardless of the material, a flat fracture surface with small fatigue striations is produced when fatigue cracks initiate on the surface of a specimen and propagate into its interior. Fatigue fracture occurs when the load-bearing area of a material is gradually reduced [19]. There are secondary cracks and features that look like fatigue striations in the area where the fatigue crack is propagating [67]. It has been estimated using a Charpy impact testing machine how tough hot-extruded AZ31B alloy is. The results show that the impact toughness can be increased by nearly half (10.7 J/cm2) when processed with four passes at an extrusion temperature of 260 °C. However, the products are anisotropic because of the directionality of the processing method [68]. There is an intermetallic phase called Mg17Al12 in AZXX’s microstructure. Due to its low thermal stability, this phase has a lower creep resistance at higher temperatures [69]. With a decrease in creep strain and a higher creep rate throughout the 120 °C and 70–90 MPa creep tests, the material’s resistance to creep decreases with increasing stress [70]. By vying with lattice diffusion, dislocation creep is found to be the dominant creep mechanism. Microcracks are seen to form and begin propagating during the acceleration creep stage [71]. Processing methods have a significant impact on the creep behaviour of magnesium alloys made via extrusion or die-casting. The strain-hardening coefficient increases as the fraction of Al and its intermetallic phases in the total metal weight rises [72]. In comparison to AZ82, AZ84, AZ124, AZ86, and AZ126, AZ122 has weaker creep properties because of its higher Zn content, and alloys with a high Al content are susceptible to softening at high temperatures due to the presence of intermetallic phase [73]. This is the stage where cracks start to form in Mg17Al12 because of the material’s brittleness. The ductility or percentage elongation of an AZXX alloy is determined by the distribution of the phase and the volume fraction of the phase [16,74]. Overall, the grain size and the presence of intermetallic phases alter the mechanical properties. It is observed that hardness increases with grain refinement; tensile strength, strain-hardening coefficient, and the yield strength improve with more saturation of Al in Mg matrix whereas creep resistance decreases.
5. Corrosion Behaviour
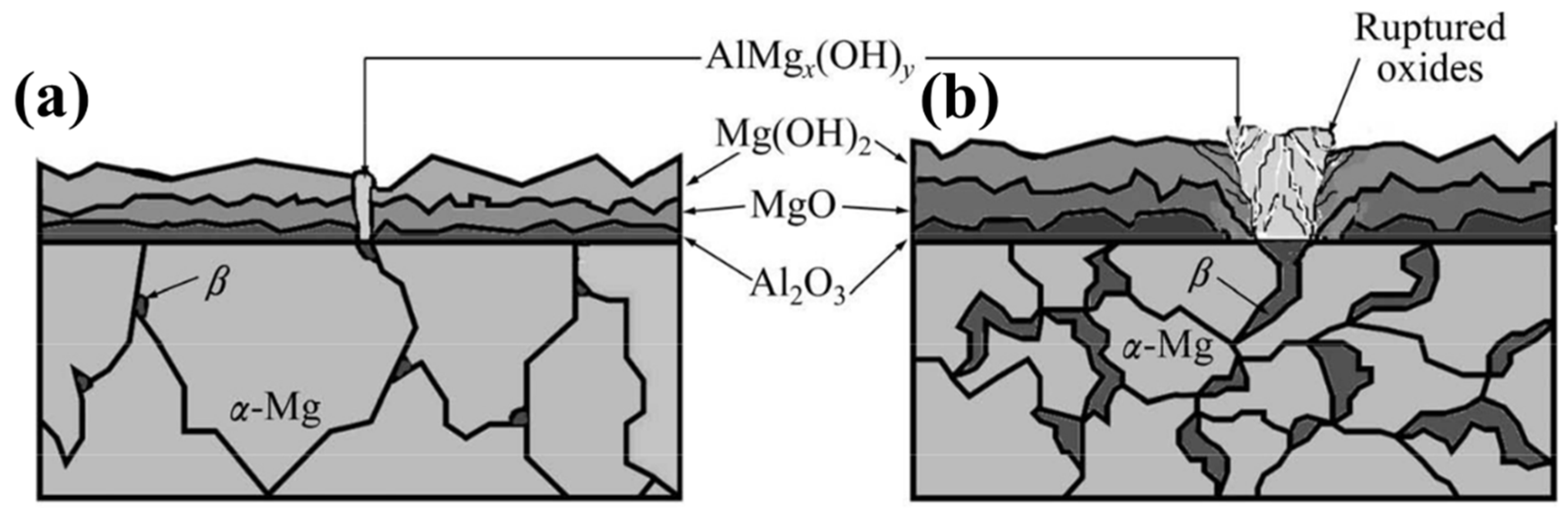
There are a number of electrochemical methods that can be used to forecast the corrosion behaviour of any given material (potentiodynamic polarisation is a commonly used technique). In potentiodynamic polarisation, the chemical reaction is kicked off by applying an electric potential difference between a reference and working electrode (ECorr), and the rate of corrosion is then calculated from the corresponding current, referred to as the corrosion current (iCorr), which is measured in amperes per square centimetre. A greater resistance to corrosion would be indicated by a larger ECorr and a smaller iCorr. In a 3.5% NaCl solution, the iCorr values for AZ01, AZ21, AZ41, AZ61, and AZ91 are found to be 65.86, 5.74, 3.16, 9.80, and 36.0 A/cm2, respectively. AZ41 has been found to have excellent corrosion resistance [75]. Corrosion resistance improves with increasing Al content up to about 4%, but then decreases as a result of coarsening intermetallic phases [75,76]. Corrosion resistance was improved in other works, regardless of Al content (≥4%) [77]. When it comes to corrosion, the distribution of the phase is more important than the weight percentage of Al [78], as demonstrated by AZ80, which has a corrosion rate of 0.03 mm/year after the T6 process. Grain refinement and α-Mg protection under the continuously distributed particles that act as a coverage make plastic deformation an effective method for reducing AZXX’s grain size and increasing the material’s resistance to corrosion. Corrosion in AZ80 increases with increasing deformation at 250 °C. Corrosion resistance at 400 °C is independent of the degree of deformation [79]. The AZXX alloys usually contain three regions such as α-Mg, β-Phase which is due to the increased percentage of Aluminum (>1%) and a eutectic region with a mixture of α and β phases. Heat treatment reduces the proportion of the second phase, which reduces corrosion in AZ31 and AZ91Mg alloys [80]. According to reports, the morphology of the secondary phase in AZ91 alloy makes it more susceptible to corrosion in 3.5% NaCl than other AZ alloys like AZ21, AZ41, and AZ61. The intermetallic phase forms AlMgx(OH)y which interrupts the normal growth of the oxide film [75]. Diagrammatically depicted in Figure 7 is the corrosion behaviour of AZXX alloys. When it comes to the corrosion behaviour of AZXX Mg alloy, the volume fraction of the intermetallic or secondary or β phase is crucial. Galvanic or localised corrosion is facilitated by the Al-rich phase acting as a cathode [81]. This limits the anodic nature of the α-Mg. Secondary phases were dissolved in the Mg matrix up to the application of heat, which may cause widespread corrosion [82]. Corrosion resistance in AZXX alloy is improved with age due to the formation of a continuous phase along the grain boundaries [78]. Corrosion rate is affected by the shape, type, and distribution of the phase due to the potential difference between the α and β phases [83]. In particular, AZ80 has a lower corrosion resistance because of the fine and discontinuous distribution of Mg17Al12 [84].
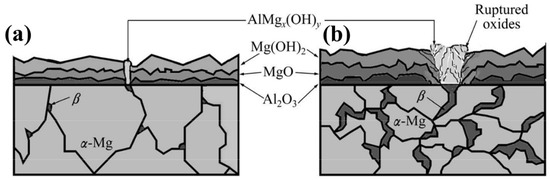
Figure 7.
Schematic illustration corrosion mechanism on (a) AZ21 and AZ41 and (b) AZ61 and AZ91 [75].
Additionally, the propensity for hydrogen embrittlement is increased by the increased transportation of hydrogen in the alloy. When NaCl medium is used instead of air, ductile fractures transform into trans-granular stress corrosion cracking [85]. The threshold stresses for AZ91 and AZ31 during Stress Corrosion Cracking (SCC) in pure water were 55–75 MPa and 105–170 MPa, respectively [86]. The corrosion rate of an alloy is affected not only by the concentration of the corrosion medium, but also by its flow rate. Both static and moving corrosion rates were reported by the studies. As the flow rate of the electrolyte (corrosive medium) is increased, the corrosion rate of the AZ80 alloy is decreased, according to the study [87]. However, in a biological setting, the fluid’s dynamic nature mitigates inflammation while hastening degradation [88]. As the flow rate of the electrolyte rises, the corrosion rate rises as well because the corrosion-protective corrosion products or passive layer on the alloy’s surface have been washed away and the new surface is in contact with the electrolyte [89,90,91]. Furthermore, hydrogen evaluation and passivation rate are affected by the pH of the electrolyte, which in turn affects corrosion behaviour [87,92]. Anodizing, heat treating, or coating with composites are all examples of surface modifications that can be used to boost corrosion resistance [93]. While many studies have focused on improving Mg alloys’ resistance to corrosion, there is still room to learn more about the material’s behaviour under the conditions encountered in practical settings. Table 2 shows the corrosion rate and electrical parameters corresponding to it. It is clearly understood that the increase in Al content leads to an increase in ICorr, which increases the anodic nature of the Mg alloy. The T6 heat treatment also shows a more promising corrosion resistance than as-fabricated and T4 alloys, due to the formation of the continuous intermetallic phases. This acts as a cathodic barrier and enhances the corrosion rate of α-Mg, as a result of galvanic corrosion between α-Mg and Mg17Al12. The corrosion of the alloy is initiated at the α-Mg phase.

Table 2.
Corrosion behaviour of AZXX Mg alloys.
6. Summary and Outlook
Because of the correlation between microstructure, mechanical properties, and corrosion behaviour, this review sheds light on the significance of the magnesium AZ series alloys. Many types of AZXX alloys are processed by extrusion and homogenization. However, there is a need for the homogenization process because the extrusion method exhibits surface-to-core grain refinement variation that must be addressed extensively. To ensure that the AZXX alloys are processed with consistent bulk properties, modern casting techniques are adapted for use with these materials, and heat treatment is often combined with casting to further improve properties. When processing coarse and dendritic Mg AZXX alloys, second phase precipitates of Mg can be seen along the grain boundaries. In the case of AZXX, the mechanical and corrosion properties are influenced by the size, shape, and distribution of the secondary phases, which are primarily a brittle Mg17Al12. For engineering applications, the presence of phases is undesirable because they improve corrosion resistance at the expense of mechanical properties. Therefore, research efforts should be redirected toward achieving an optimum volume fraction of secondary phases through possible processing techniques, which may aid in broadening the application of AZXX Mg alloys. Alloy properties are modified because of how the processing techniques modify the microstructure of the alloy. Grain size and morphology can be predicted by modelling static and dynamic recrystallization phenomena, even though there is work reported on various processing techniques of AZ31, AZ41, AZ61, AZ80, AZ91, and other AZXX alloys. Despite decades of study, processing techniques for AZ series Mg alloys can be refined further to increase their tensile, creep, and corrosion resistance.
Author Contributions
Conceptualization, A.M. and A.R.A.; methodology, S.P.B., T.U.D., S.D. and M.S.; formal analysis M.S. and A.R.A.; investigation, S.P.B., T.U.D., S.D. and M.S.; resources, S.P.B., T.U.D. and S.D.; data curation, S.P.B., T.U.D. and S.D.; writing—original draft preparation, S.P.B. and M.S.; writing—review and editing, S.P.B., M.S., A.M. and A.R.A.; supervision, A.M. and A.R.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Motallebi, R.; Savaedi, Z.; Mirzadeh, H. Post-processing heat treatment of lightweight magnesium alloys fabricated by additive manufacturing: A review. J. Mater. Res. Technol. 2022, 20, 1873–1892. [Google Scholar] [CrossRef]
- Sun, J.; Du, W.; Fu, J.; Liu, K.; Li, S.; Wang, Z.; Liang, H. A review on magnesium alloys for application of degradable fracturing tools. J. Magnes. Alloy 2022, 10, 2649–2672. [Google Scholar] [CrossRef]
- Jayasathyakawin, S.; Ravichandran, M.; Baskar, N.; Chairman, C.A.; Balasundaram, R. Mechanical properties and applications of Magnesium alloy—Review. Mater. Today Proc. 2020, 27, 909–913. [Google Scholar] [CrossRef]
- Hussein, R.O.; Northwood, D.O. Improving the performance of magnesium alloys for automotive applications. WIT Trans. Built Environ. 2014, 137, 531–544. [Google Scholar] [CrossRef]
- Ali, M.; Hussein, M.; Al-Aqeeli, N. Magnesium-based composites and alloys for medical applications: A review of mechanical and corrosion properties. J. Alloys Compd. 2019, 792, 1162–1190. [Google Scholar] [CrossRef]
- Zhang, T.; Wang, W.; Liu, J.; Wang, L.; Tang, Y.; Wang, K. A review on magnesium alloys for biomedical applications. Front. Bioeng. Biotechnol. 2022, 10, 953344. [Google Scholar] [CrossRef]
- Poinern, G.E.J.; Brundavanam, S.; Fawcett, D. Biomedical Magnesium Alloys: A Review of Material Properties, Surface Modifications and Potential as a Biodegradable Orthopaedic Implant. Am. J. Biomed. Eng. 2012, 2, 218–240. [Google Scholar] [CrossRef]
- Yartys, V.; Lototskyy, M.; Akiba, E.; Albert, R.; Antonov, V.; Ares, J.; Baricco, M.; Bourgeois, N.; Buckley, C.; von Colbe, J.B.; et al. Magnesium based materials for hydrogen based energy storage: Past, present and future. Int. J. Hydrogen Energy 2019, 44, 7809–7859. [Google Scholar] [CrossRef]
- Baran, A.; Polański, M. Magnesium-Based Materials for Hydrogen Storage—A Scope Review. Materials 2020, 13, 3993. [Google Scholar] [CrossRef]
- Yaroshevsky, A.A. Abundances of chemical elements in the Earth’s crust. Geochem. Int. 2006, 44, 48–55. [Google Scholar] [CrossRef]
- Tong, F.; Wei, S.; Chen, X.; Gao, W. Magnesium alloys as anodes for neutral aqueous magnesium-air batteries. J. Magnes. Alloy 2021, 9, 1861–1883. [Google Scholar] [CrossRef]
- Papenberg, N.P.; Gneiger, S.; Weißensteiner, I.; Uggowitzer, P.J.; Pogatscher, S. Mg-Alloys for Forging Applications—A Review. Materials 2020, 13, 985. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Cao, F.; Song, G.-L.; Zheng, D.; Shi, Z.; Dargusch, M.S.; Atrens, A. Review of the atmospheric corrosion of magnesium alloys. J. Mater. Sci. Technol. 2019, 35, 2003–2016. [Google Scholar] [CrossRef]
- Tan, J.; Ramakrishna, S. Applications of Magnesium and Its Alloys: A Review. Appl. Sci. 2021, 11, 6861. [Google Scholar] [CrossRef]
- Zhang, N.X.; Kawasaki, M.; Ding, H.; Langdon, T.G. An examination of microstructural evolution and homogeneity in a magnesium AZ80 alloy processed by high-pressure torsion. Mater. Sci. Eng. A 2021, 806, 140832. [Google Scholar] [CrossRef]
- Kleiner, S.; Beffort, O.; Wahlen, A.; Uggowitzer, P. Microstructure and mechanical properties of squeeze cast and semi-solid cast Mg–Al alloys. J. Light Met. 2002, 2, 277–280. [Google Scholar] [CrossRef]
- Rakshith, M.; Seenuvasaperumal, P. Review on the effect of different processing techniques on the microstructure and mechanical behaviour of AZ31 Magnesium alloy. J. Magnes. Alloy 2021, 9, 1692–1714. [Google Scholar] [CrossRef]
- El-Morsy, A.-W.; Ismail, A.; Waly, M. Microstructural and mechanical properties evolution of magnesium AZ61 alloy processed through a combination of extrusion and thermomechanical processes. Mater. Sci. Eng. A 2008, 486, 528–533. [Google Scholar] [CrossRef]
- Kim, Y.J.; Kim, Y.M.; Bae, J.H.; Joo, S.-H.; Park, S.H. Microstructural characteristics and low-cycle fatigue properties of AZ91 and AZ91–Ca–Y alloys extruded at different temperatures. J. Magnes. Alloy 2022. [Google Scholar] [CrossRef]
- Barnett, M.; Nave, M.; Bettles, C. Deformation microstructures and textures of some cold rolled Mg alloys. Mater. Sci. Eng. A 2004, 386, 205–211. [Google Scholar] [CrossRef]
- Zhou, X.; Zhang, J.; Chen, X.; Zhang, X.; Li, M. Fabrication of high-strength AZ80 alloys via multidirectional forging in air with no need of ageing treatment. J. Alloys Compd. 2019, 787, 551–559. [Google Scholar] [CrossRef]
- Wang, G.G.; Weiler, J. Recent developments in high-pressure die-cast magnesium alloys for automotive and future applications. J. Magnes. Alloy 2022. [Google Scholar] [CrossRef]
- Guo, Y.; Pan, H.; Ren, L.; Quan, G. Microstructure and mechanical properties of wire arc additively manufactured AZ80M magnesium alloy. Mater. Lett. 2019, 247, 4–6. [Google Scholar] [CrossRef]
- Guo, F.; Zhang, D.; Yang, X.; Jiang, L.; Chai, S.; Pan, F. Influence of rolling speed on microstructure and mechanical properties of AZ31 Mg alloy rolled by large strain hot rolling. Mater. Sci. Eng. A 2014, 607, 383–389. [Google Scholar] [CrossRef]
- Yeoh, M.K.; Tan, X.; Cheang, P.H.N.; Lim, T.-C.; Kwok, R.W.O. Effect of Solutionizing Time on Improving the Microstructure and Mechanical Properties of Aged AZ80 Mg Alloy. J. Mater. Eng. Perform. 2019, 28, 6836–6852. [Google Scholar] [CrossRef]
- Lee, G.M.; Lee, J.U.; Park, S.H. Effects of post-heat treatment on microstructure, tensile properties, and bending properties of extruded AZ80 alloy. J. Mater. Res. Technol. 2021, 12, 1039–1050. [Google Scholar] [CrossRef]
- Daud, Z.C.; Azahar, S.H.; Derman, M.N.; Adzali, N.M.S.; Zaidi, N.H.A.; Adnan, S.A. The effect of aging time on microstructure and hardness value of AZ80 Mg Alloy. IOP Conf. Ser. Mater. Sci. Eng. 2020, 957, 012051. [Google Scholar] [CrossRef]
- Yeoh, M.K.; Tan, X.; Kwok, R.W.O.; Lim, T.-C. Investigation of distinctive solutionizing process parameters on significantly increasing in strength and ductility of As-CAST AZ80 Mg alloy. Acta Met. Slovaca 2020, 26, 166–177. [Google Scholar] [CrossRef]
- Naik, G.M.; Gote, G.D.; Narendranath, S.; Kumar, S.S.S. The impact of homogenization treatment on microstructure microhardness and corrosion behavior of wrought AZ80 magnesium alloys in 3.5 wt% NaCl solution. Mater. Res. Express 2018, 5, 086513. [Google Scholar] [CrossRef]
- Chen, Q.; Lin, J.; Zhan, H.; Huang, S.; Shu, D.; Yuan, B. Microstructure Evolution and Mechanical Properties of Large-Scale AZ80 Magnesium Alloy Billets Produced by Multitemperature Multidirectional Forging. J. Mater. Eng. Perform. 2019, 28, 3498–3504. [Google Scholar] [CrossRef]
- Chen, X.; Jia, Y.; Liao, Q.; Jia, W.; Le, Q.; Ning, S.; Yu, F. The simultaneous application of variable frequency ultrasonic and low frequency electromagnetic fields in semi continuous casting of AZ80 magnesium alloy. J. Alloys Compd. 2018, 774, 710–720. [Google Scholar] [CrossRef]
- Liao, Q.; Lan, Q.; Jia, W.; Chen, X.; Le, Q.; Wang, T. Microstructure and Mechanical Property Inhomogeneities of a Modified AZ80 Magnesium Alloy Wide Plate Under Horizontal Flat Die Extrusion. J. Mater. Eng. Perform. 2019, 28, 1977–1985. [Google Scholar] [CrossRef]
- Gokalp, I.; Incesu, A. Effect of Ca Addition to the Elevated Temperature Mechanical Properties of AZ Series Magnesium Alloys. Int. J. Met. 2022, 1–11. [Google Scholar] [CrossRef]
- Niknejad, S.; Liu, L.; Lee, M.-Y.; Esmaeili, S.; Zhou, N.Y. Resistance spot welding of AZ series magnesium alloys: Effects of aluminum content on microstructure and mechanical properties. Mater. Sci. Eng. A 2014, 618, 323–334. [Google Scholar] [CrossRef]
- Cha, J.W.; Park, S.H. Variations in dynamic recrystallization behavior and mechanical properties of AZ31 alloy with extrusion temperature. J. Magnes. Alloy 2022. [Google Scholar] [CrossRef]
- Chen, T.; Hu, S.; Li, S.; Huo, Q. Uncovering the unexpected changes of creep properties in AZ-series Mg alloys. Mater. Sci. Eng. A 2022, 857, 144056. [Google Scholar] [CrossRef]
- Rong, J.; Xiao, W.; Zhao, X.; Ma, C.; Liao, H.; He, D.; Chen, M.; Huang, M.; Huang, C. High thermal conductivity and high strength magnesium alloy for high pressure die casting ultrathin-walled components. Int. J. Miner. Met. Mater. 2022, 29, 88–96. [Google Scholar] [CrossRef]
- Luo, A.A.; Fu, P.; Peng, L.; Kang, X.; Li, Z.; Zhu, T. Solidification Microstructure and Mechanical Properties of Cast Magnesium-Aluminum-Tin Alloys. Met. Mater. Trans. A 2011, 43, 360–368. [Google Scholar] [CrossRef]
- Luo, X.; Kang, L.; Liu, H.; Li, Z.; Liu, Y.; Zhang, D.; Chen, D. Enhancing mechanical properties of AZ61 magnesium alloy via friction stir processing: Effect of processing parameters. Mater. Sci. Eng. A 2020, 797, 139945. [Google Scholar] [CrossRef]
- Zou, Y.-H.; Wang, J.; Cui, L.-Y.; Zeng, R.-C.; Wang, Q.-Z.; Han, Q.-X.; Qiu, J.; Chen, X.-B.; Chen, D.-C.; Guan, S.-K.; et al. Corrosion resistance and antibacterial activity of zinc-loaded montmorillonite coatings on biodegradable magnesium alloy AZ31. Acta Biomater. 2019, 98, 196–214. [Google Scholar] [CrossRef]
- Javaid, A.; Czerwinski, F. Progress in twin roll casting of magnesium alloys: A review. J. Magnes. Alloy 2020, 9, 362–391. [Google Scholar] [CrossRef]
- Tao, J.-Q.; Zhang, Y.-P.; Fan, F.-Y.; Chen, Q. Microstructural Evolution and Mechanical Properties of AZ31 Magnesium Alloy Prepared by Casting-solid Extrusion Forging During Partial Remelting. Def. Technol. 2013, 9, 146–152. [Google Scholar] [CrossRef]
- Vyshak, T.P.; Sankar, R.; Babu, A.; Harilal, M.D.J. A review on heating and rolling effect of AZ31 magnesium alloy. Int. Res. J. Eng. 2020, 7, 4878–4883. [Google Scholar]
- Zimina, M.; Málek, P.; Bohlen, J.; Letzig, D.; Kurz, G.; Cieslar, M. Mechanical properties of homogenized twin-roll cast and conventionally cast az31 magnesium alloys. Magnes. Alloy 2015, 22, 8–15. [Google Scholar]
- Materials|Free Full-Text|Microstructure Evolution and Mechanical Properties of AZ80 Mg Alloy during Annular Channel Angular Extrusion Process and Heat Treatment. Available online: https://www.mdpi.com/1996-1944/12/24/4223 (accessed on 8 February 2023).
- Xu, H.-Y.; Wang, Q.; Zhang, Z.-M. Effect of thermal processing on microstructure and mechanical properties of AZ80 magnesium alloy. Trans. Nonferrous Met. Soc. China 2008, 18, s122–s126. [Google Scholar] [CrossRef]
- Zhao, X.; Gao, P.; Chen, G.; Wei, J.; Zhu, Z.; Yan, F.; Zhang, Z.; Wang, Q. Effects of aging treatments on low-cycle fatigue behavior of extruded AZ80 for automobile wheel disks. Mater. Sci. Eng. A 2020, 799, 140366. [Google Scholar] [CrossRef]
- Li, Z.-Z.; Yang, Y.-Q.; Zhang, Z.-M. Transformation mechanism of lamellar microstructure of AZ80 wrought Mg alloy during warm deformation. Trans. Nonferrous Met. Soc. China 2008, 18, s156–s159. [Google Scholar] [CrossRef]
- Jin, Z.-Z.; Cheng, X.-M.; Zha, M.; Rong, J.; Zhang, H.; Wang, J.-G.; Wang, C.; Li, Z.-G.; Wang, H.-Y. Effects of Mg17Al12 second phase particles on twinning-induced recrystallization behavior in Mg–Al–Zn alloys during gradient hot rolling. J. Mater. Sci. Technol. 2019, 35, 2017–2026. [Google Scholar] [CrossRef]
- Kim, S.-H.; Lee, S.W.; Moon, B.G.; Kim, H.S.; Park, S.H. Variation in dynamic deformation behavior and resultant yield asymmetry of AZ80 alloy with extrusion temperature. J. Mater. Sci. Technol. 2020, 46, 225–236. [Google Scholar] [CrossRef]
- Park, S.H.; Bae, J.H.; Kim, S.-H.; Yoon, J.; You, B.S. Effect of Initial Grain Size on Microstructure and Mechanical Properties of Extruded Mg-9Al-0.6Zn Alloy. Met. Mater. Trans. A 2015, 46, 5482–5488. [Google Scholar] [CrossRef]
- Yu, H.; Park, S.H.; You, B.S. Die angle dependency of microstructural inhomogeneity in an indirect-extruded AZ31 magnesium alloy. J. Mater. Process. Technol. 2015, 224, 181–188. [Google Scholar] [CrossRef]
- Dieter, G.E.; Bacon, D. Mechanical Metallurgy; McGraw Hill: New York, NY, USA, 1976; Volume 3. [Google Scholar]
- Huang, S.J.; Abbas, A.; Ballóková, B. Effect of CNT on microstructure, dry sliding wear and compressive mechanical properties of AZ61 magnesium alloy. J. Mater. Res. Technol. 2019, 8, 4273–4286. [Google Scholar] [CrossRef]
- Yang, Y.; Qin, Q.; Zhang, Z.; Wang, Q.; Meng, M.; Liang, M.-J.; Hu, H. Processing maps of extruded AZ80 + 0.4% Ce magnesium alloy. J. Alloys Compd. 2020, 844, 156064. [Google Scholar] [CrossRef]
- Sepahi-Boroujeni, S. Improvements in microstructure and mechanical properties of AZ80 magnesium alloy by means of an efficient, novel severe plastic deformation process. J. Manuf. Process. 2016, 24, 71–77. [Google Scholar] [CrossRef]
- Zou, H.; Zeng, X.; Zhai, C.; Ding, W. Effects of Nd on the microstructure of ZA52 alloy. Mater. Sci. Eng. A 2005, 392, 229–234. [Google Scholar] [CrossRef]
- Nam, N.; Kim, W.; Kim, J.; Shin, K.; Jung, H. Effect of mischmetal on the corrosion properties of Mg–5Al alloy. Corros. Sci. 2009, 51, 2942–2949. [Google Scholar] [CrossRef]
- Zou, Q.; Le, Q.; Chen, X.; Ban, C.; Jia, Y.; Guo, R.; Wang, A. Effect of Nd-Rich Phases on the Corrosion Behavior of AZ80 Magnesium Alloy in Alkaline Solution. Jom 2021, 73, 4376–4386. [Google Scholar] [CrossRef]
- Basu, I.; Al-Samman, T. Competitive twinning behavior in magnesium and its impact on recrystallization and texture formation. Mater. Sci. Eng. A 2017, 707, 232–244. [Google Scholar] [CrossRef]
- Pushpanathan, D.P.; Alagumurthi, N.; Devaneyan, S.P. On the microstructure and tribological properties of pulse electrodeposited Ni-B4C-TiC nano composite coating on AZ80 magnesium alloy. Surf. Interfaces 2020, 19, 100465. [Google Scholar] [CrossRef]
- Zhang, K.; Li, H.; Liang, X.; Chen, Z.; Wang, L. Discontinuous and continuous precipitation characteristics and mechanical properties of a AZ80A magnesium alloy at different aging temperatures. Mater. Charact. 2020, 161, 110146. [Google Scholar] [CrossRef]
- Marya, M.; Hector, L.G.; Verma, R.; Tong, W. Microstructural effects of AZ31 magnesium alloy on its tensile deformation and failure behaviors. Mater. Sci. Eng. A 2006, 418, 341–356. [Google Scholar] [CrossRef]
- Mechanical Properties of Energy Absorbing Magnesium Alloys. Available online: https://www.sae.org/publications/technical-papers/content/930418/ (accessed on 23 January 2023).
- Jiang, M.; Yan, H.; Chen, R. Microstructure, texture and mechanical properties in an as-cast AZ61 Mg alloy during multi-directional impact forging and subsequent heat treatment. Mater. Des. 2015, 87, 891–900. [Google Scholar] [CrossRef]
- Lin, H.; Yang, M.; Tang, H.; Pan, F. Effect of minor Sc on the microstructure and mechanical properties of AZ91 Magnesium Alloy. Prog. Nat. Sci. 2018, 28, 66–73. [Google Scholar] [CrossRef]
- Begum, S.; Chen, D.; Xu, S.; Luo, A.A. Low cycle fatigue properties of an extruded AZ31 magnesium alloy. Int. J. Fatigue 2009, 31, 726–735. [Google Scholar] [CrossRef]
- Meyer, L.W.; Hockauf, M.; Zillmann, B.; Schneider, I. Strength, ductility and impact toughness of the magnesium alloy az31b after equal-channel angular pressing. Int. J. Mater. Form. 2009, 2, 61–64. [Google Scholar] [CrossRef]
- Kashefi, N.; Mahmudi, R. The microstructure and impression creep behavior of cast AZ80 magnesium alloy with yttrium additions. Mater. Des. 2012, 39, 200–210. [Google Scholar] [CrossRef]
- Zhang, D.-D.; Liu, C.-M.; Wan, Y.-C.; Jiang, S.-N.; Zeng, G. Microstructure and anisotropy of mechanical properties in ring rolled AZ80-Ag alloy. J. Central South Univ. 2021, 28, 1316–1323. [Google Scholar] [CrossRef]
- Zeng, G.; Liu, C.; Wan, Y.; Gao, Y.; Jiang, S.; Chen, Z. Effects of aging temperature on microstructure, tensile and creep properties of ring rolled AZ80-Ag alloy. Mater. Sci. Eng. A 2018, 734, 59–66. [Google Scholar] [CrossRef]
- Unigovski, Y.; Gutman, E. Corrosion creep and fatigue behavior of magnesium (Mg) alloys. In Corrosion of Magnesium Alloys; Elsevier: Amsterdam, The Netherlands, 2011; pp. 365–402. [Google Scholar] [CrossRef]
- Wan, X.-F.; NI, H.-J.; Huang, M.-Y.; Zhang, H.-L.; Sun, J.-H. Microstructure, mechanical properties and creep resistance of Mg–(8%–12%)Zn–(2%–6%)Al alloys. Trans. Nonferrous Met. Soc. China 2013, 23, 896–903. [Google Scholar] [CrossRef]
- CSIRO Research Publications Repository-Microstructure and Properties of Magnesium Die Castings. Available online: https://publications.csiro.au/rpr/pub?list=BRO&pid=procite:85dc279a-8a01-400a-8afa-a559dfb25c15 (accessed on 23 January 2023).
- Candan, S.; Candan, E. Comparative study on corrosion behaviors of Mg-Al-Zn alloys. Trans. Nonferrous Met. Soc. China 2018, 28, 642–650. [Google Scholar] [CrossRef]
- A Cross-Sectional TEM Study of Corrosion Initiation in Rapidly Solidified Mg-Based Ribbons-Warner-1992-Surface and Interface Analysis-Wiley Online Library. Available online: https://analyticalsciencejournals.onlinelibrary.wiley.com/doi/abs/10.1002/sia.740190172 (accessed on 20 January 2023).
- The Role of Mg17Al12 Phase in the Corrosion of Mg Alloy AZ91|CORROSION. Available online: https://meridian.allenpress.com/corrosion/article-abstract/45/9/741/160574/The-Role-of-Mg17Al12-Phase-in-the-Corrosion-of-Mg (accessed on 20 January 2023).
- Dubey, D.; Kadali, K.; Kancharla, H.; Zindal, A.; Jain, J.; Mondal, K.; Singh, S.S. Effect of Precipitate Characteristics on the Corrosion Behavior of a AZ80 Magnesium Alloy. Met. Mater. Int. 2020, 27, 3282–3292. [Google Scholar] [CrossRef]
- Zhang, Z.-M.; Xu, H.-Y.; Li, B.-C. Corrosion properties of plastically deformed AZ80 magnesium alloy. Trans. Nonferrous Met. Soc. China 2010, 20, s697–s702. [Google Scholar] [CrossRef]
- Kumar, P.P.; Bharat, A.R.; Sai, B.S.; Sarath, R.P.; Akhil, P.; Reddy, G.P.K.; Kondaiah, V.; Sunil, B.R. Role of microstructure and secondary phase on corrosion behavior of heat treated AZ series magnesium alloys. Mater. Today Proc. 2019, 18, 175–181. [Google Scholar] [CrossRef]
- Dissolution Kinetics of Mg17Al12 Eutectic Phase and Its Effect on Corrosion Behavior of As-Cast AZ80 Magnesium Alloy|SpringerLink. Available online: https://link.springer.com/article/10.1007/s11837-019-03470-3 (accessed on 20 January 2023).
- Elambharathi, B.; Kumar, S.D.; Dhanoop, V.; Dinakar, S.; Rajumar, S.; Sharma, S.; Kumar, V.; Li, C.; Eldin, E.M.T.; Wojciechowski, S. Novel insights on different treatment of magnesium alloys: A critical review. Heliyon 2022, 8, e11712. [Google Scholar] [CrossRef]
- Zhou, W.; Shen, T.; Aung, N.N. Effect of heat treatment on corrosion behaviour of magnesium alloy AZ91D in simulated body fluid. Corros. Sci. 2010, 52, 1035–1041. [Google Scholar] [CrossRef]
- Liang, M.; Liu, H.; Wu, C.; Li, Y.; Guo, Z.; Murugadoss, V. Effects of rare earth neodymium (Nd) and heat treatment on anti-corrosion behaviors of the AZ80 magnesium alloy. Adv. Compos. Hybrid Mater. 2022, 5, 1460–1476. [Google Scholar] [CrossRef]
- Dubey, D.; Kadali, K.; Panda, S.S.; Kumar, A.; Jain, J.; Mondal, K.; Singh, S.S. Comparative study on the stress corrosion cracking susceptibility of AZ80 and AZ31 magnesium alloys. Mater. Sci. Eng. A 2020, 792, 139793. [Google Scholar] [CrossRef]
- Winzer, N.; Atrens, A.; Dietzel, W.; Raja, V.; Song, G.-L.; Kainer, K. Characterisation of stress corrosion cracking (SCC) of Mg–Al alloys. Mater. Sci. Eng. A 2008, 488, 339–351. [Google Scholar] [CrossRef]
- Xiong, Y.; Zhang, A. Corrosion Behavior of AZ80 Magnesium Alloy in Simulated Static and Dynamic Fluid Environments with Different pH Values. J. Mater. Eng. Perform. 2022, 1–15. [Google Scholar] [CrossRef]
- Zhu, T.; Yu, Y.; Yang, J.; Shen, Y.; He, L.; Xiong, Y. Dynamic corrosion behavior of AZ80 magnesium alloy with different orientations in simulated body fluid. Mater. Chem. Phys. 2020, 259, 124039. [Google Scholar] [CrossRef]
- Microscopic Bio-Corrosion Evaluations of Magnesium Surfaces in Static and Dynamic Conditions-BONTRAGER-2014-Journal of Microscopy-Wiley Online Library. Available online: https://onlinelibrary.wiley.com/doi/abs/10.1111/jmi.12142 (accessed on 20 January 2023).
- Investigation on Magnesium Degradation under Flow Versus Static Conditions Using a Novel Impedance-Driven Flow Apparatus-Sciencedirect. Available online: https://www.sciencedirect.com/science/article/pii/S1002007114001154 (accessed on 20 January 2023).
- Wang, J.; Giridharan, V.; Shanov, V.; Xu, Z.; Collins, B.; White, L.; Jang, Y.; Sankar, J.; Huang, N.; Yun, Y. Flow-induced corrosion behavior of absorbable magnesium-based stents. Acta Biomater. 2014, 10, 5213–5223. [Google Scholar] [CrossRef]
- Yazdani, M.; Afshar, A.; Mohammadi, N.; Paranj, B. Electrochemical evaluation of AZ 31 magnesium alloy in two simulated biological solutions. Anti-Corrosion Methods Mater. 2017, 64, 103–108. [Google Scholar] [CrossRef]
- Esmaily, M.; Svensson, J.E.; Fajardo, S.; Birbilis, N.; Frankel, G.S.; Virtanen, S.; Arrabal, R.; Thomas, S.; Johansson, L.G. Fundamentals and advances in magnesium alloy corrosion. Prog. Mater. Sci. 2017, 89, 92–193. [Google Scholar] [CrossRef]
- Ma, J.; Wang, G.; Li, Y.; Qin, C.; Ren, F. Electrochemical Investigations on AZ Series Magnesium Alloys as Anode Materials in a Sodium Chloride Solution. J. Mater. Eng. Perform. 2019, 28, 2873–2880. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).