Synthesis, Supramolecular Structural Investigations of Co(II) and Cu(II) Azido Complexes with Pyridine-Type Ligands
Abstract
:1. Introduction
2. Experiment
2.1. Physicochemical Characterizations
2.2. Synthesis of the Metal Complexes 1 and 2
2.2.1. Synthesis of [Co(4-Pic)4(H2O)(N3)]NO3*H2O*4-Pic; 1
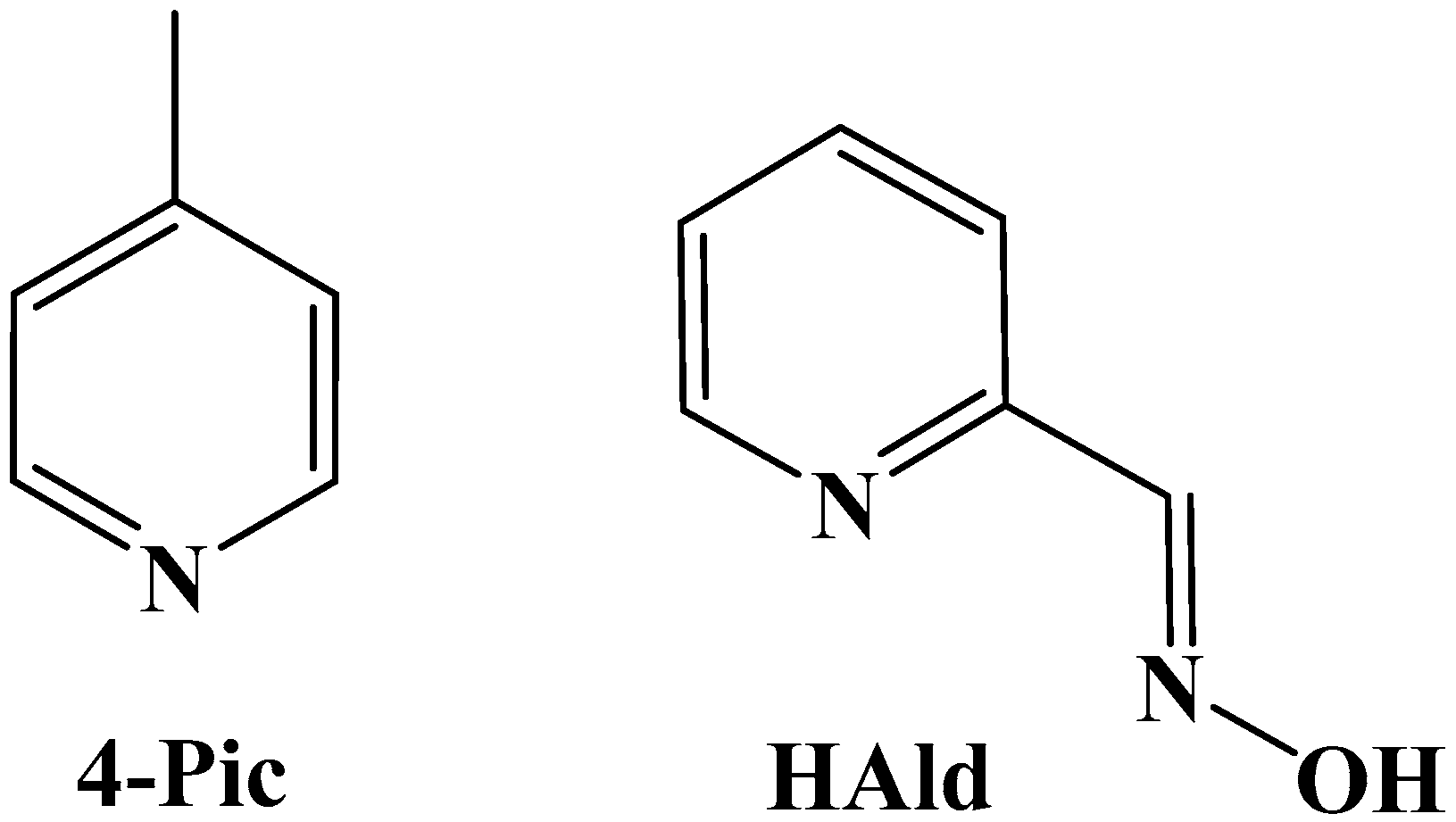
2.2.2. Synthesis of [Cu(HAld)(Ald)(N3)]; 2
2.3. X-ray Diffraction Analysis
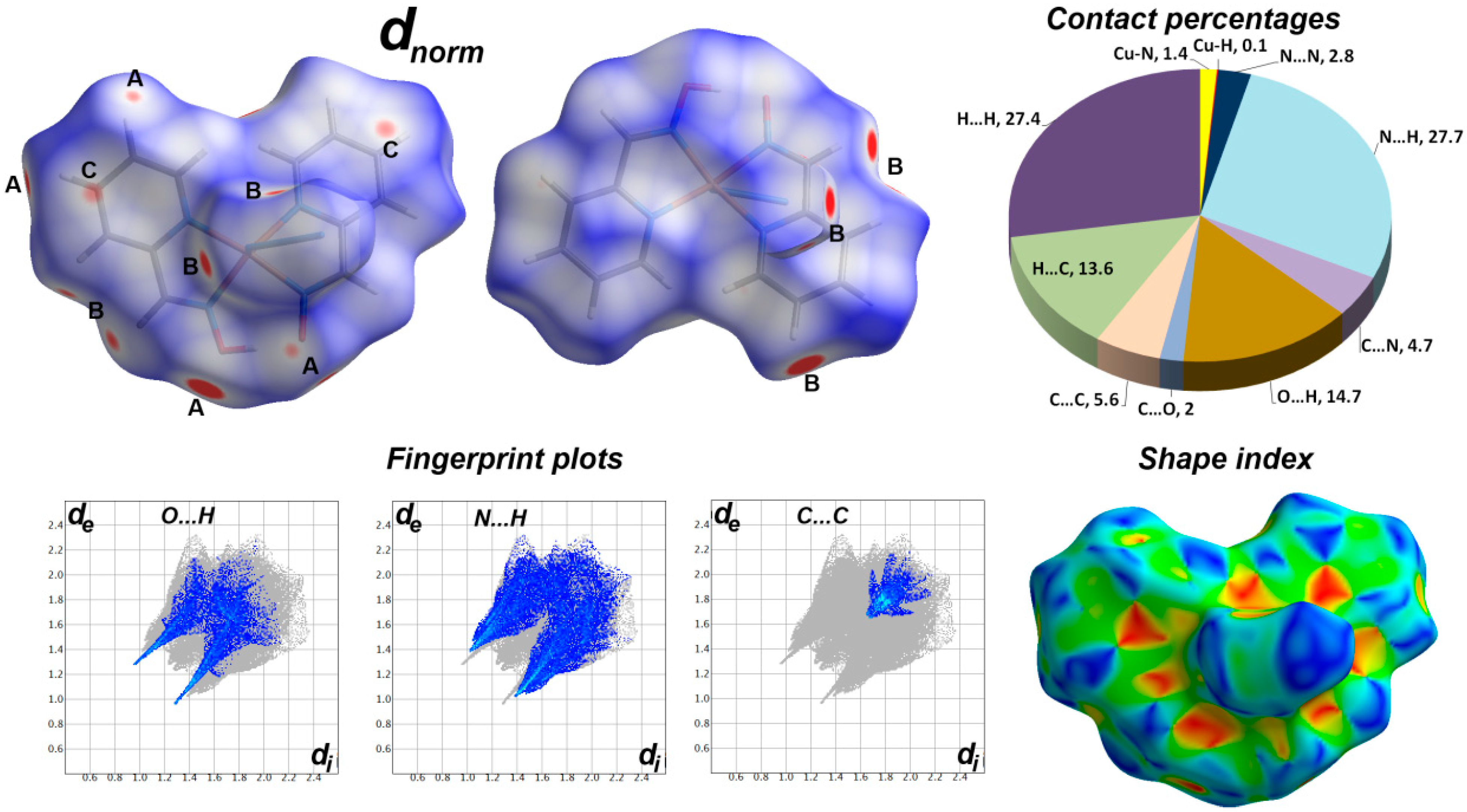
3. Hirshfeld and DFT Calculations
4. Results and Discussion
4.1. X-ray Structure Description
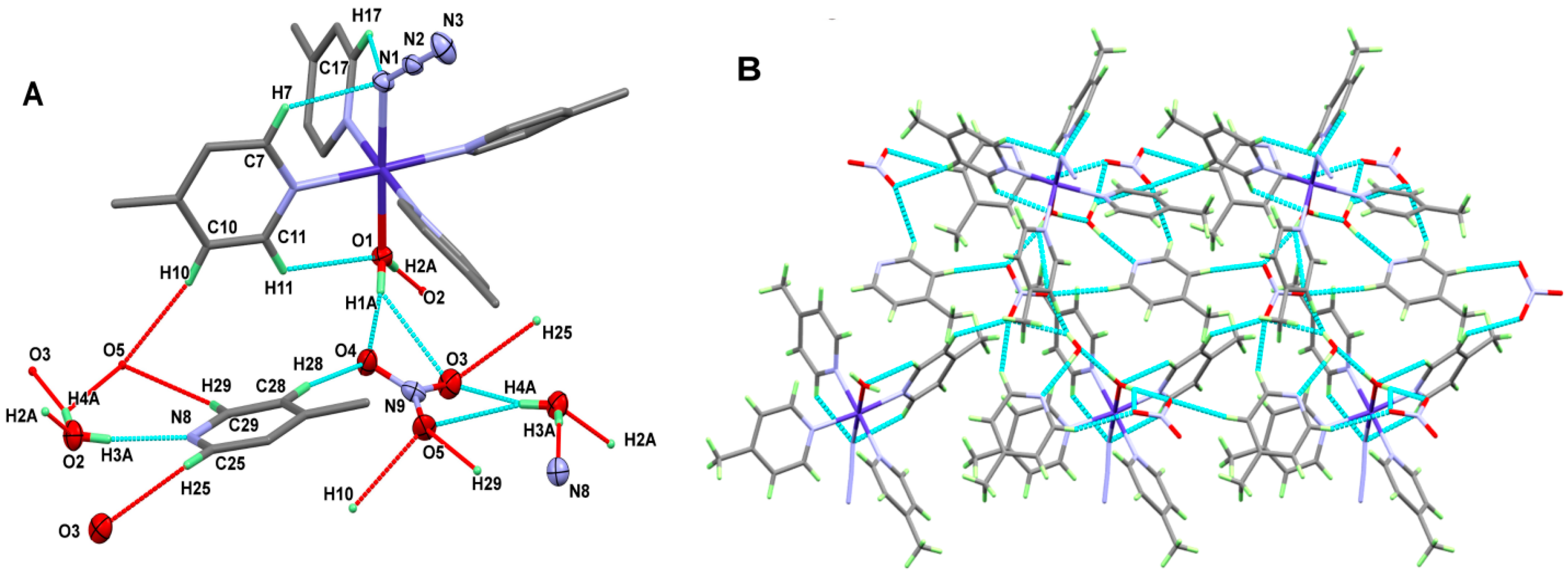
4.1.1. Structure of [Co(4-Pic)4(H2O)(N3)]NO3*H2O*4-Pic; 1
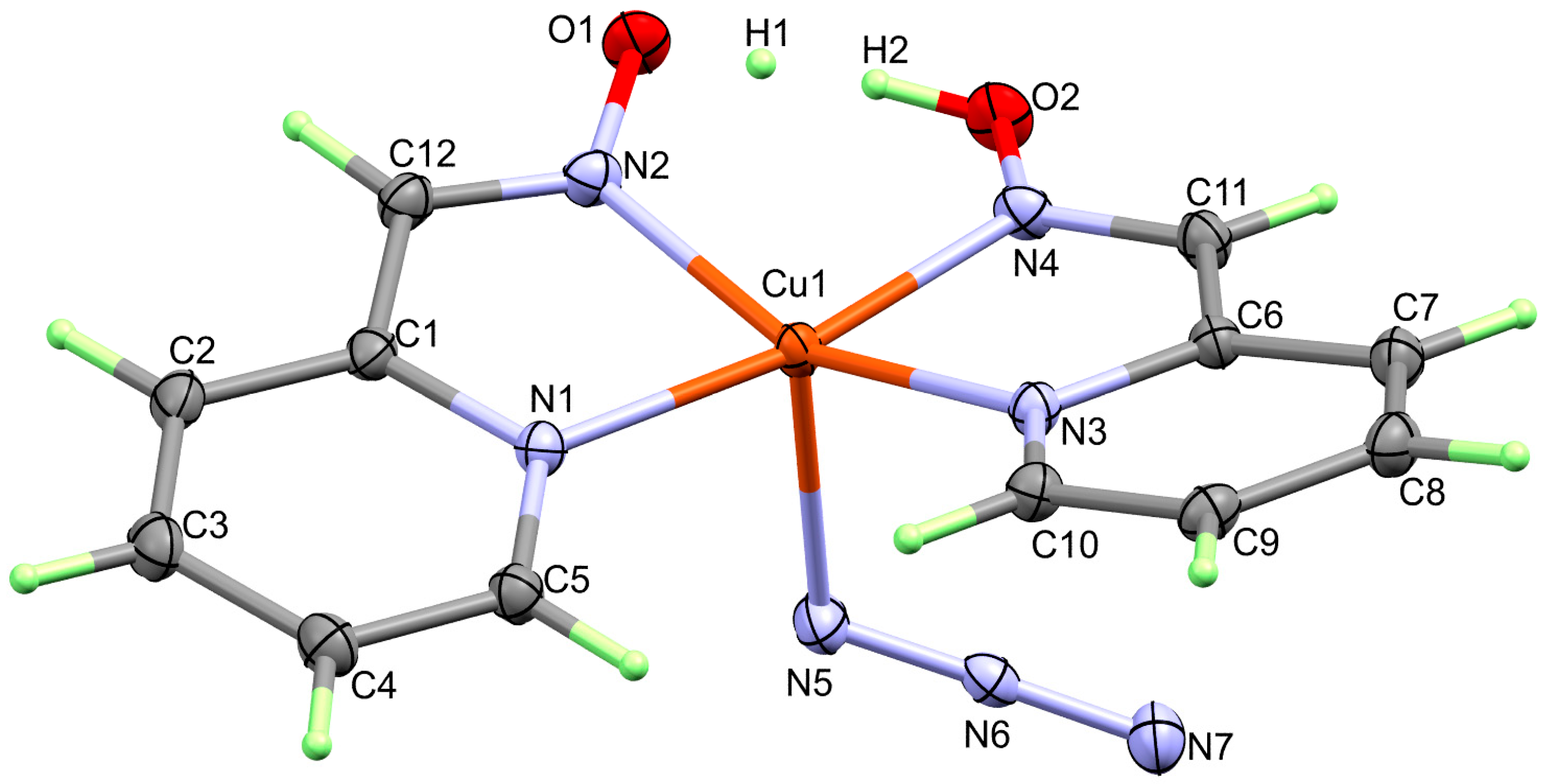
4.1.2. Structure of [Cu(HAld)(Ald)(N3)]; 2
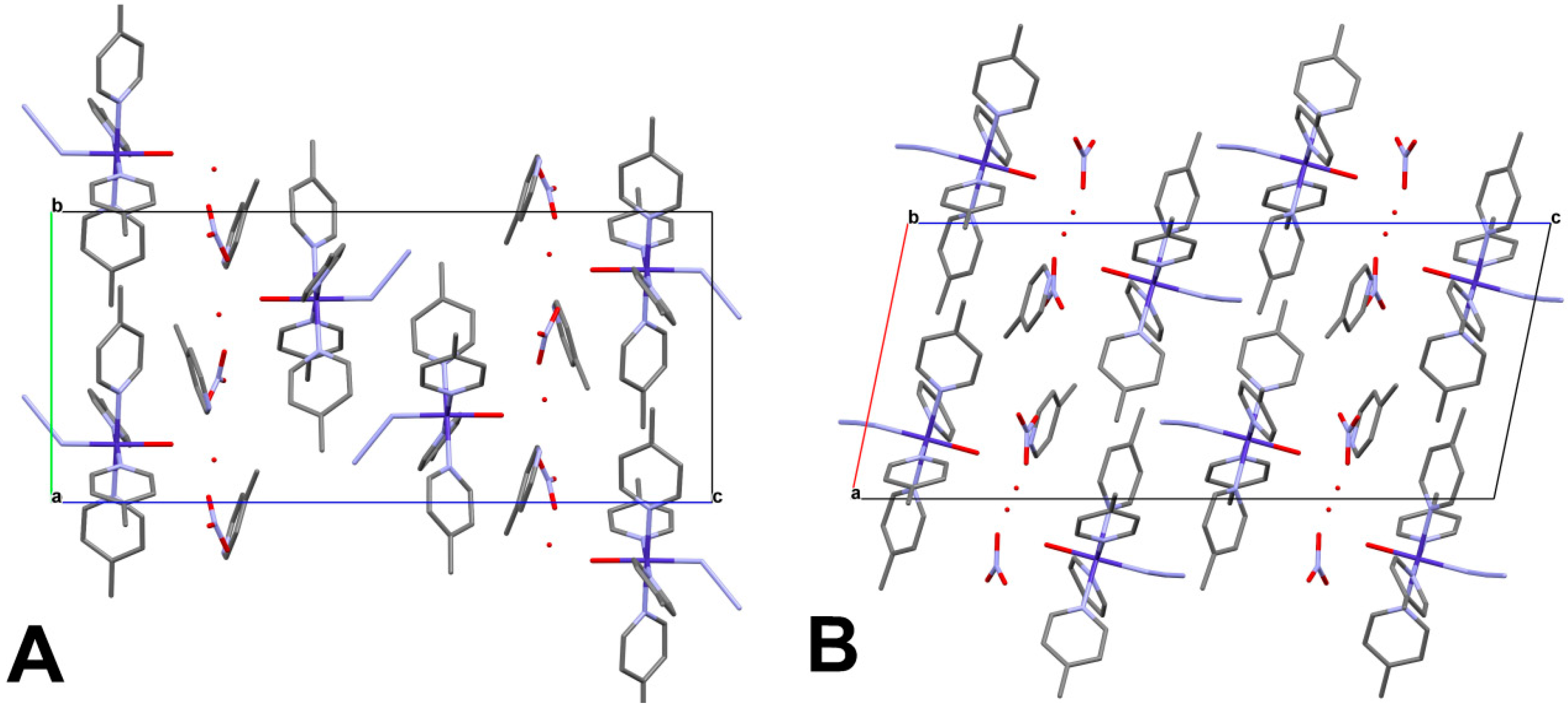
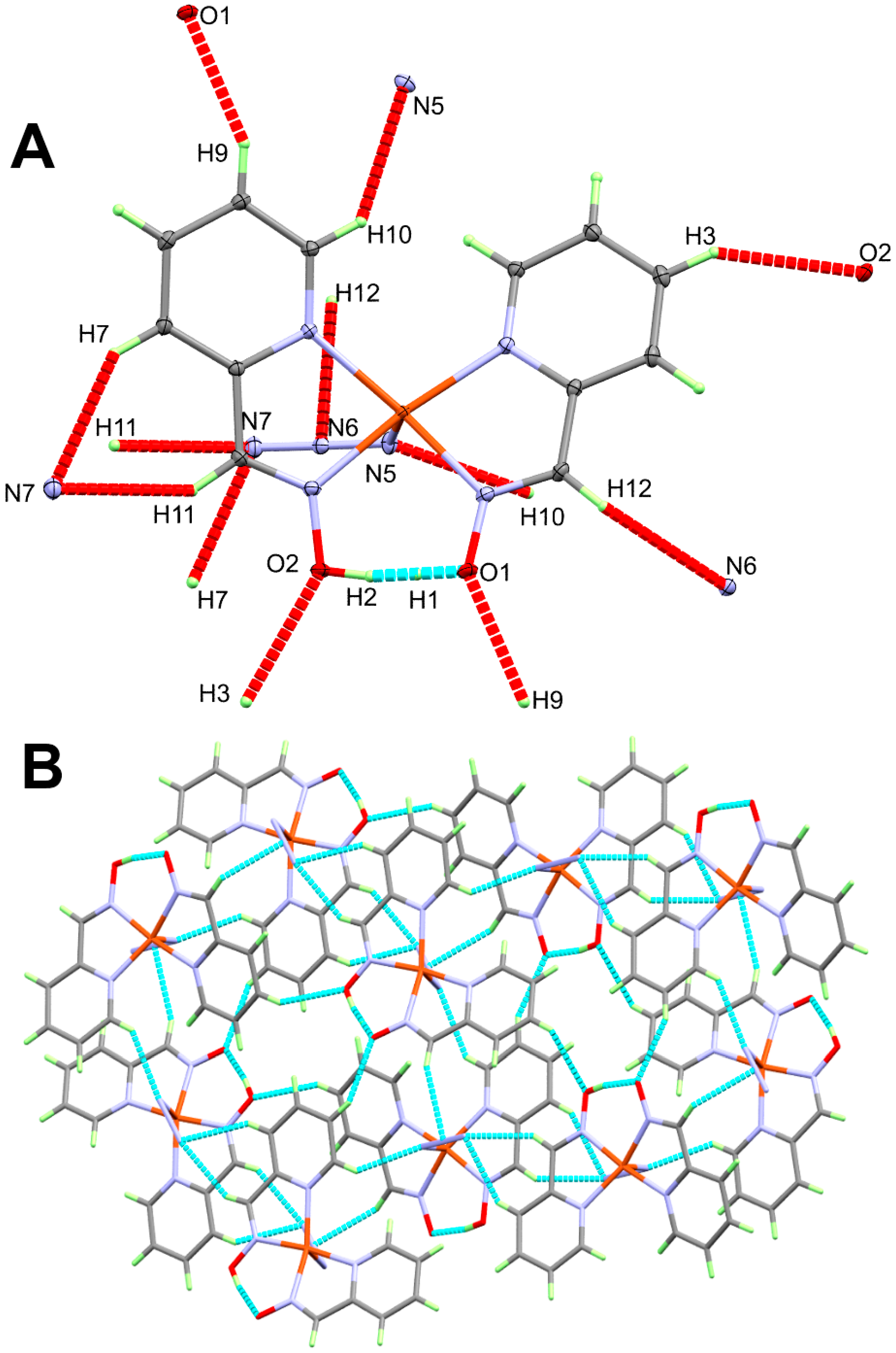
4.2. Analysis of Molecular Packing
4.3. AIM Studies
4.4. Natural Charges
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mir, M.H.; Koh, L.L.; Tan, G.K.; Vittal, J.J. Single-Crystal to Single-Crystal Photochemical Structural Transformations of Interpenetrated 3 D Coordination Polymers by [2 + 2] Cycloaddition Reactions. Angew. Chem. Int. Ed. 2010, 49, 390–393. [Google Scholar] [CrossRef] [PubMed]
- Das, L.K.; Gómez-García, C.J.; Ghosh, A. Influence of the central metal ion in controlling the self-assembly and magnetic properties of 2D coordination polymers derived from [(NiL)2M]2 + nodes (M = Ni, Zn and Cd) (H2L = salen-type di-Schiff base) and dicyanamide spacers. Dalton Trans. 2015, 44, 1292–1302. [Google Scholar] [CrossRef] [PubMed]
- Das, L.K.; Diaz, C.; Ghosh, A. Antiferromagnetic mixed-valence Cu(I)–Cu(II) two-dimensional coordination polymers constructed by double oximato bridged Cu(II) dimers and CuISCN based one-dimensional anionic chains. Cryst. Growth Des. 2015, 15, 3939–3949. [Google Scholar] [CrossRef]
- Yamada, T.; Otsubo, K.; Makiura, R.; Kitagawa, H. Designer coordination polymers: Dimensional crossover architectures and proton conduction. Chem. Soc. Rev. 2013, 42, 6655–6669. [Google Scholar] [CrossRef]
- Li, H.; Wang, Y.; He, Y.; Xu, Z.; Zhao, X.; Han, Y. Synthesis of several novel coordination complexes: Ion exchange, magnetic and photocatalytic studies. New J. Chem. 2017, 41, 1046–1056. [Google Scholar] [CrossRef]
- Mondal, M.; Jana, S.; Drew, M.G.; Ghosh, A. Application of two Cu(II)-azido based 1D coordination polymers in optoelectronic device: Structural characterization and experimental studies. Polymer 2020, 204, 122815. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhao, Y.; Gong, Q.; Li, Z.; Li, J. MOFs for CO2 capture and separation from flue gas mixtures: The effect of multifunctional sites on their adsorption capacity and selectivity. Chem. Commun. 2013, 49, 653–661. [Google Scholar] [CrossRef]
- Zeng, L.W.; Hu, K.Q.; Mei, L.; Li, F.Z.; Huang, Z.W.; An, S.W.; Chai, Z.F.; Shi, W.Q. Structural diversity of bipyridinium-based uranyl coordination polymers: Synthesis, characterization, and ion-exchange application. Inorg. Chem. 2019, 58, 14075–14084. [Google Scholar] [CrossRef]
- He, Y.; Li, B.; O’Keeffe, M.; Chen, B. Multifunctional metal–organic frameworks constructed from meta-benzenedicarboxylate units. Chem. Soc. Rev. 2014, 43, 5618–5656. [Google Scholar] [CrossRef]
- Lee, M.M.; Kim, H.Y.; Hwang, I.H.; Bae, J.M.; Kim, C.; Yo, C.H.; Kim, Y.; Kim, S.J. Cd II MOFs Constructed Using Succinate and Bipyridyl Ligands: Photoluminescence and Heterogeneous Catalytic Activity. Bull. Korean Chem. Soc. 2014, 35, 1777–1783. [Google Scholar] [CrossRef] [Green Version]
- Dutta, B.; Jana, R.; Bhanja, A.K.; Ray, P.P.; Sinha, C.; Mir, M.H. Supramolecular aggregate of Cadmium (II)-based one-dimensional coordination polymer for device fabrication and sensor application. Inorg. Chem. 2019, 58, 2686–2694. [Google Scholar] [CrossRef] [PubMed]
- Ghorai, P.; Dey, A.; Brandão, P.; Benmansour, S.; Gómez García, C.J.; Ray, P.P.; Saha, A. Multifunctional Ni(II)-Based Metamagnetic Coordination Polymers for Electronic Device Fabrication. Inorg. Chem. 2020, 59, 8749–8761. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.Q.; Luo, Z.D.; Pan, Y.; Singh, A.K.; Trivedi, M.; Kumar, A. Recent developments in luminescent coordination polymers: Designing strategies, sensing application and theoretical evidences. Coord. Chem. Rev. 2020, 406, 213145. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, W.; Hu, Z.; Wang, G.; Uvdal, K. Coordination polymers for energy transfer: Preparations, properties, sensing applications, and perspectives. Coord. Chem. Rev. 2015, 284, 206–235. [Google Scholar] [CrossRef]
- Xie, Z.; Ma, L.; de Krafft, K.E.; Jin, A.; Lin, W. Porous phosphorescent coordination polymers for oxygen sensing. J. Am. Chem. Soc. 2010, 132, 922–923. [Google Scholar] [CrossRef]
- Alsharabasy, A.M.; Pandit, A.; Farràs, P. Recent Advances in the Design and Sensing Applications of Hemin/Coordination Polymer-Based Nanocomposites. Adv. Mater. 2021, 33, 2003883. [Google Scholar] [CrossRef] [PubMed]
- Stavila, V.; Talin, A.A.; Allendorf, M.D. MOF-based electronic and opto-electronic devices. Chem. Soc. Rev. 2014, 43, 5994–6010. [Google Scholar] [CrossRef] [Green Version]
- Dhakshinamoorthy, A.; Garcia, H. Catalysis by metal nanoparticles embedded on metal–organic frameworks. Chem. Soc. Rev. 2012, 41, 5262–5284. [Google Scholar] [CrossRef]
- Allendorf, M.D.; Bauer, C.A.; Bhakta, R.K.; Houk, R.J.T. Luminescent metal–organic frameworks. Chem. Soc. Rev. 2009, 38, 1330–1352. [Google Scholar] [CrossRef]
- Roy, S.; Halder, S.; Drew, M.G.; Ray, P.P.; Chattopadhyay, S. Fabrication of an active electronic device using a hetero-bimetallic coordination polymer. ACS Omega 2018, 3, 12788–12796. [Google Scholar] [CrossRef] [Green Version]
- Kar, P.; Guha, P.M.; Drew, M.G.; Ishida, T.; Ghosh, A. Spin-Canted Antiferromagnetic Phase Transitions in Alternating Phenoxoand Carboxylato-Bridged MnIII-Salen Complexes. Eur. J. Chem. 2011, 2011, 2075–2085. [Google Scholar] [CrossRef]
- Lide, D.R. Handbook of Chemistry and Physics, 87th ed.; CRC Press: Boca Raton, FL, USA, 1998; pp. 4–55. [Google Scholar]
- Patnaik, P. Handbook of Inorganic Chemicals; The McGraw-Hill Companies Inc.: New York, NY, USA, 2003; pp. 460–461. [Google Scholar]
- Betterton, E.A. Environmental Fate of Sodium Azide Derived from Automobile Airbags. Crit. Rev. Environ. Sci. Technol. 2003, 33, 423–458. [Google Scholar] [CrossRef]
- Wang, L.-Y.; Zhao, B.; Zhang, C.-X.; Liao, D.-Z.; Jiang, Z.-H.; Yan, S.-P. The First Azide (μ1,1)-Bridged Binuclear Cobalt(II)−Imino Nitroxide Complex with Ferromagnetic Behavior. Inorg. Chem. 2003, 42, 5804–5806. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.-Y.; Wang, L.; Wang, Z.-M.; Gao, S. Solvent-Tuned Azido-Bridged Co2+ Layers: Square, Honeycomb, and Kagome. J. Am. Chem. Soc. 2006, 128, 674–675. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.-T.; Wang, Z.-M.; Gao, S. Honeycomb Layer of Cobalt(II) Azide Hydrazine Showing Weak Ferromagnetism. Inorg. Chem. 2007, 46, 10452–10454. [Google Scholar] [CrossRef] [PubMed]
- Palenik, G.H. The Structure of Coordination Compounds. I. The Crystal and Molecular Structure of AzidoPentamminecobalt(III) Azide. Acta Crystallogr. 1964, 17, 360–367. [Google Scholar] [CrossRef] [Green Version]
- Housecroft, C.E.; Constable, E.C. The Emergence of Copper(I)-based Dye Sensitized Solar Cells. Chem. Soc. Rev. 2015, 44, 8386–8398. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Safin, D.A.; Frost, J.; Murugesu, M. The Renaissance of 2,4,6-tris(2-pyrimidyl)-1,3,5-triazine (TPymT) Coordination Chemistry. Dalton Trans. 2015, 44, 20287–20294. [Google Scholar] [CrossRef]
- Case, F.H.; Koft, E.J. The Synthesis of Certain Substituted 1,3,5-Triazines Containing the Ferroin Group. Am. Chem. Soc. 1959, 81, 905–906. [Google Scholar] [CrossRef]
- Liu, X.H.; Krott, M.; Müller, P.; Hu, C.H.; Lueken, H.; Dronskowski, R. Synthesis, Crystal Structure, and Properties of MnNCN, the First Carbodiimide of a Magnetic Transition Metal. Inorg. Chem. 2005, 44, 3001–3003. [Google Scholar] [CrossRef]
- Liao, W.P.; Hu, C.H.; Kremer, R.K.; Dronskowski, R. Formation of Complex Three- and One-dimensional Interpenetrating Networks Within Carbodiimide Chemistry: NCN2−-Coordinated Rare-Earth-Metal Tetrahedra and Condensed Alkali-metal Iodide Octahedra in Two Novel Lithium Europium Carbodiimide Iodides, LiEu2(NCN)I3 and LiEu4(NCN)3I3. Inorg. Chem. 2004, 43, 5884–5890. [Google Scholar] [PubMed]
- Liao, W.P.; Dronskowski, R. Crystal Structures of Extended Europium Cyanamide−Carbodiimide Compounds Derived from Different Reaction Conditions: Temperature-Controlled Syntheses of In0.08Eu4(NCN)3I3, Eu8I9(CN)(NCN)3, and In0.28Eu12(NCN)5I14.91. Inorg. Chem. 2006, 45, 3828–3830. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, R.; Ströbele, M.; Meyer, H. Chains of [RE6] Octahedra Coupled by (NCN) Links in the Network Structure of RE2Cl(CN2)N. Synthesis and Structure of Two Novel Rare Earth Chloride Carbodiimide Nitrides with Structures Related to the RE2Cl3 Type. J. Inorg. Chem. 2003, 42, 3406–3411. [Google Scholar] [CrossRef] [PubMed]
- Gütlich, P.; Carcia, Y.; Goodwin, H.A. Spin Crossover Phenomena in Fe(II) Complexes: Dedicated to Professor F. A. Cotton on Occasion of his 70th Birthday. Chem. Soc. Rev. 2000, 29, 419–427. [Google Scholar] [CrossRef]
- Gütlich, P.; Goodwin, H.A. Spin Crossover in Transition Metal Compounds I; Springer: Berlin/Heidelberg, Germany; New York, NY, USA, 2004; pp. 1–342. [Google Scholar]
- Gaspar, A.B.; Ksenofontov, V.; Seredyuk, M.; Gütlich, P. Multifunctionality in Spin Crossover Materials. Coord. Chem. Rev. 2005, 249, 2661–2676. [Google Scholar]
- Yu, F.; Xiang, M.; Li, A.H.; Zhang, Y.M.; Li, B. Structural Diversities and Magnetic Properties of Azide-containing Coordination Polymers Based on Flexible Tetra-Pyridinate Ligands. CrystEngComm 2015, 17, 1556–1563. [Google Scholar] [CrossRef]
- Ershova, I.V.; Bogomyakov, A.S.; Rumyantsev, R.V.; Fukin, G.K.; Piskunov, A.V. Pentacoordinated Manganese(III) Bis-o-iminobenzosemiquinonates: Looking for Spin-crossover Phenomenon. J. Mol. Struct. 2021, 1225, 129092. [Google Scholar] [CrossRef]
- Ershova, I.V.; Bogomyakov, A.S.; Kubrin, S.P.; Cherkasov, A.V.; Piskunov, A.V. Iron(III) Complexes Based on N-Benzylidene-2-Hydroxy-3,5-Di-tert-Butylaniline. Russ. J. Coord. Chem. 2021, 47, 1–9. [Google Scholar] [CrossRef]
- Vasilchenko, D.B.; Zadesenets, A.V.; Baidina, I.A.; Piryazev, D.A.; Romanenko, G.V. Crystal Structures of Dis-diiododiammine Platinum and Trans-diazidodiammine Platinum. J. Struct. Chem. 2017, 58, 1689–1692. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SADABS, Program for Empirical Absorption Correction of Area Detector Data; ScienceOpen: Burlington, VT, USA, 1996. [Google Scholar]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef] [Green Version]
- Spackman, M.A.; Jayatilaka, D. Hirshfeld Surface Analysis. CrystEngComm 2009, 11, 19–32. [Google Scholar] [CrossRef]
- Hirshfeld, F.L. Bonded-Atom Fragments for Describing Molecular Charge Densities. Theor. Chim. Acta 1977, 44, 129–138. [Google Scholar] [CrossRef]
- Turner, M.J.; McKinnon, J.J.; Wolff, S.K.; Grimwood, D.J.; Spackman, P.R.; Jayatilaka, D.; Spackman, M.A. Crystal Explorer17; University of Western Australia: Crawley, Australia, 2017; Available online: https://crystalexplorer.net/download/ (accessed on 30 July 2019).
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. GAUSSIAN 09; Revision A02; Gaussian Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Glendening, E.D.; Reed, A.E.; Carpenter, J.E.; Weinhold, F. NBO; Version 3.1, CI; University of Wisconsin: Madison, WI, USA, 1998. [Google Scholar]
- Chai, J.D.; Head-Gordon, M. Long-range corrected hybrid density functionals with damped atom–atom dispersion corrections. Phys. Chem. Chem. Phys. 2008, 10, 6615–6620. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bader, R.F.W. Atoms in Molecules: A Quantum Theory; Oxford University Press: Oxford, UK, 1990. [Google Scholar]
- Lu, T.; Chen, F. Multiwfn: A Multifunctional Wavefunction Analyzer. J. Comp. Chem. 2012, 33, 580–592. [Google Scholar] [CrossRef] [PubMed]
- Addison, A.W.; Rao, T.N.; Reedijk, J.; Rijn, J.V.; Verschoor, G.C. Synthesis, Structure, and Spectroscopic Properties of Copper(II) Compounds Containing Nitrogen–Sulphur Donor Ligands; The Crystal and Molecular Structure of Aqua [1,7-bis(N-methylbenzimidazol-2′-yl)-2,6-dithiaheptane]copper(II) Perchlorate. J. Chem. Soc. Dalton Trans. 1984, 7, 1349–1356. [Google Scholar] [CrossRef]
- Evans, L.B.; Yofee, A.D.; Gray, P. Physics and Chemistry of the Inorganic Azides. Chem. Rev. 1959, 59, 515–568. [Google Scholar] [CrossRef]
- Pauling, L. The Natural of the Chemical Bond; Cornell University Press: Ithaca, NY, USA, 1967. [Google Scholar]
- Matta, C.F.; Hernandez-Trujillo, J.; Tang, T.-H.; Bader, R.F.W. Hydrogen–Hydrogen Bonding: A Stabilizing Interaction in Molecules and Crystals. Chem. Eur. J. 2003, 9, 1940–1951. [Google Scholar] [CrossRef]
- Grabowski, S.J.; Pfitzner, A.; Zabel, M.; Dubis, A.T.; Palusiak, M.; Intramolecular, H.H. Interactions for the Crystal Structures of [4-((E)-But-1-enyl)-2,6-dimethoxyphenyl]pyridine-3-carboxylate and [4-((E)-Pent-1-enyl)-2,6-dimethoxyphenyl]pyridine-3-carboxylate; DFT Calculations on Modeled Styrene Derivatives. J. Phys. Chem. B 2004, 108, 1831–1837. [Google Scholar] [CrossRef]
- Matta, C.F.; Castillo, N.; Boyd, R.J. Characterization of a Closed-Shell Fluorine−Fluorine Bonding Interaction in Aromatic Compounds on the Basis of the Electron Density. J. Phys. Chem. A 2005, 109, 3669–3681. [Google Scholar] [CrossRef]
- Pendás, A.M.; Francisco, E.; Blanco, M.A.; Gatti, C. Bond Paths as Privileged Exchange Channels. Chem. Eur. J. 2007, 13, 9362–9371. [Google Scholar] [CrossRef]
- Gibbs, G.V.; Cox, D.F.; Crawford, T.D.; Rosso, K.M.; Ross, N.L.; Downs, R.T. Classification of Metal-oxide Bonded Interactions Based on Local potential and Kinetic-energy Densities. J. Chem. Phys. 2006, 124, 084704. [Google Scholar] [CrossRef] [PubMed]
- Dinda, S.; Samuelson, A.G. The Nature of Bond Critical Points in Dinuclear Copper(I) Complexes. Chem. Eur. J. 2012, 18, 3032–3042. [Google Scholar] [CrossRef] [PubMed]
- Cremer, D.; Kraka, E. Chemical Bonds without Bonding Electron Density—Does the Difference Electron-Density Analysis Suffice for a Description of the Chemical Bond? Angew. Chem. Int. Ed. Engl. 1984, 23, 627–628. [Google Scholar] [CrossRef]
- Jenkins, V.; Morrison, I. The Chemical Character of The Intermolecular Bonds of Seven Phases of Ice as Revealed by AB initio Calculation of Electron Densities. Chem. Phys. Lett. 2000, 317, 97–102. [Google Scholar] [CrossRef]
- Espinosa, E.; Alkorta, I.; Elguero, J.; Molins, E. From Weak to Strong interactions: A Comprehensive Analysis of the Topological and Energetic Properties of the Electron Density Distribution Involving X–H…F–Y Systems. J. Chem. Phys. 2002, 117, 5529–5542. [Google Scholar] [CrossRef]
- Varadwaj, P.R.; Marques, H.M. The Physical Chemistry of Coordinated Aqua-, Ammine-, and Mixed-ligand Co2+ Complexes: DFT Studies on the Structure, Energetics, and Topological Properties of the Electron Eensity. Phys. Chem. Chem. Phys. 2010, 12, 2126–2138. [Google Scholar] [CrossRef]



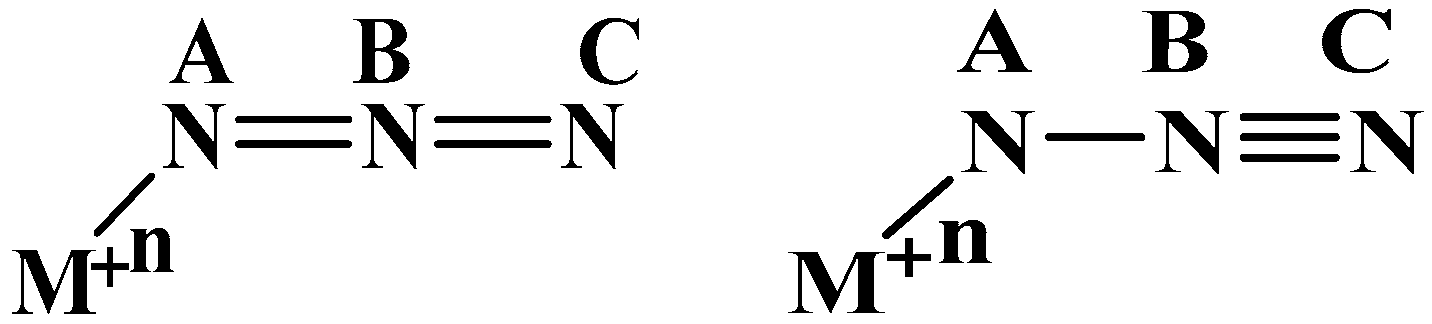
| Compound | 1 | 2 |
|---|---|---|
| Empirical formula | C30H39CoN9O5 | C12H11CuN7O2 |
| Fw | 664.63 | 348.82 |
| T (K) | 293(2) K | 100(2) K |
| λ (Å) | 0.71073 Å | 0.71073 Å |
| cryst syst | Monoclinic | Monoclinic |
| Space group | P21/c | P21/n |
| a (Å) | 11.3305(7) | 6.8277(2) |
| b (Å) | 11.3887(15) | 10.2203(2) |
| c (Å) | 25.9243(16) | 18.8056(6) |
| β (°) | 101.672(2) | 94.700(2) |
| V (Å3) | 3276.1(5) | 1307.86(6) |
| Z | 4 | 4 |
| ρcalc (Mg/m3) | 1.347 Mg/m3 | 1.772 Mg/m3 |
| μ (Mo Kα) (mm−1) | 0.576 mm−1 | 1.690 mm−1 |
| F(000) | 1396 | 708 |
| θ-range | 2.180 to 26.341° | 2.173 to 32.498° |
| No. reflns. | 7685 | 28,102 |
| Unique reflns. | 4249 [R(int) = 0.0414] | 4745 [R(int) = 0.0310] |
| Completeness to theta = 25.242° | 64.10% | 100.00% |
| GOOF (F2) | 1.037 | 1.032 |
| Final R indices [I > 2sigma(I)] | R1 = 0.0392, wR2 = 0.0930 | R1 = 0.0229, wR2 = 0.0635 |
| R indices (all data) | R1 = 0.0754, wR2 = 0.1144 | R1 = 0.0264, wR2 = 0.0657 |
| CCDC | 2,158,205 | 2,158,206 |
| Bond | Bond Length | Bond | Bond Length |
| Co(1)-O(1) | 2.091(2) | Co(1)-N(6) | 2.181(4) |
| Co(1)-N(1) | 2.102(3) | Co(1)-N(7) | 2.198(3) |
| Co(1)-N(4) | 2.160(4) | Co(1)-N(5) | 2.211(3) |
| Bonds | Angle | Bonds | Angle |
| O(1)-Co(1)-N(1) | 178.79(16) | N(1)-Co(1)-N(7) | 89.77(11) |
| O(1)-Co(1)-N(4) | 88.80(12) | N(4)-Co(1)-N(7) | 91.54(14) |
| N(1)-Co(1)-N(4) | 90.50(15) | N(6)-Co(1)-N(7) | 87.04(14) |
| O(1)-Co(1)-N(6) | 91.72(12) | O(1)-Co(1)-N(5) | 89.67(9) |
| N(1)-Co(1)-N(6) | 88.96(15) | N(1)-Co(1)-N(5) | 91.35(11) |
| N(4)-Co(1)-N(6) | 178.48(10) | N(4)-Co(1)-N(5) | 93.06(13) |
| O(1)-Co(1)-N(7) | 89.27(9) | N(6)-Co(1)-N(5) | 88.37(14) |
| O(5)-Co(1)-N(7) | 175.25(10) |
| D-H…A | d(D-H) | d(H…A) | <DHA | d(D…A) | Symmetry Code |
|---|---|---|---|---|---|
| O1-H1A…O3 | 0.853 | 2.568 | 133.64 | 3.216 | |
| O1-H1A…O4 | 0.853 | 1.909 | 170.04 | 2.753 | |
| O1-H2A…O2 | 0.807 | 1.859 | 178.01 | 2.665 | [x + 1, y, z] |
| O2-H3A…N8 | 0.819 | 1.973 | 173.17 | 2.788 | |
| O2-H4A…O3 | 0.866 | 1.953 | 167.15 | 2.803 | [−x + 1, y − 1/2, −z + 1/2] |
| O2-H4A…O5 | 0.866 | 2.611 | 135.83 | 3.288 | [−x + 1, y − 1/2, −z + 1/2] |
| C7-H7…N1 | 0.93 | 2.483 | 124.23 | 3.103 | |
| C10-H10…O5 | 0.93 | 2.513 | 139.78 | 3.278 | [−x + 1, y − 1/2, −z + 1/2] |
| C11-H11…O1 | 0.93 | 2.496 | 121.54 | 3.086 | |
| C17-H17…N1 | 0.93 | 2.468 | 120.95 | 3.052 | |
| C25-H25…O3 | 0.93 | 2.658 | 158.25 | 3.538 | [x − 1, y, z] |
| C28-H28…O4 | 0.93 | 2.584 | 168.86 | 3.501 | |
| C29-H29…O5 | 0.93 | 2.586 | 139.48 | 3.348 | [−x + 1, y − 1/2, −z + 1/2] |
| Bond | Bond Length | Bond | Bond length |
| Cu(1)-N(4) | 1.9946(9) | Cu(1)-N(3) | 2.0480(9) |
| Cu(1)-N(2) | 1.9967(9) | Cu(1)-N(5) | 2.2158(10) |
| Cu(1)-N(1) | 2.0341(9) | ||
| Bonds | Angle | Bonds | Angle |
| N(4)-Cu(1)-N(2) | 90.94(4) | N(1)-Cu(1)-N(3) | 106.57(4) |
| N(4)-Cu(1)-N(1) | 170.83(4) | N(4)-Cu(1)-N(5) | 90.34(4) |
| N(2)-Cu(1)-N(1) | 80.57(4) | N(2)-Cu(1)-N(5) | 101.56(4) |
| N(4)-Cu(1)-N(3) | 79.98(4) | N(1)-Cu(1)-N(5) | 94.74(4) |
| N(2)-Cu(1)-N(3) | 156.74(4) | N(3)-Cu(1)-N(5) | 99.88(4) |
| D-H | d(D-H) | d(H…A) | <DHA | d(D…A) | Symmetry Code |
|---|---|---|---|---|---|
| O1-H1…O2 | 0.84(3) | 1.61(3) | 2.4431(13) | 174(4) | |
| C3-H3…O2 | 0.95 | 2.54 | 3.3761(15) | 148 | −1/2 + x, 3/2 − y, 1/2 + z |
| C7-H7…N7 | 0.95 | 2.57 | 3.3751(15) | 143 | 1 − x, 2 − y, −z |
| C9-H9…O1 | 0.95 | 2.35 | 3.2252(14) | 152 | x, 1 + y, z |
| C10-H10…N5 | 0.95 | 2.54 | 3.2872(15) | 136 | 1/2 − x, 1/2 + y, 1/2 − z |
| C11-H11…N7 | 0.95 | 2.51 | 3.2984(15) | 141 | 1 − x, 2 − y, −z |
| C12-H12…N6 | 0.95 | 2.62 | 3.4037(15) | 140 | 1/2 − x, −1/2 + y, 1/2 − z |
| Contact | Distance | Contact | Distance | Contact | Distance |
|---|---|---|---|---|---|
| 1 | 2 | ||||
| N3…H24B | 2.498 | O3…H25 | 2.517 | C3…C8 | 3.325 |
| N3…H4 | 2.591 | O2…H2A | 1.682 | N7…H7 | 2.461 |
| N9…H1A | 2.445 | O3…H4A | 1.839 | N7…H11 | 2.406 |
| H13…C25 | 2.684 | O5…H4A | 2.529 | N7…H2B | 2.521 |
| H3A…C25 | 2.676 | O5…H12C | 2.513 | N6…H12 | 2.519 |
| H20…C9 | 2.749 | O5…H10 | 2.397 | N5…H10 | 2.442 |
| O4…H1A | 1.781 | O5…H29 | 2.472 | O2…H3 | 2.424 |
| O3…H1A | 2.482 | O4…H28 | 2.453 | O2…H4 | 2.531 |
| O1…H9 | 2.236 | ||||
| Bond | dN-N | Δd | ρ(r), a.u | ρ(r) a | V(r)/G(r) b |
|---|---|---|---|---|---|
| Complex 1 | |||||
| NA-NB | 1.189(6) | 0.04 | 0.4847 | −1.3846 | 2.884 |
| NB-NC | 1.149(7) | 0.5424 | −1.4216 | 2.670 | |
| Complex 2 | |||||
| NA-NB | 1.192(1) | 0.02 | 0.4833 | −1.2346 | 2.743 |
| NB-NC | 1.172(1) | 0.5105 | −1.2270 | 2.623 |
| Bond | Bond Length | ρ(r); a.u. | H(r) a; a.u. | V(r)/G(r) b | ρ(r) c |
|---|---|---|---|---|---|
| Complex 1 | |||||
| Co1-O1 | 2.091(2) | 0.0401 | 0.0023 | 0.974 | 0.3519 |
| Co1-N1 | 2.102(3) | 0.0616 | −0.0088 | 1.104 | 0.3040 |
| Co1-N4 | 2.159(4) | 0.0419 | 0.0008 | 0.991 | 0.3589 |
| Co1-N5 | 2.211(3) | 0.0360 | 0.0017 | 0.975 | 0.2868 |
| Co1-N6 | 2.181(4) | 0.0396 | 0.0008 | 0.991 | 0.3380 |
| Co1-N7 | 2.197(3) | 0.0377 | 0.0013 | 0.982 | 0.2989 |
| Complex 1 | |||||
| Cu1-N1 | 2.034(1) | 0.0553 | −0.0050 | 1.041 | 0.4637 |
| Cu1-N2 | 1.997(1) | 0.0588 | −0.0043 | 1.032 | 0.5258 |
| Cu1-N3 | 2.048(1) | 0.0552 | −0.0049 | 1.042 | 0.4513 |
| Cu1-N4 | 1.994(1) | 0.0798 | −0.0191 | 1.175 | 0.3597 |
| Cu1-N5 | 2.216(1) | 0.0381 | 0.0000 | 1.000 | 0.2947 |
| 1 | 2 | ||
|---|---|---|---|
| Co | 0.9623 | Cu | 0.7655 |
| 4-Pic | 0.5804 | HAld | 0.3354 |
| H2O | 0.1278 | Ald− | −0.7207 |
| N3− | −0.7138 | N3− | −0.7207 |
| NO3− | −0.9567 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Altowyan, M.S.; Albering, J.H.; Barakat, A.; Soliman, S.M.; Abu-Youssef, M.A.M. Synthesis, Supramolecular Structural Investigations of Co(II) and Cu(II) Azido Complexes with Pyridine-Type Ligands. Crystals 2023, 13, 346. https://doi.org/10.3390/cryst13020346
Altowyan MS, Albering JH, Barakat A, Soliman SM, Abu-Youssef MAM. Synthesis, Supramolecular Structural Investigations of Co(II) and Cu(II) Azido Complexes with Pyridine-Type Ligands. Crystals. 2023; 13(2):346. https://doi.org/10.3390/cryst13020346
Chicago/Turabian StyleAltowyan, Mezna Saleh, Jörg H. Albering, Assem Barakat, Saied M. Soliman, and Morsy A. M. Abu-Youssef. 2023. "Synthesis, Supramolecular Structural Investigations of Co(II) and Cu(II) Azido Complexes with Pyridine-Type Ligands" Crystals 13, no. 2: 346. https://doi.org/10.3390/cryst13020346







