Ion-Beam Synthesis of Structure-Oriented Iron Nanoparticles in Single-Crystalline Rutile TiO2
Abstract
1. Introduction
2. Experimental Part
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dietl, T. Spintronics and ferromagnetism in wide-band-gap semiconductors. AIP Conf. Proc. 2005, 772, 56–64. [Google Scholar] [CrossRef]
- Chambers, S.A. Ferromagnetism in doped thin-film oxide and nitride semiconductors and dielectrics. Surf. Sci. Rep. 2006, 61, 345–381. [Google Scholar] [CrossRef]
- Hirohata, A.; Yamada, K.; Nakatani, Y.; Prejbeanu, I.-L.; Diény, B.; Pirro, P.; Hillebrands, B. Review on spintronics: Principles and device applications. J. Magn. Magn. Mater. 2020, 509, 166711. [Google Scholar] [CrossRef]
- Gupta, A.; Zhang, R.; Kumar, P.; Kumar, V.; Kumar, A. Nano-Structured Dilute Magnetic Semiconductors for Efficient Spintronics at Room Temperature. Magnetochemistry 2020, 6, 15. [Google Scholar] [CrossRef]
- Coey, J.M.D.; Chambers, S.A. Oxide Dilute Magnetic Semiconductors—Fact or Fiction? MRS. Bull. 2011, 33, 1053–1058. [Google Scholar] [CrossRef]
- Ryssel, H.; Ruge, I. Ion Implantation; Wiley: Chichester, UK, 1986. [Google Scholar]
- Potzger, K. Ion-beam synthesis of magnetic semiconductors. Nucl. Instruments Methods Phys. Res. Sect. B Beam Interact. Mater. Atoms 2012, 272, 78–87. [Google Scholar] [CrossRef]
- Afify, N.; Abbady, G.; Hamad, D.; Abdelbaki, R.F.; Yousef, E.S.; Shaaban, E.R.; Abd-el Salam, M.N. The effective role of dilute Co on SnO2 nanoparticles: Structural, optical and magnetic characterization properties for spintronics. Sens. Actuator Phys. 2021, 331, 112984. [Google Scholar] [CrossRef]
- Morozov, I.G.; Belousova, O.V.; Blanco-Andujar, C.; Ortega, D.; Kuznetsov, M.V. Structural, optical, magnetic, and XPS properties of SnOx nanoparticles. Solid State Sci. 2022, 126, 106854. [Google Scholar] [CrossRef]
- Ahsan, H.F.; Lal, K.; Saleem, M.; Mustafa, G.M.; Khan, M.A.; Haidyrah, A.S.; Atiq, S. Tuning the dielectric behavior and energy storage properties of Mn/Co co-doped ZnO. Mater. Sci. Semicond. Process. 2021, 134, 105977. [Google Scholar] [CrossRef]
- Naseem, S.; Pinchuk, I.V.; Luo, Y.K.; Kawakami, R.K.; Khan, S.; Husain, S.; Khan, W. Epitaxial growth of cobalt doped TiO2 thin films on LaAlO3 substrate by molecular beam epitaxy and their opto-magnetic based applications. Appl. Surf. Sci. 2019, 493, 691–702. [Google Scholar] [CrossRef]
- Waseem, S.; Anjuma, S.; Zeeshan, T.; Mustafa, L.; Kayani, Z.N.; Majid, F.; Warsi, A.; Shah, S.I. Pulsed laser deposited Cobalt doped Ti0.9Fe0.1-xO2 thin films: Structural, morphological, magnetic, optical and electrical properties. Ceram. Int. 2021, 47, 8555–8565. [Google Scholar] [CrossRef]
- Chen, H.; Zhang, Y.; Wu, Z.; Qin, Z.; Wu, S.; Hu, W. Substrate temperature dependence of chemical state and magnetoresistance characteristics of Co–TiO2 nanocomposite films. Trans. Nonferrous Met. Soc. China 2020, 30, 2502–2509. [Google Scholar] [CrossRef]
- Ho, J.; de Boer, T.; Braun, P.M.; Leedahl, B.; Manikandan, D.; Murugan, R.; Moewes, A. Origin and control of room temperature ferromagnetism in Co,Zn-doped SnO2: Oxygen vacancies and their local environment. J. Mater. Chem. C 2020, 8, 4902–4908. [Google Scholar] [CrossRef]
- Garnet, N.S.; Ghodsi, V.; Hutfluss, L.N.; Yin, P.; Hegde, M.; Radovanovic, P.V. Probing the Role of Dopant Oxidation State in the Magnetism of Diluted Magnetic Oxides Using Fe-Doped In2O3 and SnO2 Nanocrystals. J. Phys. Chem. C 2017, 121, 1918–1927. [Google Scholar] [CrossRef]
- Jiang, F.; Chen, D.; Zhou, G.; Wang, Y.; Xu, X. The dramatic enhancement of ferromagnetism and band gap in Fe-doped In2O3 nanodot arrays. Sci. Rep. 2018, 8, 2417. [Google Scholar] [CrossRef] [PubMed]
- Dhamodaran, M.; Karuppannan, R.; Murugan, R.; Boukhvalov, D.W.; Pandian, M.S.; Perumalsamy, R. Morphology controlled synthesis of Fe and Mn co-doped In2O3 nanocubes and their Dopant-Atom effects on electronic structure and magnetic properties. J. Magn. Magn. Mater. 2022, 560, 169547. [Google Scholar] [CrossRef]
- Matsumoto, Y.; Murakami, M.; Shono, T.; Hasegawa, T.; Fukuruma, T.; Kawasaki, M.; Ahmet, P.; Chikyow, T.; Koshihara, S.; Koinuma, H. Room-Temperature Ferromagnetism in Transparent Transition Metal-Doped Titanium Dioxide. Science 2001, 291, 854–856. [Google Scholar] [CrossRef]
- Dietl, T. A ten-year perspective on dilute magnetic semiconductors and oxides. Nat. Mater. 2010, 9, 965–974. [Google Scholar] [CrossRef]
- Yakout, S.M. Room Temperature Ferromagnetism: Nonmagnetic Semiconductor Oxides and Nonmagnetic Dopants. J. Electron. Mater. 2021, 50, 1922–1941. [Google Scholar] [CrossRef]
- Vokoun, D.; Svatuška, M.; Olejníček, J.; Kohout, M.; Drahokoupil, J.; Rameš, M.; Vejpravová, J.; Mantlíková, A.; Fekete, L.; Kopeček, J.; et al. Ni–TiO2 nanocomposite films and their magnetic properties. Phys. B Condens. Matter. 2016, 503, 44–50. [Google Scholar] [CrossRef]
- Darapaneni, P.; Moura, N.S.; Harry, D.; Cullen, D.A.; Dooley, K.M.; Dorman, J.A. Effect of Moisture on Dopant Segregation in Solid Hosts. J. Phys. Chem. C 2019, 123, 12234–12241. [Google Scholar] [CrossRef]
- Vokoun, D.; Vronka, M.; Rameš, M.; Olejníček, J.; Kohout, M.; Heczko, O. Ni nanoparticles in TiO2 films and their magnetic properties. Physica B Condens. Matter. 2020, 578, 411862. [Google Scholar] [CrossRef]
- Saadaoui, H.; Luo, X.; Salman, Z.; Cui, X.Y.; Bao, N.N.; Bao, P.; Zheng, R.K.; Tseng, L.T.; Du, Y.H.; Prokscha, T.; et al. Intrinsic Ferromagnetism in the Diluted Magnetic Semiconductor Co:TiO2. Phys. Rev. Lett. 2016, 117, 227202. [Google Scholar] [CrossRef]
- Zhang, H.; Huang, W.; Lin, R.; Wang, Y.; Long, B.; Hu, Q.; Wu, Y. Room temperature ferromagnetism in pristine TiO2 nanoparticles triggered by singly ionized surface oxygen vacancy induced via calcining in different air pressure. J. Alloy Compd. 2021, 860, 157913. [Google Scholar] [CrossRef]
- Joshi, S.R.; Padmanabhan, B.; Chanda, A.; Shukla, N.; Malik, V.K.; Kanjilal, D.; Verma, S. Anisotropic super-paramagnetism in cobalt implanted rutile-TiO2 single crystals. J. Magn. Magn. Mater. 2018, 465, 122–127. [Google Scholar] [CrossRef]
- Shutthanandan, V.; Thevuthasan, S.; Heald, S.M.; Droubay, T.; Engelhard, M.H.; Kaspar, T.C.; McCready, D.E.; Saraf, L.; Chambers, S.A. Room-temperature ferromagnetism in ion-implanted Co-doped TiO2 rutile. Appl. Phys. Lett. 2004, 84, 4466–4468. [Google Scholar] [CrossRef]
- Luk, W.Y.; Wong, S.P.; Ke, N.; Li, Q.; Lindner, J.K.N. Structural and magnetic properties of cobalt implanted TiO2 thin films. Proc. SPIE 2006, 6415, 162–170. [Google Scholar] [CrossRef]
- Zhu, S.; Wang, L.M.; Zu, X.T.; Xiang, X. Optical and magnetic properties of Ni nanoparticles in rutile formed by Ni ion implantation. Appl. Phys. Lett. 2006, 88, 43107. [Google Scholar] [CrossRef]
- Zhou, S.; Talut, G.; Potzger, K.; Shalimov, A.; Grenzer, J.; Skorupa, W.; Helm, M.; Fassbender, J.; Čižmár, E.; Zvyagin, S.A.; et al. Crystallographically oriented Fe nanocrystals formed in Fe-implanted TiO2. J. Appl. Phys. 2008, 103, 83907. [Google Scholar] [CrossRef]
- Cheng, F.; Ding, B.; Pan, F.; Yao, S.; Potzger, K.; Zhou, S. Investigation on the structural and magnetic properties of Co+ implanted rutile TiO2. Nucl. Instruments Methods Phys. Res. Sect. B Beam Interact. Mater. Atoms. 2012, 286, 180–183. [Google Scholar] [CrossRef]
- Ding, B.; Cheng, F.; Pan, F.; Fa, T.; Yao, S.; Potzger, K.; Zhou, S. The correlation between structure and magnetism of Ni-implanted TiO2 annealed at different temperatures. J. Magn. Magn. Mater. 2012, 324, 33–36. [Google Scholar] [CrossRef]
- Cruz, M.M.; da Silva, R.C.; Pinto, J.V.; Borges, R.P.; Franco, N.; Casaca, A.; Alves, E.; Godinho, M. Formation of oriented nickel aggregates in rutile single crystals by Ni implantation. J. Magn. Magn. Mater. 2013, 340, 102–108. [Google Scholar] [CrossRef]
- Silva, C.; Costa, A.R.G.; da Silva, R.C.; Alves, L.C.; Ferreira, L.P.; Carvalho, M.D.; Franco, N.; Godinho, M.; Cruz, M.M. Magnetic and electrical characterization of TiO2 single crystals co-implanted with iron and cobalt. J. Magn. Magn. Mater. 2014, 364, 106–116. [Google Scholar] [CrossRef]
- Cortie, D.L.; Khaydukov, Y.; Keller, T.; Sprouster, D.J.; Hughes, J.S.; Sullivan, J.P.; Wang, X.L.; Le Brun, A.P.; Bertinshaw, J.; Callori, S.J.; et al. Enhanced Magnetization of Cobalt Defect Clusters Embedded in TiO2−δ Films. ACS Appl. Mater. Interfaces 2017, 9, 8783–8795. [Google Scholar] [CrossRef] [PubMed]
- Khaibullin, R.I.; Tagirov, L.R.; Rameev, B.Z.; Ibragimov, S.Z.; Yildiz, F.; Aktaş, B. High Curie-temperature ferromagnetism in cobalt-implanted single-crystalline rutile. J. Phys. Condens. Matter. 2004, 16, L443. [Google Scholar] [CrossRef]
- Khaibullin, R.I.; Ibragimov, S.Z.; Tagirov, L.R.; Popok, V.N.; Khaibullin, I.B. Formation of anisotropic ferromagnetic response in rutile (TiO2) implanted with cobalt ions. Nucl. Instruments Methods Phys. Res. Sect. B Beam Interact. Mater. Atoms. 2007, 257, 369–373. [Google Scholar] [CrossRef]
- Akdogan, N.; Nefedov, A.; Zabel, H.; Westerholt, K.; Becker, H.-W.; Somsen, C.; Gök, Ş.; Bashir, A.; Khaibullin, R.; Tagirov, L. High-temperature ferromagnetism in Co-implanted TiO2 rutile. J. Phys. D Appl. Phys. 2009, 42, 115005. [Google Scholar] [CrossRef]
- Achkeev, A.A.; Khaibullin, R.I.; Tagirov, L.R.; Mackova, A.; Hnatowicz, V.; Cherkashin, N. Specific features of depth distribution profiles of implanted cobalt ions in rutile TiO2. Phys. Solid State 2011, 53, 543–553. [Google Scholar] [CrossRef]
- Okay, C.; Vakhitov, I.R.; Valeev, V.F.; Khaibullin, R.I.; Rameev, B. Magnetic Resonance Study of Fe-Implanted TiO2 Rutile. Appl. Magn. Reson. 2017, 48, 347–360. [Google Scholar] [CrossRef]
- Vakhitov, I.R.; Lyadov, N.M.; Valeev, V.F.; Nuzhdin, V.I.; Tagirov, L.R.; Khaibullin, R.I. Effects of nickel ions implantation and subsequent thermal annealing on structural and magnetic properties of titanium dioxide. J. Phys. Conf. Ser. 2014, 572, 012048. [Google Scholar] [CrossRef]
- Vakhitov, I.R.; Shemukhin, A.A.; Gumarov, A.I.; Lyadov, N.M.; Nuzhdin, V.I.; Faizrakhmanov, I.A.; Okay, C.; Rameev, B.Z.; Tagirov, L.R.; Khaibullin, R.I. Structural and magnetic studies of TiO2 rutile implanted with vanadium ions. Mater. Res. Express 2019, 6, 116103. [Google Scholar] [CrossRef]
- Fairley, N.; Fernandez, V.; Richard-Plouet, M.; Guillot-Deudon, C.; Walton, J.; Smith, E.; Flahaut, D.; Greiner, M.; Biesinger, M.; Tougaard, S.; et al. Systematic and collaborative approach to problem solving using X-ray photoelectron spectroscopy. Appl. Surf. Sci. Adv. 2021, 5, 100112. [Google Scholar] [CrossRef]
- Was, G.S.; Taller, S.; Jiao, Z.; Monterrosa, A.M.; Woodley, D.; Jennings, D.; Kubley, T.; Naab, F.; Toader, O.; Uberseder, E. Resolution of the carbon contamination problem in ion irradiation experiments. Nucl. Instruments Methods Phys. Res. Sect. B Beam Interact. Mater. Atoms. 2017, 412, 58–65. [Google Scholar] [CrossRef]
- Drouin, D.; Couture, A.R.; Joly, D.; Tastet, X.; Aimez, V.; Gauvin, R. CASINO V2.42—A Fast and Easy-to-use Modeling Tool for Scanning Electron Microscopy and Microanalysis Users. Scanning 2007, 29, 92–101. [Google Scholar] [CrossRef] [PubMed]
- Ziegler, J.F.; Ziegler, M.D.; Biersack, J.P. SRIM—The stopping and range of ions in matter (2010). Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. Atoms. 2010, 268, 1818–1823. [Google Scholar] [CrossRef]
- NIST X-ray Photoelectron Spectroscopy Database, NIST Standard Reference Database Number 20; National Institute of Standards and Technology: Gaithersburg, MD, USA, 2000. [CrossRef]
- Bean, C.P.; Livingston, J.D. Superparamagnetism. J. Appl. Phys. 1959, 30, S120–S129. [Google Scholar] [CrossRef]
- Majetich, S.A.; Sachan, M. Magnetostatic interactions in magnetic nanoparticle assemblies: Energy, time and length scales. J. Phys. D Appl. Phys. 2006, 39, R407–R422. [Google Scholar] [CrossRef]
- Kittel, C. Introduction to Solid State Physics; Wiley: New York, NY, USA, 1976. [Google Scholar]
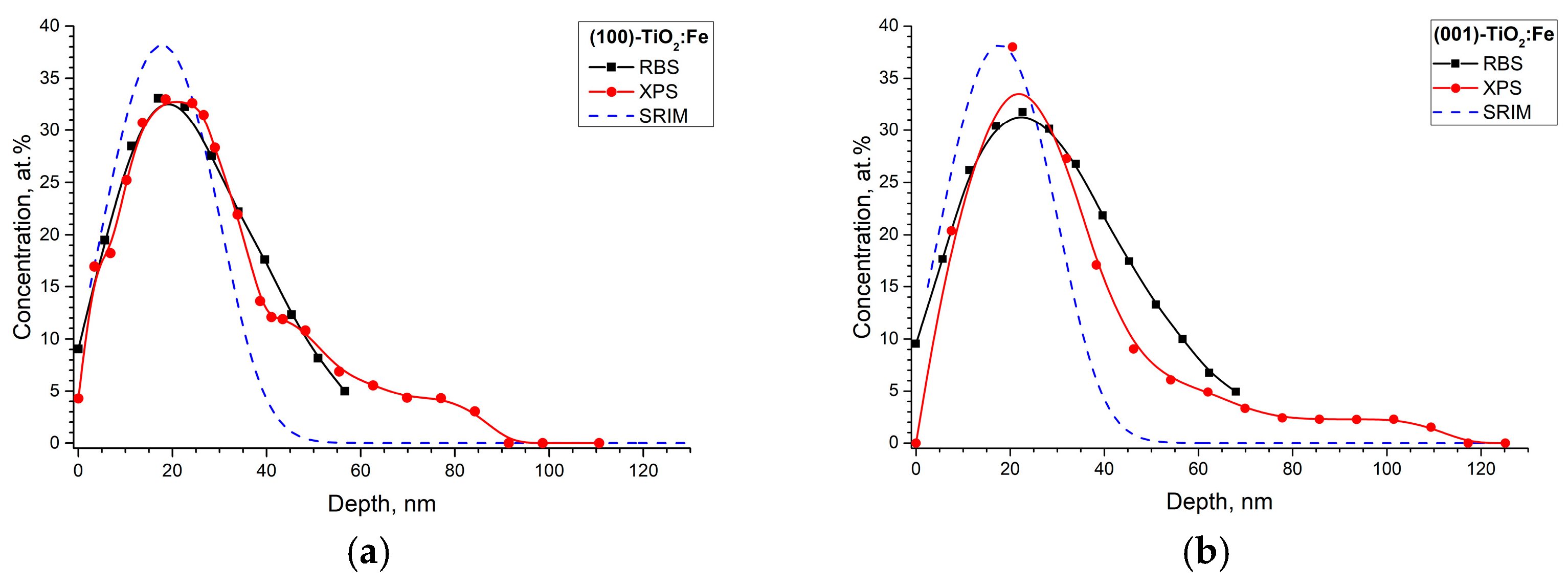
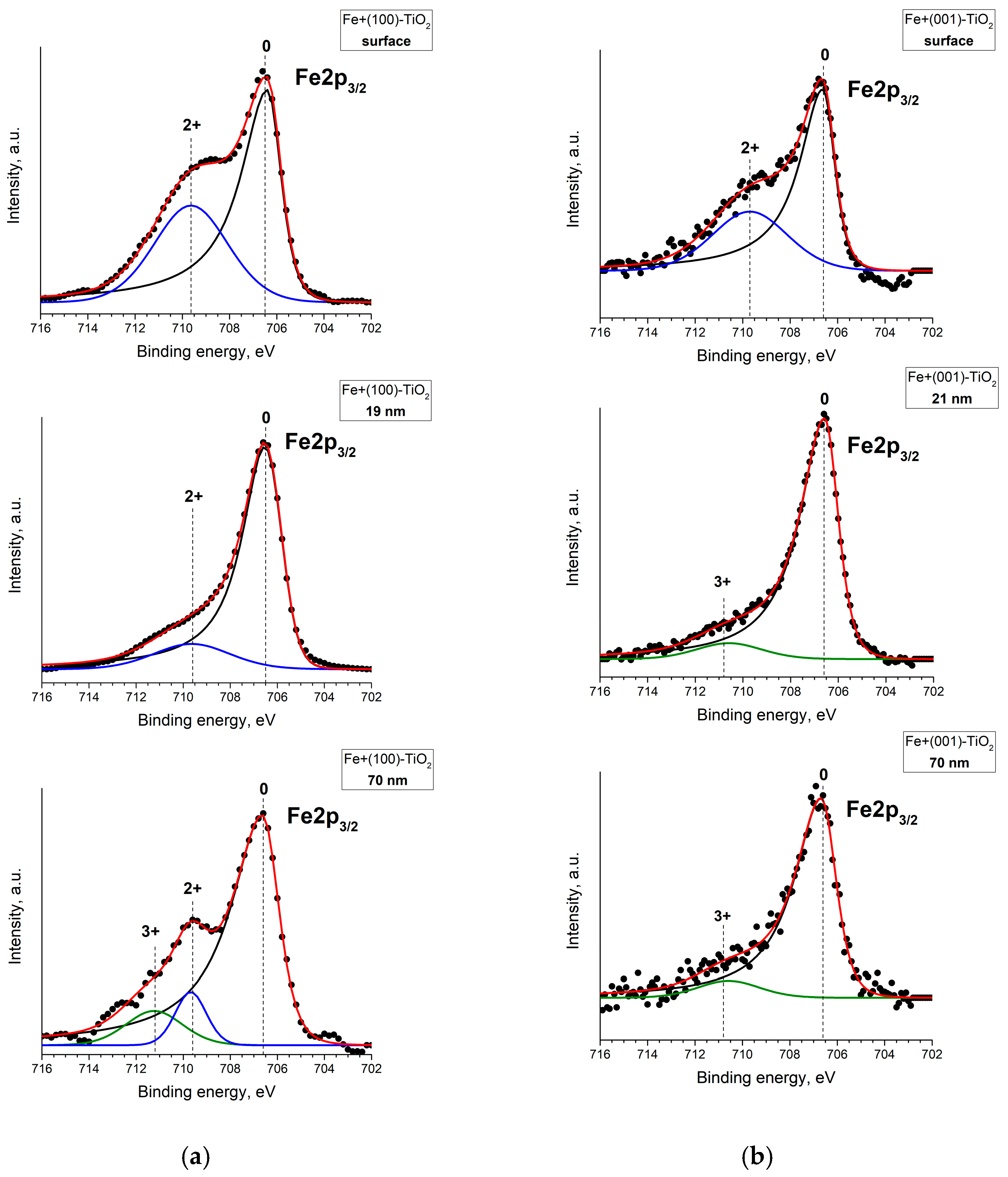
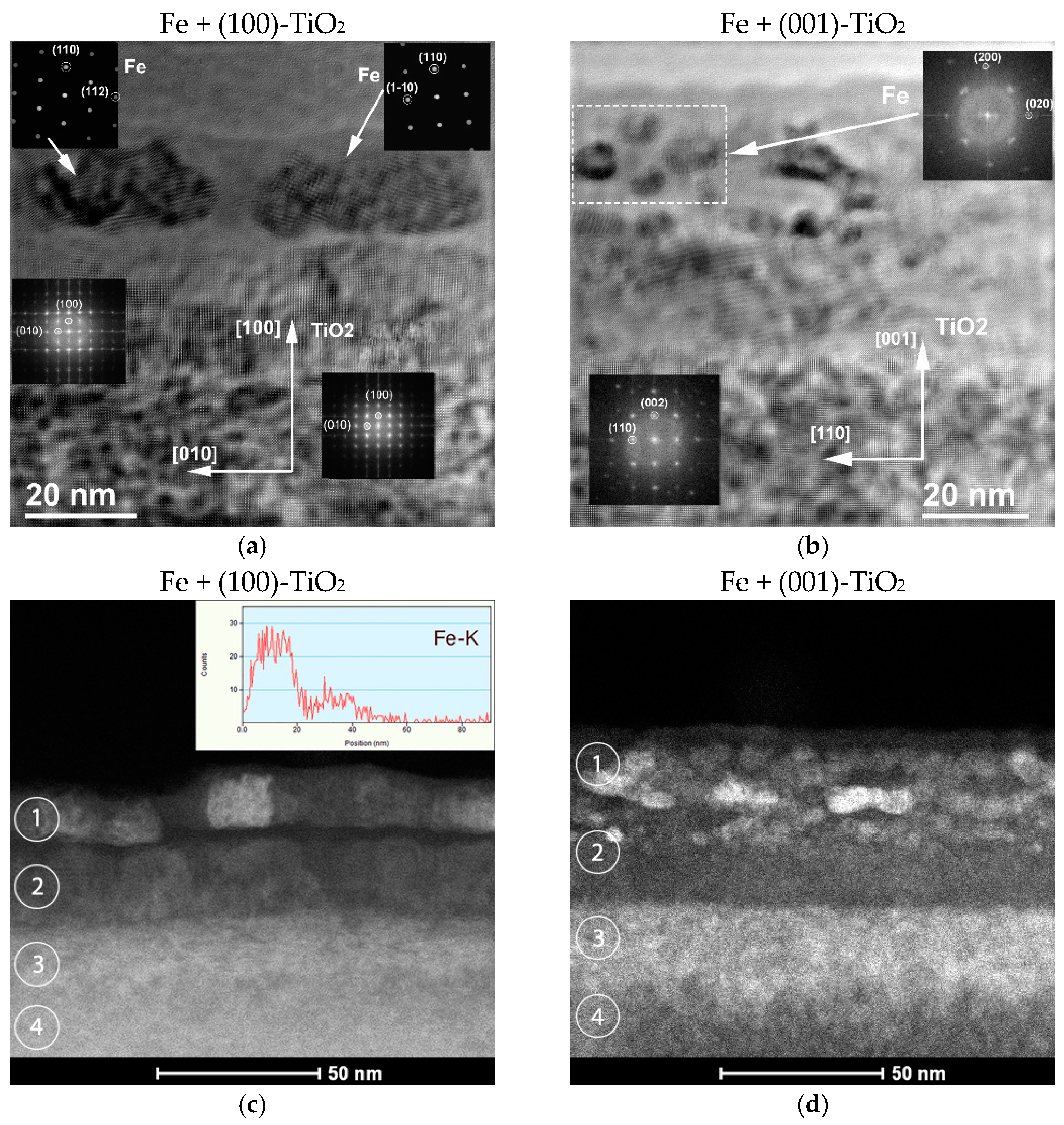



| No. | Plate Orientation | Fluence, Ion/cm2 | Elemental Composition, at. % | Fe/Ti | |||
|---|---|---|---|---|---|---|---|
| C | O | Ti | Fe | ||||
| 1 | (100) | - | 1.88 | 70.36 | 27.75 | - | - |
| 2 | (100) | 0.5 × 1017 | 2.85 | 65.84 | 27.20 | 4.11 | 0.15 |
| 3 | (001) | 2.13 | 65.1 | 28.18 | 4.6 | 0.16 | |
| 4 | (100) | 1.0 × 1017 | 4.68 | 61.68 | 26.03 | 7.61 | 0.29 |
| 5 | (001) | 3.07 | 61.04 | 27.39 | 8.5 | 0.31 | |
| 6 | (100) | 1.5 × 1017 | 4.12 | 58.77 | 26.7 | 10.4 | 0.39 |
| 7 | (001) | 2.63 | 58.81 | 26.9 | 11.66 | 0.43 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vakhitov, I.R.; Lyadov, N.M.; Vdovin, V.I.; Gutakovskii, A.K.; Nuzhdin, V.I.; Tagirov, L.R.; Khaibullin, R.I. Ion-Beam Synthesis of Structure-Oriented Iron Nanoparticles in Single-Crystalline Rutile TiO2. Crystals 2023, 13, 355. https://doi.org/10.3390/cryst13020355
Vakhitov IR, Lyadov NM, Vdovin VI, Gutakovskii AK, Nuzhdin VI, Tagirov LR, Khaibullin RI. Ion-Beam Synthesis of Structure-Oriented Iron Nanoparticles in Single-Crystalline Rutile TiO2. Crystals. 2023; 13(2):355. https://doi.org/10.3390/cryst13020355
Chicago/Turabian StyleVakhitov, Iskander R., Nikolay M. Lyadov, Vladimir I. Vdovin, Anton K. Gutakovskii, Vladimir I. Nuzhdin, Lenar R. Tagirov, and Rustam I. Khaibullin. 2023. "Ion-Beam Synthesis of Structure-Oriented Iron Nanoparticles in Single-Crystalline Rutile TiO2" Crystals 13, no. 2: 355. https://doi.org/10.3390/cryst13020355
APA StyleVakhitov, I. R., Lyadov, N. M., Vdovin, V. I., Gutakovskii, A. K., Nuzhdin, V. I., Tagirov, L. R., & Khaibullin, R. I. (2023). Ion-Beam Synthesis of Structure-Oriented Iron Nanoparticles in Single-Crystalline Rutile TiO2. Crystals, 13(2), 355. https://doi.org/10.3390/cryst13020355








