1. Introduction
In the automotive industry, particularly in agricultural and road vehicle manufacturing, corrosion-resistant multi-layer coatings that encounter metal usually have a phosphating layer at the bottom layer. This adheres tightly to the underlying metal, protecting the base metal from corrosion and improving the correct adhesion of the paints and organic finishes to be applied subsequently [
1,
2]. For vehicle manufacturing applications, this layer is usually zinc phosphate as it provides the best corrosion protection in outdoor and extreme (salty, wet, marine) conditions [
2,
3,
4,
5].
Complex agricultural vehicle bodies usually contain a phosphate layer with a varying composition and microstructure on the surface of the raw materials, depending on their physical and chemical properties. This affects the corrosion protection properties of the final coating [
6]. Vehicle manufacturers assemble parts of complex design from various base metals, such as aluminum and steel alloys, that undergo different machining stages (welding, machining, sheet metalworking, forging, etc.) and mechanical pre-treatments [
1,
3]. Therefore, a process of pre-treatment before painting must be developed to ensure that the coating systems formed by the multi-metal treatments meet corrosion resistance requirements [
3]. Zinc phosphate coatings on metal surfaces are very often the first layer of multi-layer corrosion protection coating and are expected to uniformly cover the base metal surface, thereby protecting the base metal from corrosion effects in the event of damage to the corrosion protection coating. This zinc phosphate layer is also intended to ensure proper adhesion of the applied paint layer [
2]. On the other hand, phosphate coatings on the zinc surfaces, e.g., on the hot-dip galvanized or electrogalvanized zinc surface of the car body, must not only provide excellent corrosion resistance but also ensure good paintability and good adhesion of the paint layer [
7,
8,
9,
10]. During surface protection by phosphating, a phosphate layer consisting of crystals and/or mixed crystals containing iron, zinc, and manganese is formed on the metal surface, depending on the composition of the phosphating solution [
6]. The quality of the base metal, the method of surface pretreatment, and the technological and operational conditions of the phosphating process (composition of baths, addition of metals to the modified phosphating bath, temperature, treatment time, pH, acceleration, etc.) influence the formation and structure of the phosphate layer [
11,
12,
13,
14,
15,
16,
17].
The main components of zinc-phosphate baths are water, phosphoric acid, zinc dihydrogen phosphate and metallic salts (nickel, manganese salts), oxidizers (accelerators), and agents to improve corrosion resistance and adhesion [
1]. An important development of zinc-phosphate baths was the modern tri-cationic (containing Zn
2+, Mn
2+, and Ni
2+) bath, that is suitable for depositing excellent phosphate coating on “multimetal” vehicle manufacturing structures containing steel and aluminum alloys, and with superior alkali resistance of the phosphate coating. These coatings are highly suitable for operation in cathodic electrophoretic paints (KTL/CED) [
2,
3,
6].
There are normal and low zinc phosphate baths (2000 to 4000 ppm zinc and 6000 to 14,000 ppm phosphate, 400 to 1700 ppm zinc and 16,000 to 22,000 ppm phosphate, respectively [
4]). For the low zinc baths, manganese is usually added to the bath to improve the coating’s resistance to external influences. Due to the modified crystal structure, these layers are suited for outdoor use [
4].
Phosphatization is an electrochemical process in which the metals (e.g., iron and aluminum) dissolve as the micro-anode H
+ is reduced and the insoluble phosphate precipitates on the micro-cathode [
6]. The phosphate coating is deposited on the metal surfaces as a result of interfacial reactions between the metal surface and the phosphating solution [
2,
4].
By immersing iron-based alloys and steels in baths containing water soluble dihydrogen phosphate compounds of an appropriate composition, or by spraying the bath contents onto the surface of the metals, fine crystalline conversion coatings are formed [
16,
17].
Light metals (most often aluminum alloys) are already widely used in car manufacturing [
18]. Agricultural vehicle and truck production are forced to reduce the total weight of vehicles, so manufacturers began to replace steel with light metals. Nowadays, complex vehicle bodies contain both steel and aluminum alloy elements, since replacing steel alloys with aluminum alloys provides weight reduction and energy savings for drivers [
18,
19]. On aluminum alloy surfaces, the paints adhere poorly without proper surface modification. During vehicle manufacturing, steel and aluminum alloy elements are usually phosphatized simultaneously [
20,
21].
During the pre-treatment of aluminum alloys, the pickling reaction results in the release of aluminum ions into the phosphating bath. This prevents the formation of a crystalline phosphate layer on both aluminum alloy surfaces and steel and galvanized steel surfaces [
17]. The inhibitory effect is already present at aluminum ion contents of 5 to 10 ppm [
22]. The formation of aluminum phosphate tends to inhibit the formation of zinc phosphate coatings, but this is limited when sodium fluoride (NaF) is added to the bath [
21,
22].
In the phosphating process, so-called accelerators (usually oxidizers) are used to accelerate the formation of the coating [
4]. Oxidizing agents added to the phosphating solution react with H
+ ions and electrons and further reduce the solution’s acidity, thereby preventing the formation of hydrogen gas bubbles that block contact between the phosphating solution and the metal surface [
4,
23]. The most used compounds are nitrites, nitrates, chlorates, peroxides, metal salts, and inorganic or organic nitrogen compounds such as nitroguanidine [
3,
17,
22,
23] or rare earth element e.g., REN (Rare Earth Nitrate) [
24]. Nitrite, usually in the form of sodium nitrite (NaNO
2), is often chosen as an accelerator in zinc phosphating processes [
13]. Narayan [
16] described phosphating with the nitroguanidine (CH
4N
4O
2) accelerator. It allowed the formation of the fine-grained and uniform phosphate layers and the softer, easily removable sludge.
This study investigates the simultaneous phosphating of aluminum and steel alloy surfaces on the same technological line using nitrite (N) and nitroguanidine (NG) accelerators. Nitrite is the most used and most effective accelerator and is particularly beneficial for steel surfaces [
1,
8,
13]. However, the disadvantage of these systems is that, usually, nitrite levels must be kept high, especially for spraying processes, thus polluting the effluent and producing toxic nitrous gases [
4]. Organic nitrogen compounds (e.g., nitroguanidine) are used to reduce or eliminate the effects of nitrous gases generated when using nitrite accelerator, especially for low zinc baths, resulting in a softer, easily removable sludge [
4]. The disadvantage of nitroguanidine accelerator is that it must be used at relatively high concentrations, can only be determined with sufficient accuracy by complex analysis in the phosphate solution, and can only be dosed most accurately in the ratio of the phosphating agent. It has the advantage that it does not decompose spontaneously and can be used over a very wide dosage range, allowing robust processes to be developed [
4].
In the case of simultaneous zinc phosphating of steel and aluminum alloys, special attention must be paid to the fluoride content, even when using “multimetal” processes, to ensure that a phosphate layer with a suitable structure is formed on both steel and aluminum alloy surfaces. Fluoride dosing is also necessary if the surface pretreatment line is set up for the pretreatment of iron and steel surfaces, even if only a small proportion of aluminum alloy surfaces are pretreated. Dosing of the fluoride component must be ensured continuously, as the fluoride content must be constant in the phosphating bath [
1].
The aim of the experiment series is to characterize the structure and properties of the zinc phosphate-based coatings formed on aluminum alloys and steel plate (important in the vehicle industry) formed simultaneously with different accelerators (N and NG). The novelty of the research is to investigate the effect of different base alloys and accelerators on phosphate coatings in a process line typically designed for the phosphating of iron and steel alloys under identical process parameters.
2. Materials and Methods
2.1. Methods
The surface structure of raw metals was visualized using a Keyence VHX-2000 type digital light microscope (LM).
The chemical composition of raw metals was analyzed by a PMI-MASTER Pro2 spark exacted optical emission spectrometer (OES) from Oxford Instruments. The samples were pre-polished with P80-grit sandpaper and dusted at the test site to exclude the influence of other contaminants on the measurement results. The measurements were performed using 99.99% argon as a protective gas because, during the vaporizing, a small amount of material was obtained by sparking as the plasma state required for the measurement could be achieved using a protective gas. Three measurements were performed on each sample due to the inhomogeneity of the raw material.
Morphology, crystal shape, and size were tested by FEI/Thermo Fisher Apreo S scanning electron microscope (SEM). Observation by SEM was carried out in low vacuum mode with an accelerating voltage of 20.0 kV. To acquire the best resolution for back-scattered electron imaging of the phosphate-based layers, the samples were washed in ultrasonic bath using ethanol. The samples were embedded in epoxy resin (NXMET XF40), cross sectioned, and finally polished with a 1 µm diamond suspension. The elemental compositions of the samples were determined by an EDAX AMETEK Octane Elect Plus Energy Dispersive X-ray Analyzer. The accelerating voltage was 20 kV and the data collection time was 180 s.
An image analyzer software (Image Color Summarizer 0.76) was used to calculate the surface coverage of the phosphate coating. Color analysis was based on elemental maps obtained by SEM. The pixels of the images were classified into two different clusters, where green pixels represent the phosphor-containing coating and black pixels refer to the aluminum- or iron-based substrate.
The X-ray diffraction (XRD) analyses of the samples were performed using a Philips PW 3710 diffractometer. The measurement parameters were as follows: CuKα radiation (50 kV, 40 mA), 0.02 °2θ/s speed, the range of 10 to 70°2θ, and a curved graphite monochromator. The X’Pert Data Collector software was used to collect data of XRD pattern data. The HighScore Plus 5.0 software was applied to identify phases and to perform quantitative phase analysis using the Rietveld method. The crystalline phases were identified by comparing the XRD patterns with the 2021 Powder Diffraction Files (PDF-2 2021) of the International Centre of Diffraction Data (ICDD).
A modified scratch-test was used to investigate the adhesion of the coatings. During the examination, an Anton Paar TRB3 type tribometer was used with a total stroke length of 10 mm and a normal force of 1 N throughout the entire measurement. The normal force was kept constant during the tests and the diamond indentation marks on the surface were evaluated using a scanning electron microscope.
2.2. Materials
A wheel hub was chosen as one of the aluminum alloy samples (AL1) as wheel hubs are most often made of aluminum alloy. The sample was sliced without using a cooling medium prior the phosphating process.
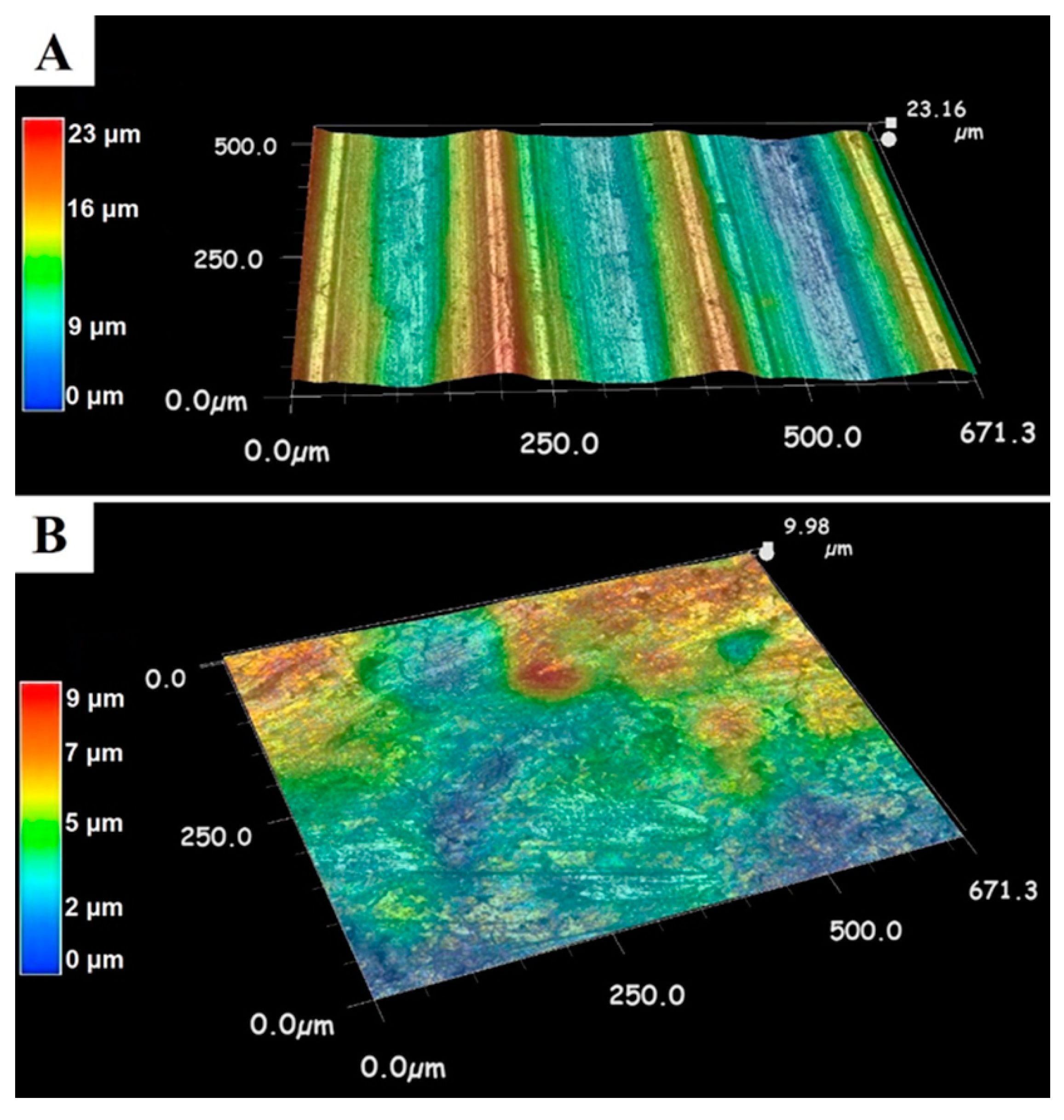
Figure 1 shows the surfaces of the AL1 base alloy, which is a high-strength AlSi1MgMn alloy (EN-AW-6082, Forging Products Trading Spain/CAMARA) designed for high-load structural applications. Thanks to its fine-grained structure, this alloy exhibits good resistance to dynamic loading conditions. This #6000 series aluminum alloy (Si 1~2%; Mg~%) is also suitable for the manufacture of vehicle bodies and for casting [
18,
25]; it is easy to work and has good corrosion resistance but is difficult to phosphatize [
1]. The machined rough surface of the sample (
Figure 1A) becomes smoother by forging (
Figure 1B). The 3-dimensional LM images (
Figure 1) show that the highest roughness value of the raw material is 23 μm, decreasing to 9 μm after forging. During forging, the surface roughness can be significantly reduced. This can help to produce a more uniform zinc phosphate coating. By selecting the appropriate technology, the grain arrangement can be adjusted to the subsequent use to improve the mechanical properties of the piece. This aluminum alloy is also suitable for the manufacture of vehicle bodies; it is easy to machine and it has good corrosion resistance [
18] but it is difficult to phosphatize [
1]. The elemental composition of AL1 sample measured by OES is given in
Table 1, in weight percent (wt.%).
The other raw alloy (AL2) chosen was an AA6014 (AlMg0.6Si0.6V) aluminum alloy panel (Gardobond, Chemetall, Frankfurt am Main, Germany) formed in the T4 temper. The thickness of the rolled aluminum alloy panels was 1.0 mm. The LM image (
Figure 2) shows that the highest roughness value for the raw AL2 sample is 5 μm. The #6000 series (Al-Mg-Si) alloys are the most used aluminum alloy plates in automotive bodywork as they possess excellent mechanical properties, are easy to machine and weld, and have good corrosion resistance. They respond well to the high-temperature heating treatment with the burning of electrophoretic paints [
25,
26] but are difficult to phosphatize [
1]. The elemental composition of the AL2 alloy measured by OES is shown in
Table 1.
The third studied raw alloy (ST) was a standard low-carbon, cold-rolled, CRS SAE 1008/1010 Type R46 steel panel (Q-Panel) with a thickness of 0.81 mm, temper 1/4 hard. In the case of this rolled steel plate (ST), the highest roughness value is 7.6 μm (
Figure 3), which is similar to the AL2 sample. Steel test panels have been recognized as the world standard for a uniform and consistent test surface for paints, adhesives, sealants, and other coatings. These panels are made from standard low-carbon, cold-rolled steel and they have a clean, consistent, and matte surface produced by roughened rolls. The chemical composition of the raw ST sample used in the tests is given in
Table 1.
2.3. Preparation of Zinc Phosphate Coating
Phosphating of the alloys was carried out on an industrial production line, thus allowing for the simulation of most of the interfering factors occurring under industrial conditions. The test plates and the test pieces were zinc phosphated without any mechanical surface treatment (polishing, sandblasting, grit blasting, grinding).
The samples were subjected to surface pretreatment and phosphating in the same technological step, using dipping process according to
Table A1 for nitroguanidine and
Table A2 for nitrite accelerator. After pretreatment, the samples were dried in a laboratory oven.
Important parameters of the phosphate baths are the free acid value (FA), that refers to the free H
+ ions present, and the total acid value (TA), that represents the total phosphate content of the phosphating bath [
17].
The samples were degreased, rinsed, activated, phosphated and rinsed again in successive steps through different baths. In the degreasing bath(s), oils and greases were removed from the metal surface. In the rinsing bath(s), the degreasing chemicals were removed with water and in the subsequent bath the metal surface was activated. In the phosphating bath, the formation of insoluble heavy metal tertiary phosphates on the surface was carried out, and in the rinsing bath, the removal of acid residues, soluble salts, and non-adhesive particles on the metal was carried out.
Gardobond 2600 zinc phosphate mixture (BASF, Chemetall, Ltd., Frankfurt am Main, Germany) was used for the experiments. This mixture contains zinc, manganese, and nickel (tri-cation bath). This chemical can be used for phosphating steel, galvanized steel, and aluminum alloy surfaces using the dipping and spraying process (“multimetal” process). This is a nitrite (N)-accelerated system but, according to the manufacturer’s technical data sheet (TDS), it can also work with nitroguanidine (NG) accelerator. For both series of experiments with the accelerators, we used a zinc-phosphating solution with identical settings and the accelerators were dosed at the average value according to the technical data sheet. The free fluoride ion content of the phosphating bath used for treating the sample plates was between 140 and 150 ppm, and the total fluoride (SiF62−) was 1.2 to 1.4 g/L during the process, determined by a pH-mV measuring device with fluoride selective electrode (HACH ISEF121; WTW ISE F 500 DIN).
Before the N-accelerated dipping phosphatization process, the samples were degreased with Gardoclean S 5197 (alkaline cleaner for spray and immersion applications; BASF, Chemetall Ltd.), a moderately alkaline, liquid degreasing solution with silicate and borate. It is primarily used for cleaning aluminum alloy, but it is also suitable for cleaning steel and galvanized steel. It can be applied by dipping or spraying. During our tests, it was applied using the dipping method with Gardobond Additive H7400 (BASF, Chemetall, Ltd.) as surfactant, at 60 °C for 600 s, with intensive mixing of the bath. After a water rinse, the surface was activated in the activation bath with Gardolene V6513 solution (BASF, Chemetall Ltd.) at a pH value of 8.9. The zinc phosphating step was carried out using Gardobond 2600 solution (BASF, Chemetall, Ltd.), at a temperature of 53 °C, with 180 s of exposure time. The bath contained 1.3 g/L zinc and 2.4 gas points N accelerator. After the phosphatizing process, a two-step water rinse was completed. To remove residual salt content from the surface, a cascade system of deionized water rinse was used. Finally, the pre-treated pieces were air-dried (
Table A2).
Before the NG-accelerated dipping phosphatization process, the samples were degreased with Gardoclean S 5197. It can be applied by dipping or spraying. During our tests, it was applied using the dipping method with Gardobond Additive H7400 (BASF, Chemetall, Ltd.) as surfactant, at 55 °C for 600 s, with intensive mixing of the bath. After a water rinse, the surface was activated in the activation bath with Gardolene V6513 solution (BASF, Chemetall, Ltd.) at a pH value of 8.9. The zinc phosphating step was carried out using Gardobond 2600 solution (BASF, Chemetall, Ltd.) at a temperature of 53 °C, with 180 s of exposure time. The bath contained 500 mg/L NG of accelerator measured by photometry. After the phosphatizing process, a two-step water rinse was completed. To remove residual salt content from the surface, a cascade system of deionized water rinse was used. Finally, the pretreated pieces were air-dried (
Table A2).
The compositions of the phosphating baths with different accelerators, as measured by ICP, are summarized in
Table 2. In
Table 3, the symbols of the phosphate samples are given.