The Impact of Ammonium Fluoride on Structural, Absorbance Edge, and the Dielectric Properties of Polyvinyl Alcohol Films: Towards a Novel Analysis of the Optical Refractive Index, and CUT-OFF Laser Filters
Abstract
:1. Introduction
2. Specifics of the Experiment
2.1. Test Experimentation
2.2. Equipment and Characteristics
3. Discussions and Consequences
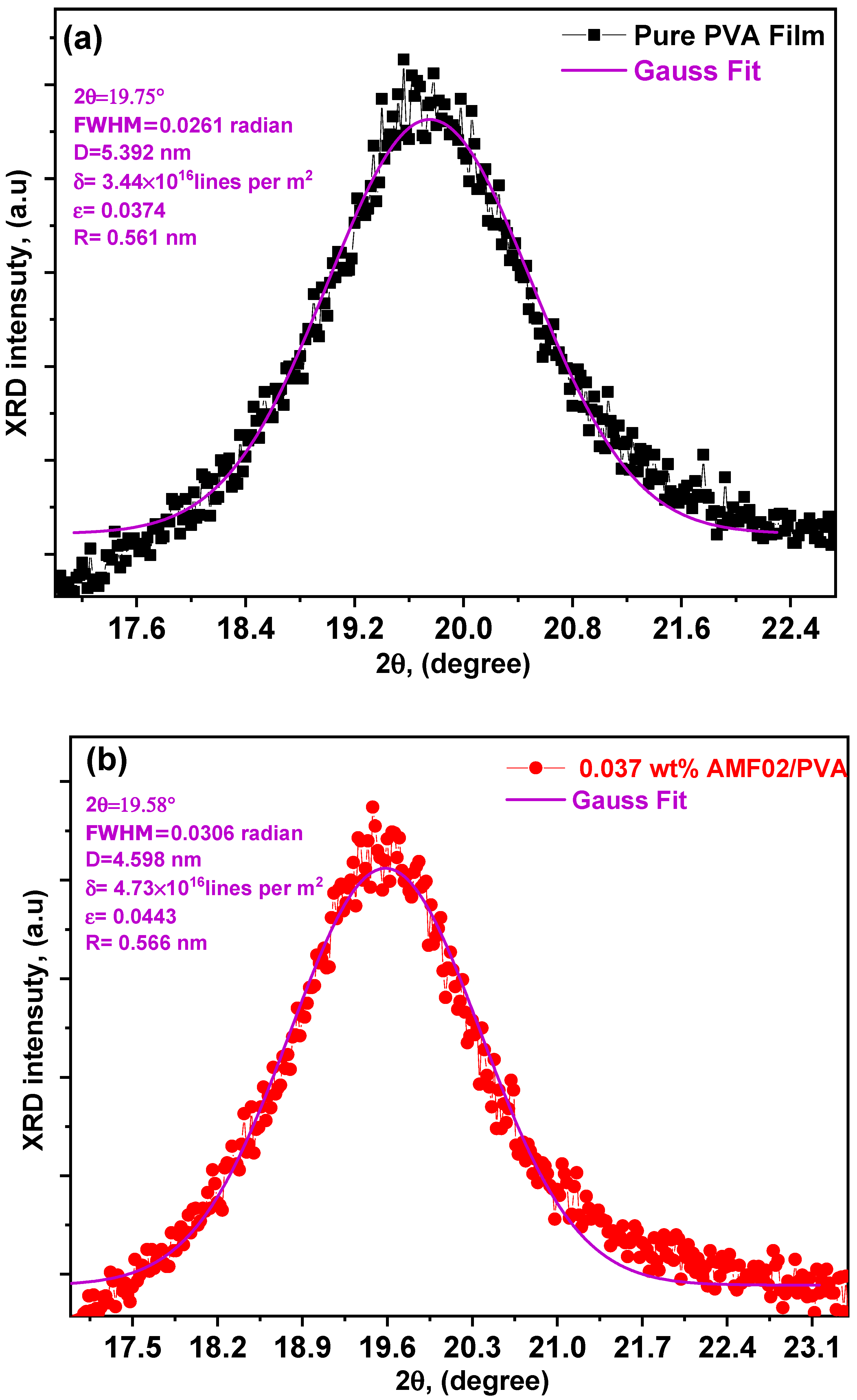
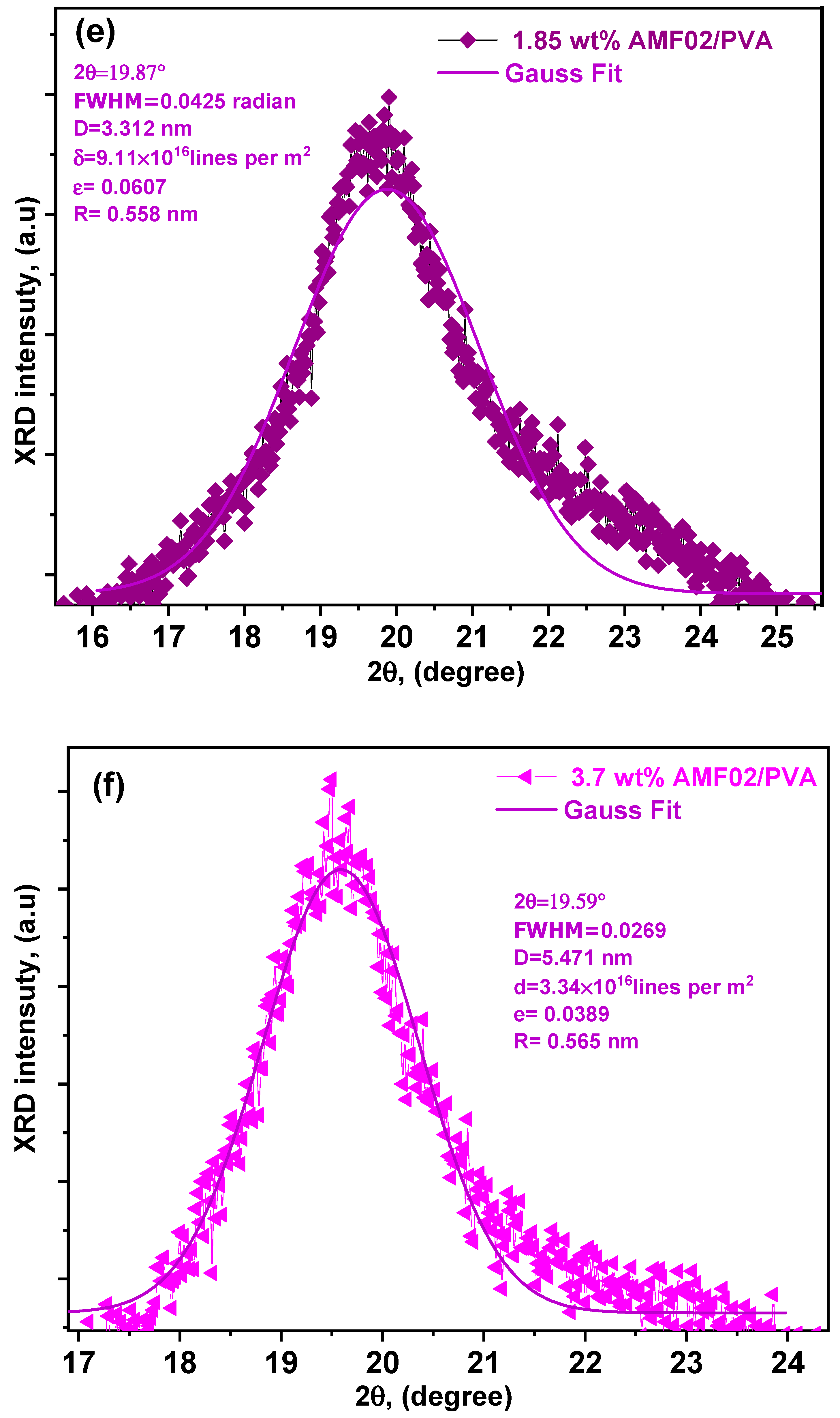
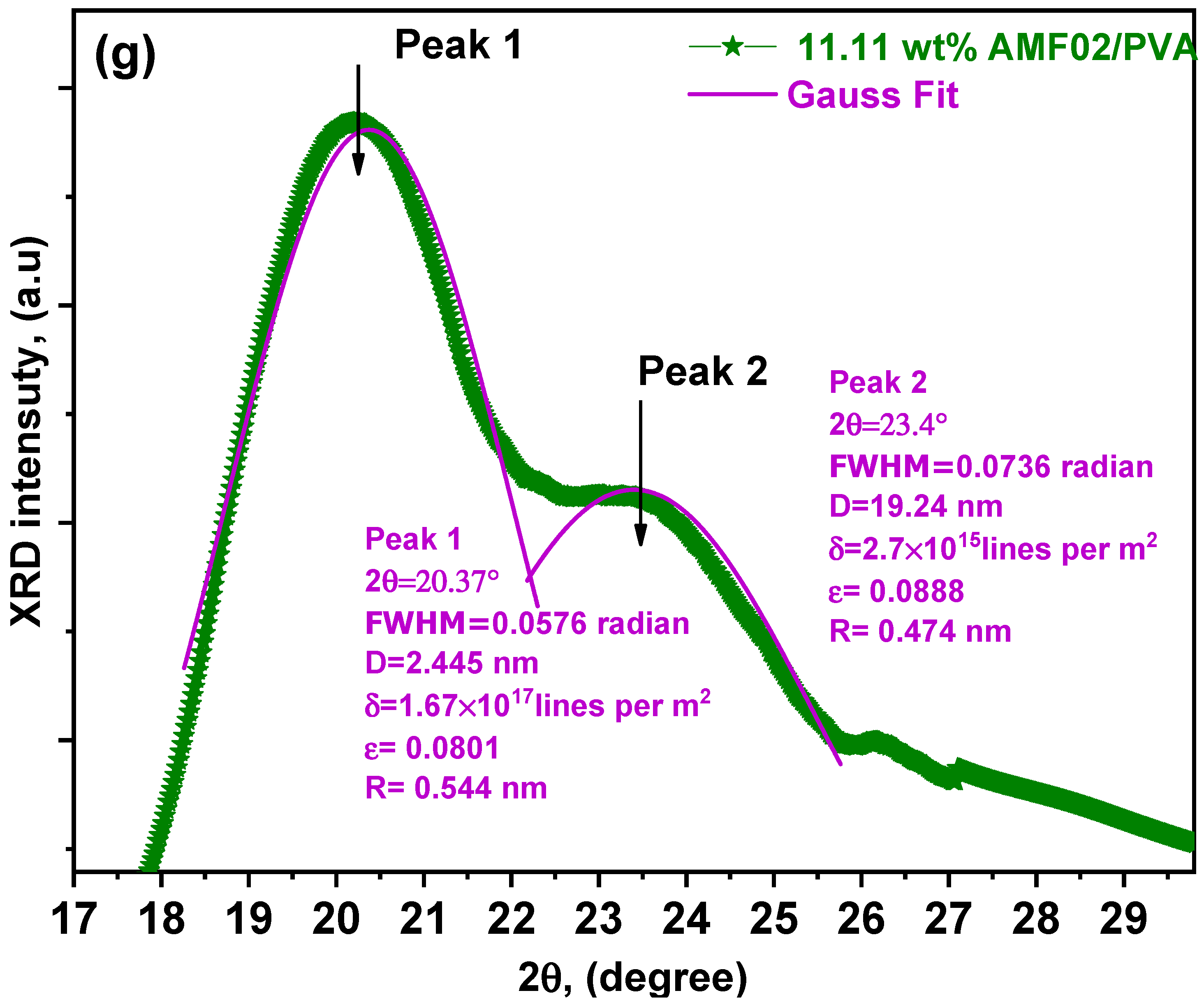
3.1. XRD Analysis of Innovative PCCEFs Made from AMF02/PVA
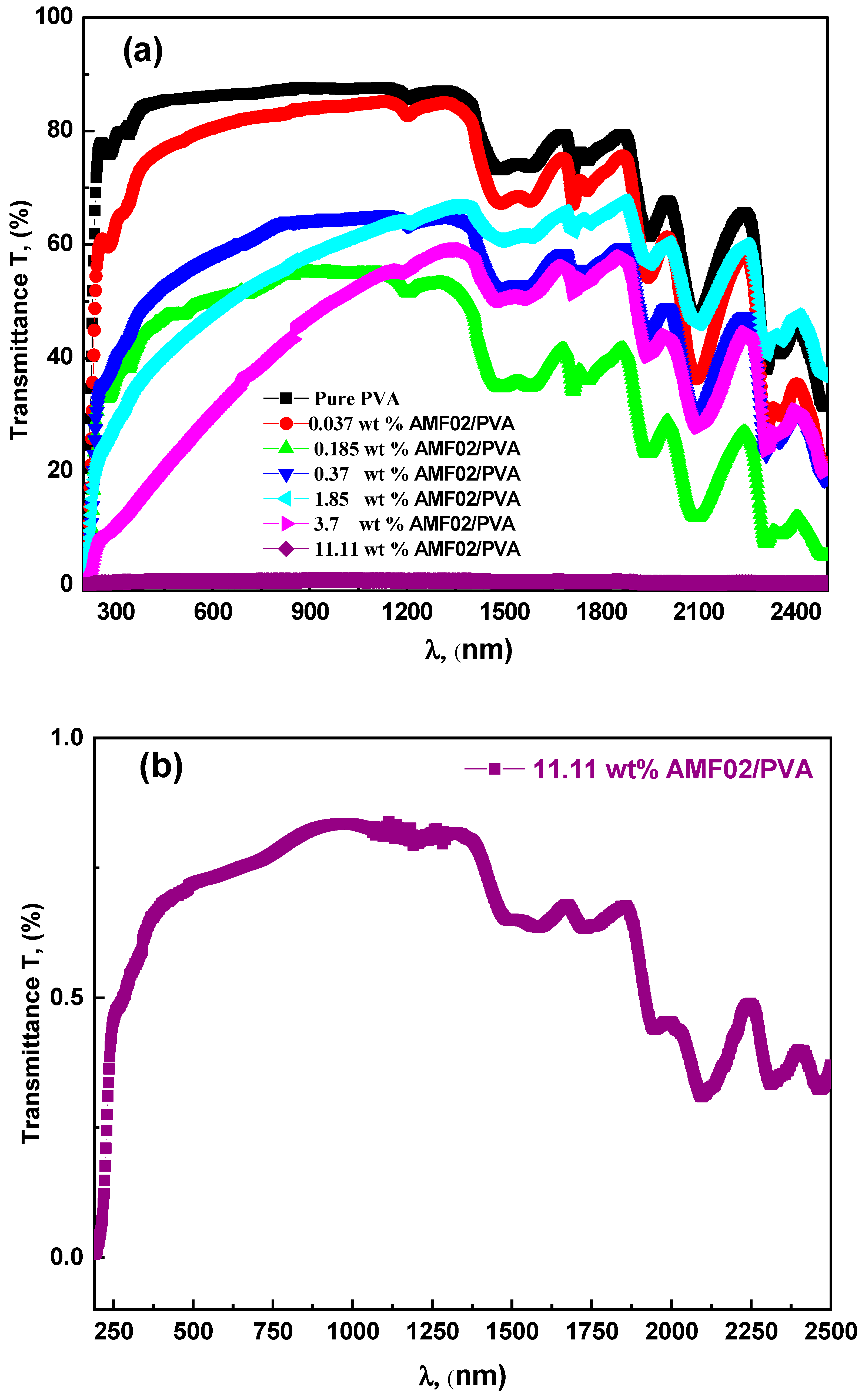
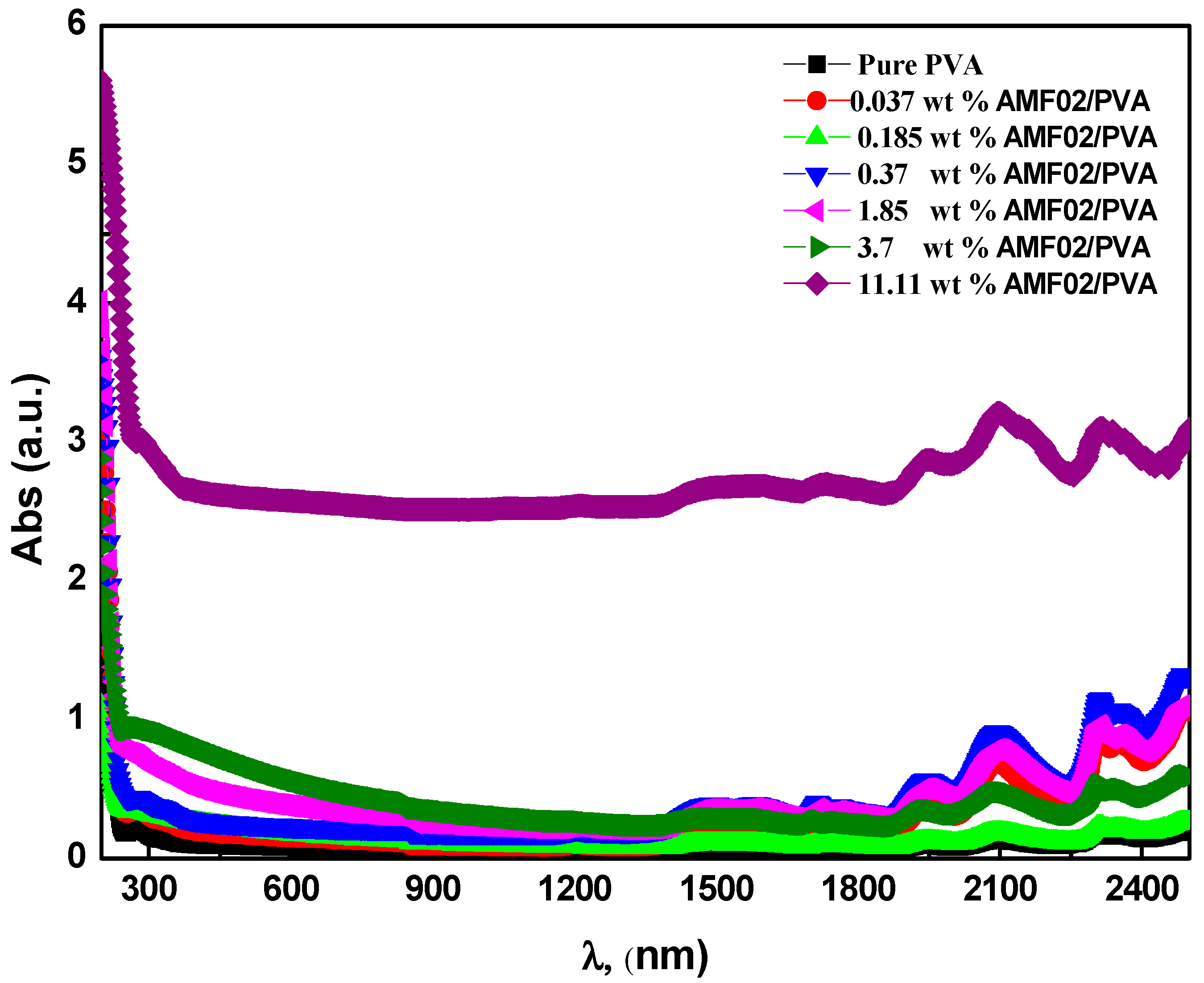
3.2. Optical Transmittance, Absorption, and Absorption Edge Examination for AMF02/PVA Novel Proton-Conducting Composite Electrolyte Films
3.3. Bandgap Energy and the Refractive Index Relationships for AMF02/PVA Novel PCCEFs
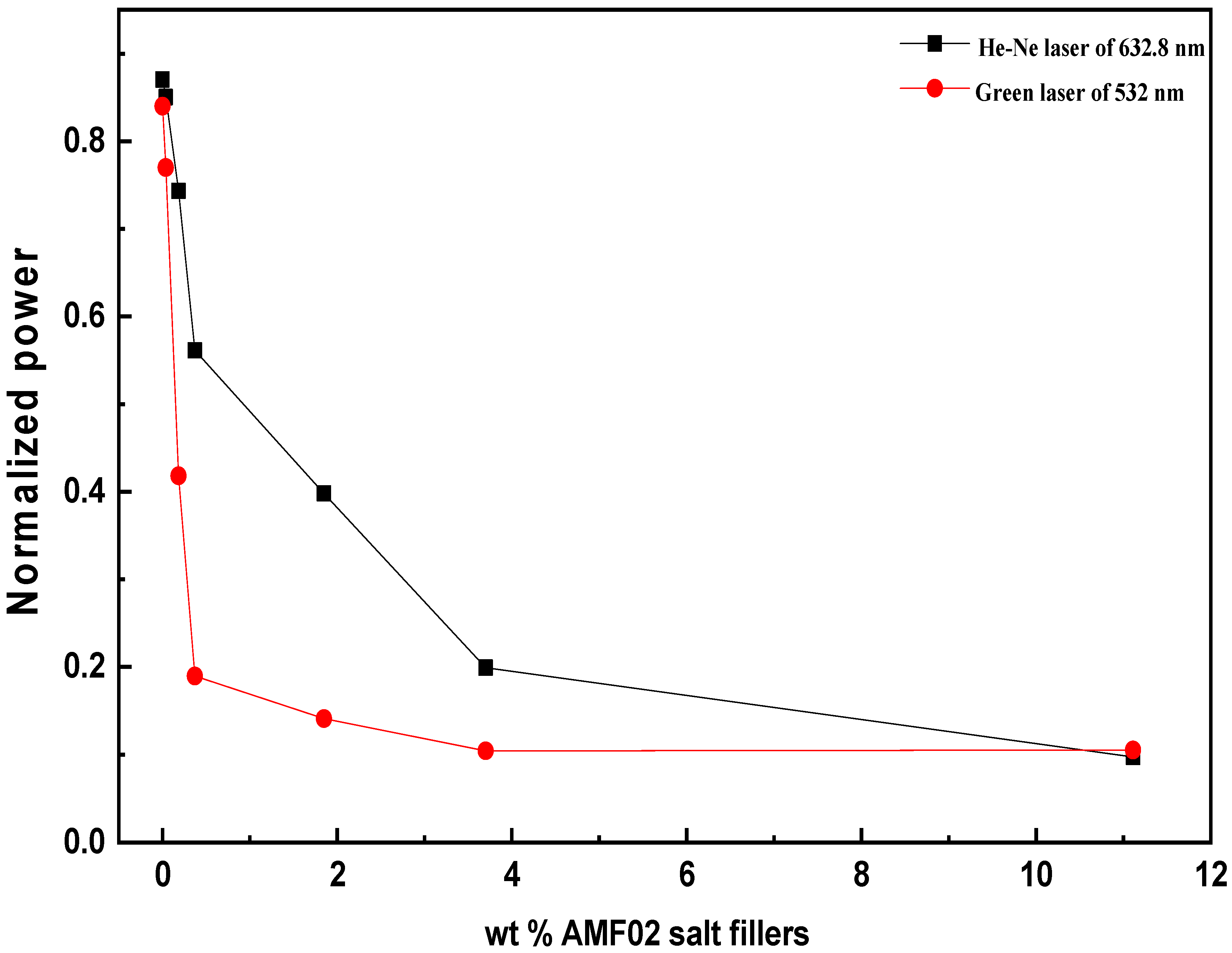
3.4. Laser Power Attenuation Analysis for AMF02/PVA Novel PCCEFs
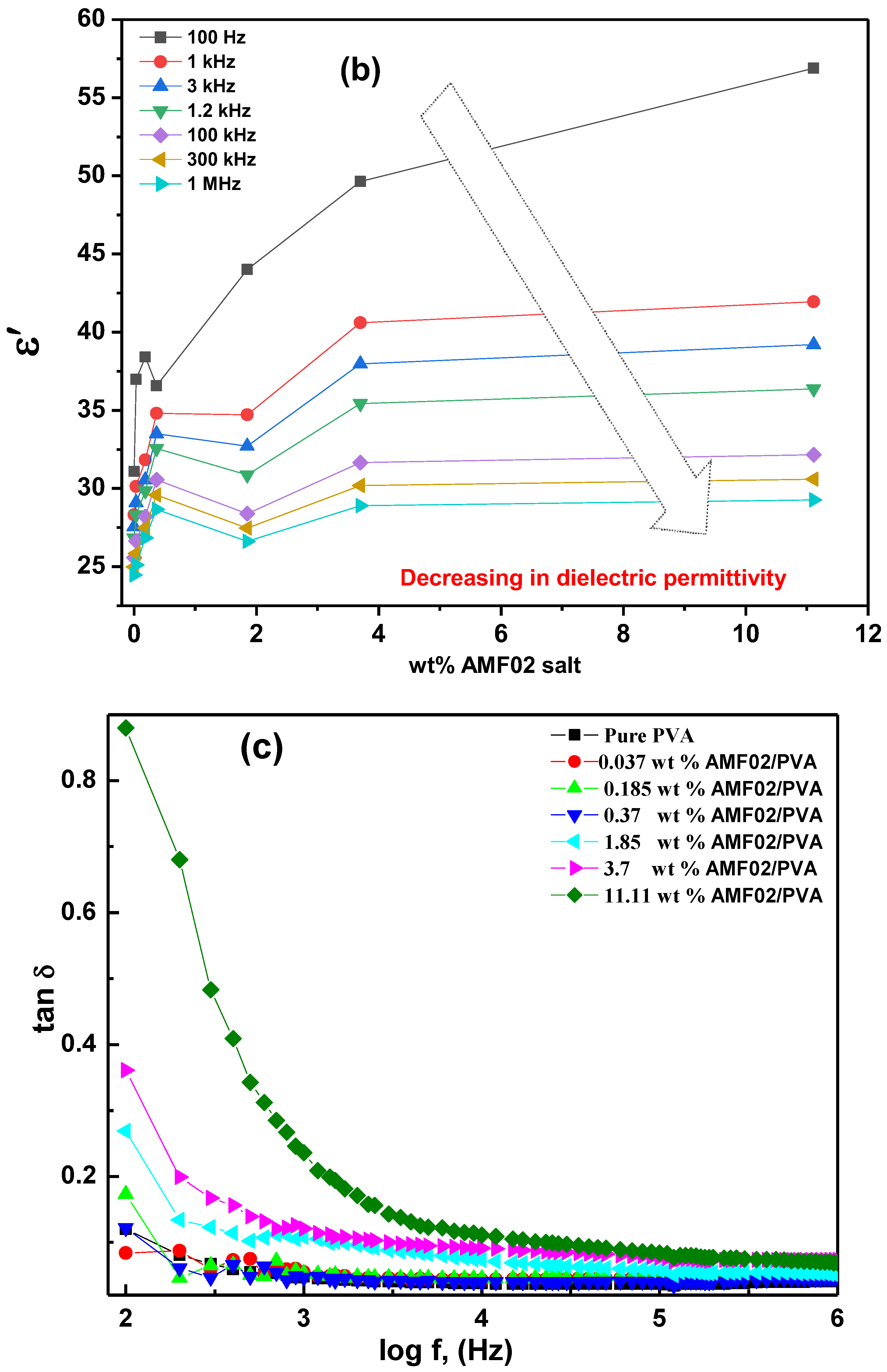
3.5. Frequency Necessity of Electric Permittivity ε′, Dielectric Loss ε″, and Loss Tangent tan δ for AMF02/PVA Novel PCCEFs
3.5.1. Frequency Dependence of ε′, ε″, and tan δ at Various AMF02 Salt Fillers
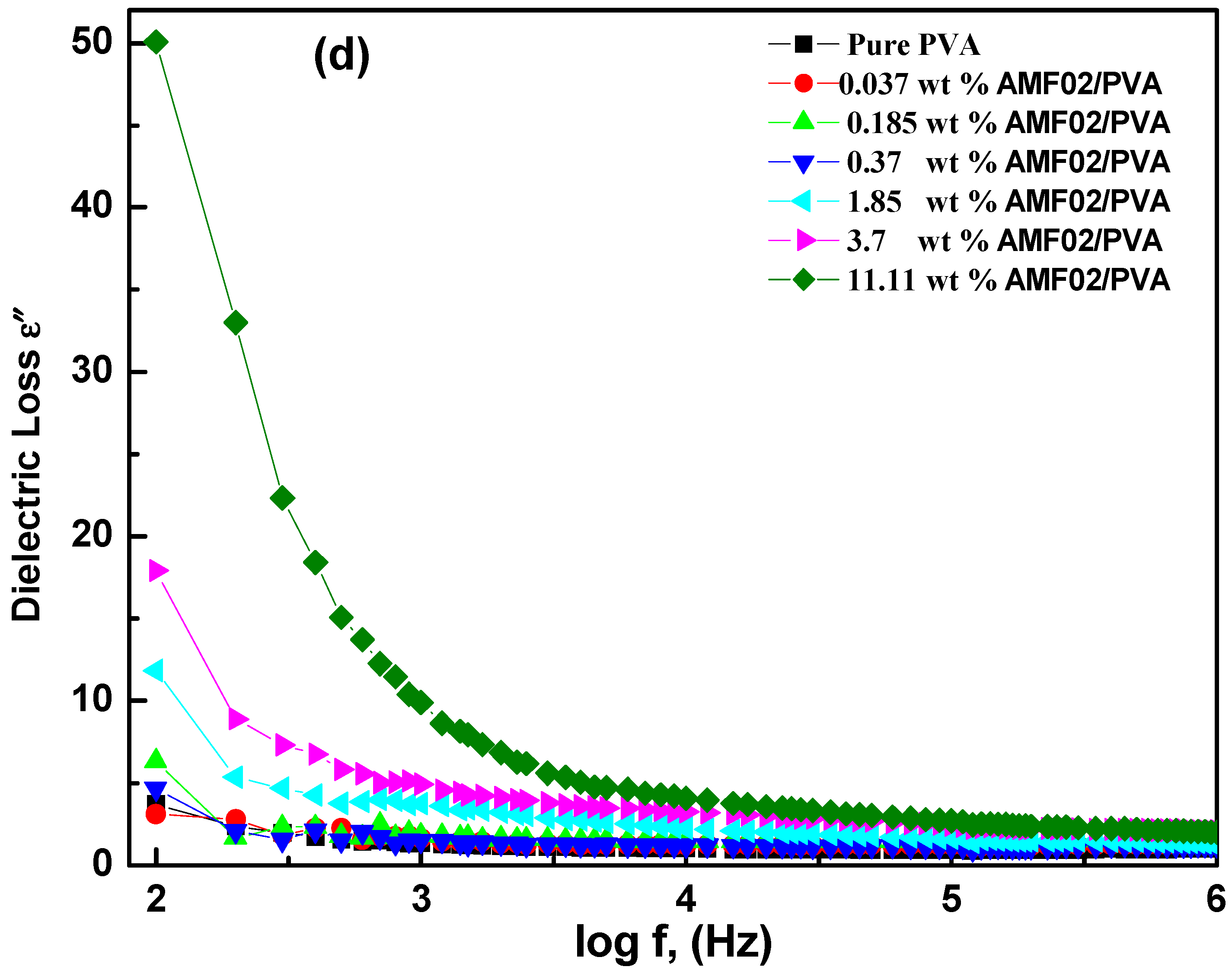
3.5.2. Frequency Dependence of Electric Modulus Analysis at Various AMF02 Salt Fillers
3.5.3. Frequency Dependence of AC Conductivity
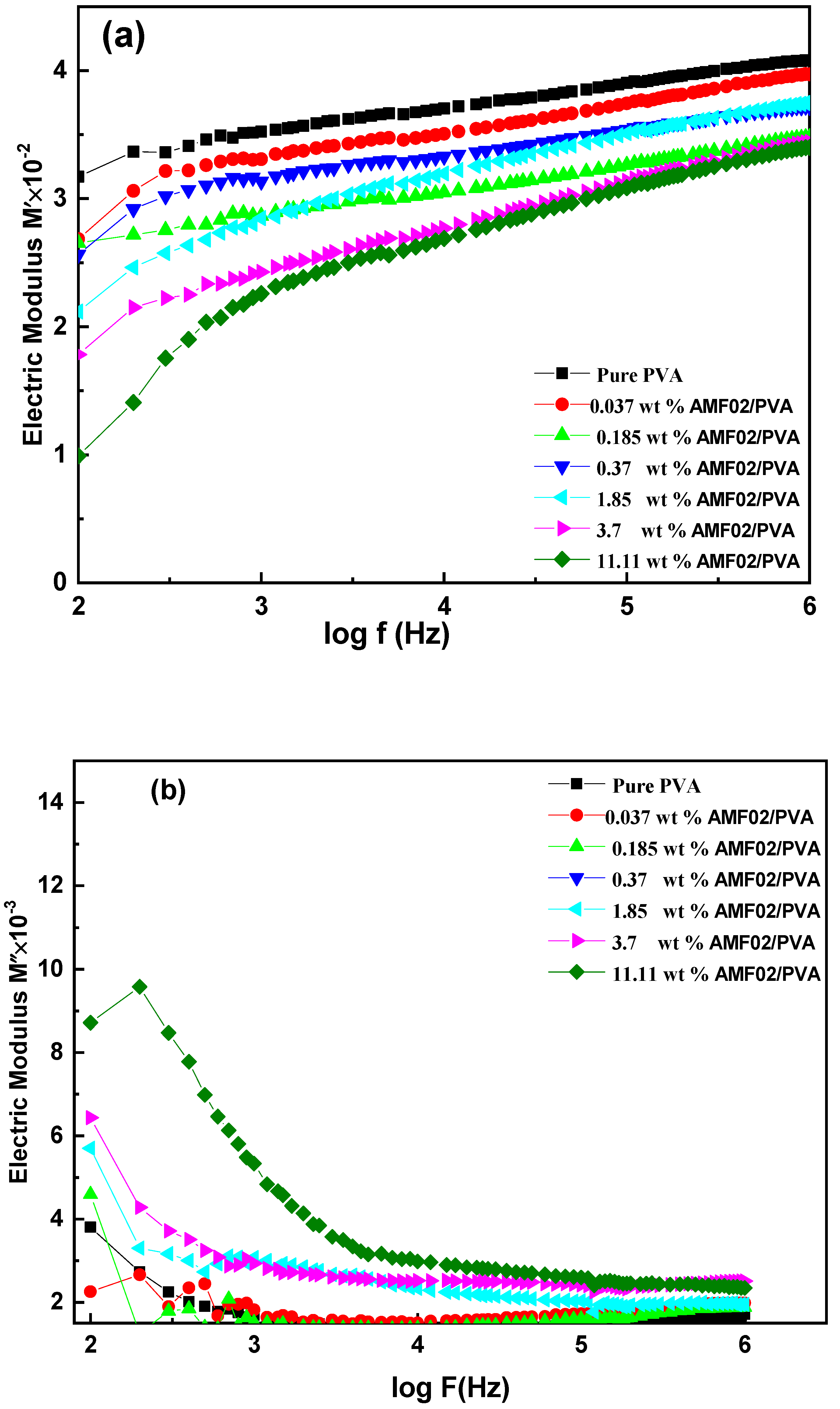
3.5.4. Frequency Dependence of Impedance Measurements
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, A.; Tu, Y.; Wang, S.; Zhang, H.; Yu, F.; Chen, Y.; Li, D. A PEGylated Chitosan as Gel Polymer Electrolyte for Lithium Ion Batteries. Polymers 2022, 14, 4552. [Google Scholar] [CrossRef]
- Thanganathan, U.; Nogami, M. Investigations on effects of the incorporation of various ionic liquids on PVA based hybrid membranes for proton exchange membrane fuel cells. Int. J. Hydrogen Energy 2015, 40, 1935–1944. [Google Scholar] [CrossRef]
- Uma, T.; Yin, F. High proton conductivity of poly (methyl methacrylate)-based hybrid membrane for PEMFCs. Chem. Phys. Lett. 2011, 512, 104–107. [Google Scholar] [CrossRef]
- Flora, X.H.; Ulaganathan, M.; Rajendran, S. Role of Different Plasticizers in Li-Ion Conducting Poly(Acrylonitrile)-Poly(Methyl Methacrylate) Hybrid Polymer Electrolyte. Int. J. Polym. Mater. Polym. Biomater. 2013, 62, 737–742. [Google Scholar] [CrossRef]
- Radha, K.P.; Selvasekarapandian, S.; Karthikeyan, S.; Hema, M.; Sanjeeviraja, C. Synthesis and impedance analysis of proton-conducting polymer electrolyte PVA:NH4F. Ionics 2013, 19, 1437–1447. [Google Scholar] [CrossRef]
- Kawai, R.; Yada, S.; Yoshimura, T. Characterization and Solution Properties of Quaternary-Ammonium-Salt-Type Amphiphilic Gemini Ionic Liquids. ACS Omega 2019, 4, 14242–14250. [Google Scholar] [CrossRef]
- Sekhon, S.; Kumar, M.; Yamada, K.; Okuda, T. On the ionic diffusive motion in some fluoride doped polymer electrolytes. Chem. Phys. Lett. 2003, 379, 466–470. [Google Scholar] [CrossRef]
- Nik Aziz, N.A.; Idris, N.K.; Isa, M.I.N. PC polymer electrolytes of methylcellulose doped ammonium fluoride: Conductivity and ionic transport studies. Int. J. Phys. Sci. 2010, 5, 748. [Google Scholar]
- Mohammed, M.I.; Bouzidi, A.; Zahran, H.Y.; Jalalah, M.; Harraz, F.A.; Yahia, I.S. Ammonium iodide salt-doped polyvinyl alcohol polymeric electrolyte for UV-shielding filters: Synthesis, optical and dielectric characteristics. J. Mater. Sci. Mater. Electron. 2021, 32, 4416–4436. [Google Scholar] [CrossRef]
- Hema, M.; Selvasekarapandian, S.; Arunkumar, D.; Sakunthala, A.; Nithya, H. FTIR, XRD and ac impedance spectroscopic study on PVA based polymer electrolyte doped with NH4X (X = Cl, Br, I). J. Non-Cryst. Solids 2009, 355, 84–90. [Google Scholar] [CrossRef]
- Huang, J.; Zhu, J.; Yang, G.; Zhu, Y.; Xu, X.; Pan, G. Lifetime-tunable green room temperature phosphorescence of carbon dots by the multi-step modification. Opt. Express 2021, 29, 41014–41022. [Google Scholar] [CrossRef]
- Jiang, L.; Ding, H.; Xu, M.; Hu, X.; Li, S.; Zhang, M.; Zhang, Q.; Wang, Q.; Lu, S.; Tian, Y.; et al. UV–Vis–NIR Full-Range Responsive Carbon Dots with Large Multiphoton Absorption Cross Sections and Deep-Red Fluorescence at Nucleoli and In Vivo. Small 2020, 16, 2000680. [Google Scholar] [CrossRef]
- Saeed, M.A.M.; Abdullah, O.G. Effect of High Ammonium Salt Concentration and Temperature on the Structure, Morphology, and Ionic Conductivity of Proton-Conductor Solid Polymer Electrolytes Based PVA. Membranes 2020, 10, 262. [Google Scholar] [CrossRef]
- Yahia, I.S.; Mohammed, M.; Nawar, A.M. Multifunction applications of TiO2/poly(vinyl alcohol) nanocomposites for laser attenuation applications. Phys. B Condens. Matter 2019, 556, 48–60. [Google Scholar] [CrossRef]
- Ali, H.E.; Abdel-Aziz, M.; Nawar, A.M.; Algarni, H.; Zahran, H.; Yahia, I.; Khairy, Y. Structural, electrical, and nonlinear optical performance of PVAL embedded with Li+ -ions for multifunctional devices. Polym. Adv. Technol. 2020, 32, 1011–1025. [Google Scholar] [CrossRef]
- Bhajantri, R.; Ravindrachary, V.; Harisha, A.; Crasta, V.; Nayak, S.P.; Poojary, B. Microstructural studies on BaCl2 doped poly(vinyl alcohol). Polymer 2006, 47, 3591–3598. [Google Scholar] [CrossRef]
- Abdelaziz, M.; Abdelrazek, E. Effect of dopant mixture on structural, optical and electron spin resonance properties of polyvinyl alcohol. Phys. B Condens. Matter 2007, 390, 1–9. [Google Scholar] [CrossRef]
- Kumar, N.B.R.; Crasta, V.; Bhajantri, R.F.; Praveen, B.M. Microstructural and Mechanical Studies of PVA Doped with ZnO and WO3 Composites Films. J. Polym. 2014, 2014, 846140. [Google Scholar] [CrossRef] [Green Version]
- Li, K.; Lu, X.; Zhang, Y.; Liu, K.; Huang, Y.; Liu, H. Bi3TaO7/Ti3C2 heterojunctions for enhanced photocatalytic removal of water-borne contaminants. Environ. Res. 2020, 185, 109409. [Google Scholar] [CrossRef]
- Madhuri, S.N.; Hemalatha, K.S.; Rukmani, K. Preparation and investigation of suitability of gadolinium oxide nanoparticle doped polyvinyl alcohol films for optoelectronic applications. J. Mater. Sci. Mater. Electron. 2019, 30, 9051–9063. [Google Scholar] [CrossRef]
- Cho, S.-D.; Lee, J.-Y.; Paik, K.-W. Effects of particle size on dielectric constant and leakage current of epoxy/barium titanate (BaTiO3) composite films for embedded capacitors. In Advances in Electronic Materials and Packaging 2001 (Cat. No.01EX506); IEEE: Piscataway, NJ, USA, 2001; pp. 63–68. [Google Scholar] [CrossRef]
- Aziz, S.B.; Ahmed, H.M.; Hussein, A.M.; Fathulla, A.B.; Wsw, R.M.; Hussein, R.T. Tuning the absorption of ultraviolet spectra and optical parameters of aluminum doped PVA based solid polymer composites. J. Mater. Sci. Mater. Electron. 2015, 26, 8022–8028. [Google Scholar] [CrossRef]
- Al-Osaimi, J.; Al-Hosiny, N.; Abdallah, S.; Badawi, A. Characterization of optical, thermal and electrical properties of SWCNTs/PMMA nanocomposite films. Iran. Polym. J. 2014, 23, 437–443. [Google Scholar] [CrossRef]
- Bhowmick, M.; Xiz, H.; Ullrich, B. Optical Bandgap Definition via a Modified Form of Urbach’s Rule. Materials 2021, 14, 1639. [Google Scholar] [CrossRef]
- Abdullah, O.G.; Saber, D.R.; Taha, S.A. The Optical Characterization Of Polyvinyl Alcohol: Cobalt Nitrate Solid Polymer Electrolyte Films. Adv. Mater. Lett. 2015, 6, 153–157. [Google Scholar] [CrossRef]
- Norouzi, M.A.; Montazer, M.; Harifi, T.; Karimi, P. Flower buds like PVA/ZnO composite nanofibers assembly: Antibacterial, in vivo wound healing, cytotoxicity and histological studies. Polym. Test. 2021, 93, 106914. [Google Scholar] [CrossRef]
- Aziz, S.B.; Abdullah, O.G.; Rasheed, M.A. A novel polymer composite with a small optical band gap: New approaches for photonics and optoelectronics. J. Appl. Polym. Sci. 2017, 134, 44847. [Google Scholar] [CrossRef]
- Coskun, D.; Gunduz, B.; Coskun, M.F. Synthesis, characterization and significant optoelectronic parameters of 1-(7-methoxy-1-benzofuran-2-yl) substituted chalcone derivatives. J. Mol. Struct. 2018, 1178, 261–267. [Google Scholar] [CrossRef]
- Soliman, T.S.; Hessien, M.M.; Elkalashy, S.I. Structural, Thermal, and Optical Properties of Polyvinyl Alcohol Films Doped with La2ZnOx Nanoparticles. J Non Cryst Solids 2022, 580, 121405. [Google Scholar] [CrossRef]
- Ahmed, A.A.A.; Al-Hussam, A.M.; Abdulwahab, A.M.; Ahmed, A.N.A.A. The impact of sodium chloride as dopant on optical and electrical properties of polyvinyl alcohol. AIMS Mater. Sci. 2018, 5, 533–542. [Google Scholar] [CrossRef]
- Abdullah, O.G.; Aziz, S.B.; Rasheed, M.A. Structural and optical characterization of PVA:KMnO4 based solid polymer electrolyte. Results Phys. 2016, 6, 1103–1108. [Google Scholar] [CrossRef] [Green Version]
- Muhammad, F.F.; Aziz, S.; Hussein, S.A. Effect of the dopant salt on the optical parameters of PVA:NaNO3 solid polymer electrolyte. J. Mater. Sci. Mater. Electron. 2015, 26, 521–529. [Google Scholar] [CrossRef]
- Nemade, K.R.; Waghuley, S.A. Synthesis of MgO nanoparticles by solvent mixed spray pyrolysis technique for optical in-vestigation. Int. J. Met. 2014, 2014, 389416. [Google Scholar]
- Moss, T.S. Relations between the Refractive Index and Energy Gap of Semiconductors. Phys. Status Solidi (B) 1985, 131, 415–427. [Google Scholar] [CrossRef]
- Ali, F.M. Synthesis and Characterization of a Novel Erbium Doped Poly(vinyl alcohol) Films for Multifunctional Optical Materials. J. Inorg. Organomet. Polym. Mater. 2020, 30, 2418–2429. [Google Scholar] [CrossRef]
- Reddy, R.R.; Anjaneyulu, S. Analysis of the Moss and Ravindra relations. Phys. Status Solidi (B) 1992, 174, K91–K93. [Google Scholar] [CrossRef]
- Abdel-Baset, T.; Elzayat, M.; Mahrous, S. Characterization and Optical and Dielectric Properties of Polyvinyl Chloride/Silica Nanocomposites Films. Int. J. Polym. Sci. 2016, 2016, 1707018. [Google Scholar] [CrossRef] [Green Version]
- Anani, M.; Mathieu, C.; Lebid, S.; Amar, Y.; Chama, Z.; Abid, H. Model for calculating the refractive index of a III–V semiconductor. Comput. Mater. Sci. 2007, 41, 570–575. [Google Scholar] [CrossRef]
- Kumar, V.; Singh, J.K. Model for calculating the refractive index of different materials. J. Pure Appl. Phys. 2010, 48, 571–574. Available online: http://nopr.niscair.res.in/handle/123456789/9962 (accessed on 2 February 2023).
- Hervé, P.; Vandamme, L. General relation between refractive index and energy gap in semiconductors. Infrared Phys. Technol. 1994, 35, 609–615. [Google Scholar] [CrossRef]
- Li, Y.; Xia, Y.; Liu, K.; Ye, K.; Wang, Q.; Zhang, S.; Huang, Y.; Liu, H. Constructing Fe-MOF-Derived Z-Scheme Photocatalysts with Enhanced Charge Transport: Nanointerface and Carbon Sheath Synergistic Effect. ACS Appl. Mater. Interfaces 2020, 12, 25494–25502. [Google Scholar] [CrossRef]
- Cabuk, M.; Gündüz, B. Controlling the optical properties of polyaniline doped by boric acid particles by changing their doping agent and initiator concentration. Appl. Surf. Sci. 2017, 424, 345–351. [Google Scholar] [CrossRef]
- Abdel-Aziz, M.M.; Algarni, H.; Alshehri, A.M.; Yahia, I.S.; Ali, H.E. Study the impact of terbium additions in the microstructure, optical and electrical properties of polyvinyl alcohol. Mater. Res. Express 2019, 6, 125321. [Google Scholar] [CrossRef]
- Ali, H.E.; Khairy, Y. Facile synthesis, structure, AFM, thermal, and optical analysis of BiI3/PVAL nanocomposite films for laser CUT-OFF optical devices. Vacuum 2020, 180, 109640. [Google Scholar] [CrossRef]
- Bouzidi, A.; Jilani, W.; Yahia, I.S.; Zahran, H.Y.; Assiri, M.A. Optical Analysis and UV-Blocking Filter of Cadmium Iodide-Doped Polyvinyl Alcohol Polymeric Composite Films: Synthesis and Dielectric Properties. J. Inorg. Organomet. Polym. Mater. 2020, 30, 3940–3952. [Google Scholar] [CrossRef]
- Singh, P.K.; Nagarale, R.; Pandey, S.P.; Rhee, H.W.; Bhattacharya, B. Present status of solid state photoelectrochemical solar cells and dye sensitized solar cells using PEO-based polymer electrolytes. Adv. Nat. Sci. Nanosci. Nanotechnol. 2011, 2, 023002. [Google Scholar] [CrossRef]
- Muthupandeeswari, A.; Kalyani, P.; Vickraman, P. Evaluation of vital features of PVA–CaCO3 nanocomposite films for biodegradable packaging applications. Polym. Bull. 2022, 79, 65–85. [Google Scholar] [CrossRef]
- Kao, K.C. Dielectric Phenomena in Solids; Elsevier Academic Press: San Diego, CA, USA, 2004. [Google Scholar] [CrossRef]
- Aziz, S.B.; Abdullah, R.M. Crystalline and amorphous phase identification from the tanδ relaxation peaks and impedance plots in polymer blend electrolytes based on [CS:AgNt]x:PEO(x-1) (10 ≤ x ≤ 50). Electrochim. Acta 2018, 285, 30–46. [Google Scholar] [CrossRef]
- Aziz, S.B. The Study of Dielectric Properties and Conductivity Relaxation of Ion Conducting Chitosan:NaTf Based Solid Electrolyte. Int. J. Electrochem. Sci. 2018, 13, 10274–10288. [Google Scholar] [CrossRef]
- Ramya, C.; Selvasekarapandian, S.; Hirankumar, G.; Savitha, T.; Angelo, P. Investigation on dielectric relaxations of PVP–NH4SCN polymer electrolyte. J. Non-Cryst. Solids 2008, 354, 1494–1502. [Google Scholar] [CrossRef]
- Eldeeb, T.M.; El Nemr, A.; Khedr, M.H.; El-Dek, S.; Imam, N.G. Novel three-dimensional chitosan-carbon nanotube–PVA nanocomposite hydrogel for removal of Cr6+ from wastewater. Desalination Water Treat. 2020, 184, 163–177. [Google Scholar] [CrossRef]
- Aziz, S.B. Modifying Poly(Vinyl Alcohol) (PVA) from Insulator to Small-Bandgap Polymer: A Novel Approach for Organic Solar Cells and Optoelectronic Devices. J. Electron. Mater. 2016, 45, 736–745. [Google Scholar] [CrossRef]
- Aziz, S.B. Role of Dielectric Constant on Ion Transport: Reformulated Arrhenius Equation. Adv. Mater. Sci. Eng. 2016, 2016, 2527013. [Google Scholar] [CrossRef] [Green Version]
- Mallakpour, S.; Khadem, E. Facile and cost-effective preparation of PVA/modified calcium carbonate nanocompo-sites via ultrasonic irradiation: Application in adsorption of heavy metal and oxygen permeation property. Ultrason. Sonochem. 2017, 39, 430–438. [Google Scholar] [CrossRef]
- Aziz, S.B.; Abdullah, R.M.; Rasheed, M.A.; Ahmed, H.M. Role of Ion Dissociation on DC Conductivity and Silver Nanoparticle Formation in PVA:AgNt Based Polymer Electrolytes: Deep Insights to Ion Transport Mechanism. Polymers 2017, 9, 338. [Google Scholar] [CrossRef] [Green Version]
- Bouzidi, A.; Jilani, W.; Guermazi, H.; Yahia, I.S.; Zahran, H.Y.; Sakr, G.B. The effect of zinc iodide on the physicochemical properties of highly flexible transparent poly (vinyl alcohol) based polymeric composite films: Opto-electrical performance. J. Mater. Sci. Mater. Electron. 2019, 30, 11799–11806. [Google Scholar] [CrossRef]
- Jilani, W.; Bouzidi, A.; Yahia, I.S.; Guermazi, H.; Zahran, H.Y.; Saker, G. Effect of organic dyes on structural properties, linear optics and impedance spectroscopy of methyl orange (C.I. acid orange 52) doped polyvinyl alcohol composite thin films. J. Mater. Sci. Mater. Electron. 2018, 29, 16446–16453. [Google Scholar] [CrossRef]
- El-Sayed, H.E.A.; Ali, H.A.M.; Salem, G.F.; Mahmoud, M.A. Characteristics of Dielectric Behavior and AC Electrical Con-ductivity of Bulk Antimony Sulfide (Sb2S3). Arab J. Nucl. Sci. Appl. 2020, 53, 93–101. [Google Scholar]
- Aziz, S.B.; Abidin, Z.H.Z. Electrical Conduction Mechanism in Solid Polymer Electrolytes: New Concepts to Arrhenius Equation. J. Soft Matter 2013, 2013, 323868. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, H.T.; Abdullah, O.G. Structural and ionic conductivity characterization of PEO:MC-NH4I proton-conducting polymer blend electrolytes based films. Results Phys. 2020, 16, 102861. [Google Scholar] [CrossRef]
- Manjunath, A.; Deepa, T.; Supreetha, N.K.; Irfan, M. Studies on AC Electrical Conductivity and Dielectric Properties of PVA/NH4NO3 Solid Polymer Electrolyte Films. Adv. Mater. Phys. Chem. 2015, 5, 295–301. [Google Scholar] [CrossRef] [Green Version]
- Marf, A.S.; Abdullah, R.M.; Aziz, S.B. Structural, Morphological, Electrical and Electrochemical Properties of PVA: CS-Based Proton-Conducting Polymer Blend Electrolytes. Membranes 2020, 10, 71. [Google Scholar] [CrossRef]
- Irfan, M.; Manjunath, A.; Mahesh, S.S. Studies on structural characterization and electrical properties of NaF doped PVA-PVP blend electrolyte films. AIP Conf. Proc. 2020, 2244, 100009. [Google Scholar] [CrossRef]
- Bouzidi, A.; Jilani, W. Study the effect of sodium molybdate dihydrate powders on the structural, optical, and impedance parameters of NA-MOAT-04-C.2HYD/PolVA polymeric composite electrolyte films. Mater. Sci. Eng. B 2022, 280, 115693. [Google Scholar] [CrossRef]
- Asandulesa, M.; Chibac-Scutaru, A.L.; Culica, M.E.; Melinte, V.; Coseri, S. Cellulose-based films with enhanced load of nitrogen containing heterocycles: The impact on the surface morphology and proton conductivity. Appl. Surf. Sci. 2023, 607, 155077. [Google Scholar] [CrossRef]
- Asandulesa, M.; Kostromin, S.; Tameev, A.; Aleksandrov, A.; Bronnikov, S. Molecular Dynamics and Conductivity of a PTB7:PC71BM Photovoltaic Polymer Blend: A Dielectric Spectroscopy Study. ACS Appl. Polym. Mater. 2021, 3, 4869–4878. [Google Scholar] [CrossRef]


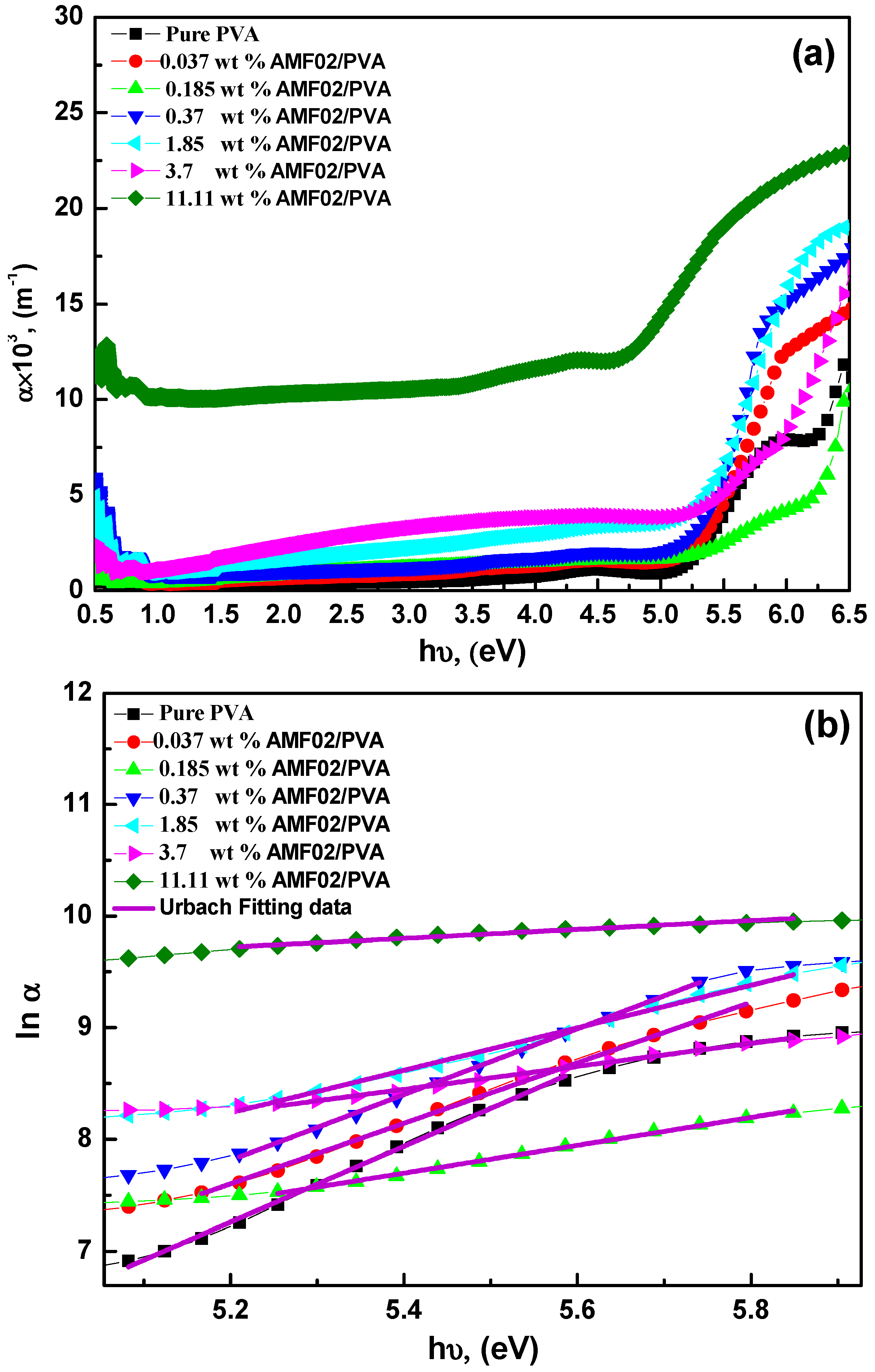
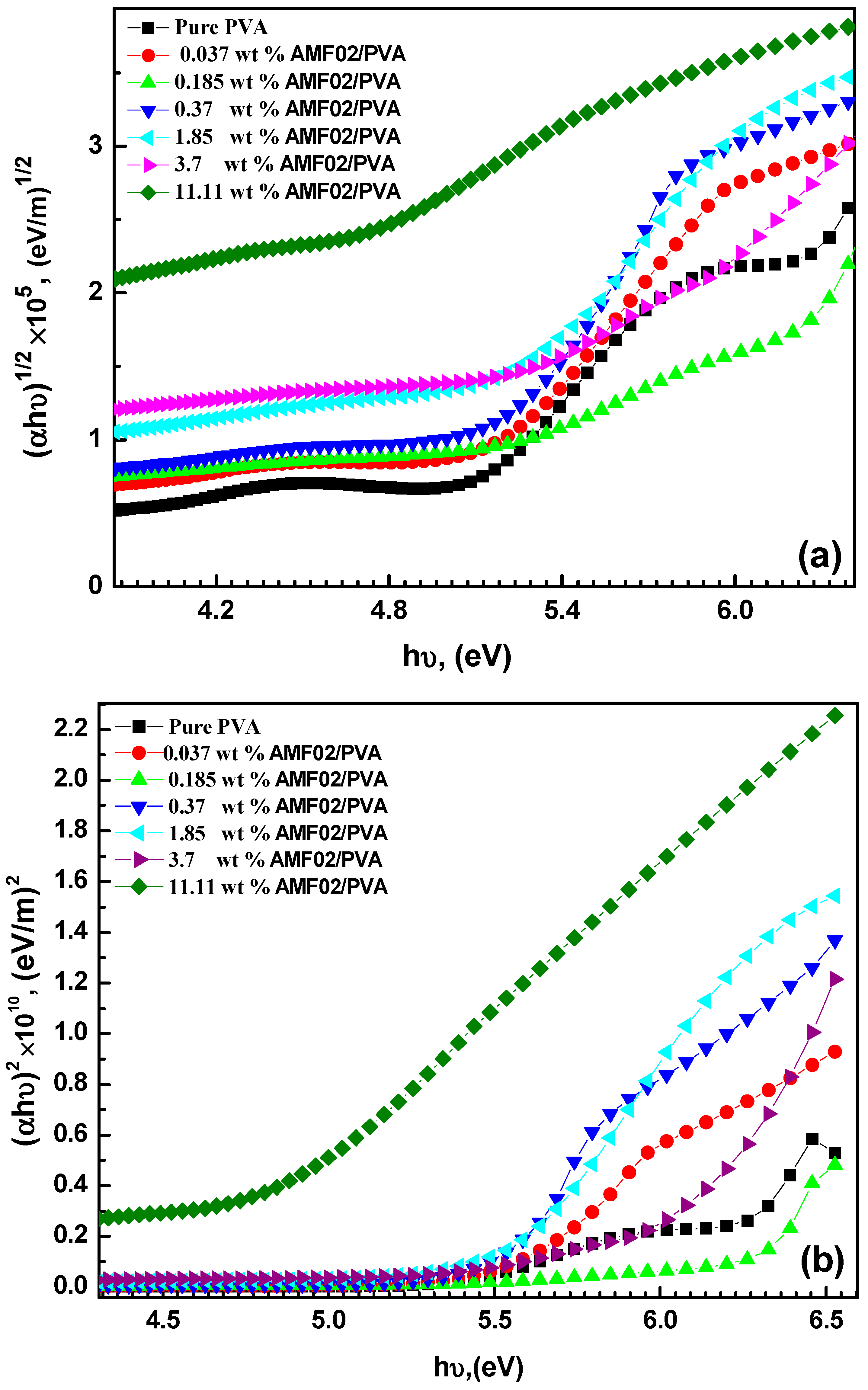





| Samples | Eu, (eV) | Eg (eV) Indirect | Eg (eV) Direct | References |
|---|---|---|---|---|
| Pure PVA | 0.294 | 5.08 | 5.25 | Present work |
| 0.037 wt% AMF02 | 0.368 | 4.95 | 5.33 | |
| 0.185 wt% AMF02 | 0.800 | 4.59 | 5.33 | |
| 0.37 wt% AMF02 | 0.339 | 4.81 | 5.21 | |
| 1.85 wt% AMF02 | 0.524 | 4.57 | 5.18 | |
| 3.7 wt% AMF02 | 0.971 | 4.16 | 5.63 | |
| 11.11 wt% AMF02 | 2.508 | 2.86 | 4.58 | |
| NH4I/PVA (Pure-3.7 wt%) | 0.812 − 0.369 | 5.268 − 4.797 | 5.89 −5.202 | [9] |
| LiNO3-doped PVA (Pure-4 wt%) | 0.596 −0.418 | 5.014 −4.614 | 5.576 −5.386 | [15] |
| Samples | Refractive index (n) | ||||||
|---|---|---|---|---|---|---|---|
| Moss | Ravindra | Hervé and Vandamme | Reddy | Anani | Kumar and Singh | Average | |
| (A) | |||||||
| Pure PVA | 2.079 | 2.147 | 1.889 | 1.966 | 2.384 | 1.993 | 2.076 |
| 0.037 wt%AMF02 | 2.093 | 2.161 | 1.911 | 1.992 | 2.41 | 2.0105 | 2.096 |
| 0.185 wt%AMF02 | 2.132 | 2.202 | 1.974 | 2.0679 | 2.482 | 2.060 | 2.153 |
| 0.37 wt%AMF02 | 2.108 | 2.176 | 1.934 | 2.021 | 2.438 | 2.029 | 2.118 |
| 1.85 wt%AMF02 | 2.135 | 2.204 | 1.977 | 2.072 | 2.486 | 2.063 | 2.156 |
| 3.7 wt%AMF02 | 2.186 | 2.257 | 2.058 | 2.166 | 2.568 | 2.126 | 2.227 |
| 11.11 wt%AMF02 | 2.400 | 2.478 | 2.391 | 2.540 | 2.828 | 2.399 | 2.506 |
| (B) | |||||||
| Pure PVA | 2.062 | 2.129 | 1.863 | 1.933 | 2.35 | 1.972 | 2.051 |
| 0.037 wt%AMF02 | 2.054 | 2.121 | 1.851 | 1.918 | 2.334 | 1.963 | 2.040 |
| 0.185 wt%AMF02 | 2.054 | 2.121 | 1.851 | 1.918 | 2.334 | 1.963 | 2.040 |
| 0.37 wt%AMF02 | 2.0664 | 2.133 | 1.869 | 1.941 | 2.358 | 1.977 | 2.057 |
| 1.85 wt%AMF02 | 2.069 | 2.136 | 1.874 | 1.947 | 2.364 | 1.981 | 2.062 |
| 3.7 wt%AMF02 | 2.0267 | 2.092 | 1.807 | 1.863 | 2.274 | 1.928 | 1.998 |
| 11.11 wt%AMF02 | 2.134 | 2.203 | 1.975 | 2.070 | 2.484 | 2.061 | 2.154 |
| Samples | σDC × 10−9, (siemens/m) | Frequency Exponent, (s) |
|---|---|---|
| Pure PVA | 0.545 ± 0.11 | 0.61 ± 0.02 |
| 0.037 wt% AMF02 | 0.620 ± 0.16 | 0.58 ± 0.03 |
| 0.185 wt% AMF02 | 0.639 ± 0.3 | 0.54 ± 0.05 |
| 0.37 wt% AMF02 | 0.813 ± 0.21 | 0.52 ± 0.04 |
| 1.85 wt% AMF02 | 2.727 ± 0.16 | 0.47 ± 0.03 |
| 3.7 wt% AMF02 | 4.451 ± 0.17 | 0.53 ± 0.04 |
| 11.11 wt% AMF02 | 73.205 ± 0.10 | 1.19 ± 0.06 |
| Samples | Rb (Ω) | Q (F) | s | σDC × 10−9, (siemens/m) |
|---|---|---|---|---|
| Pure PVA | 1.13 × 1010 | 7.5 × 10−11 | 0.976 | 0.361 |
| 0.037 wt% AMF02 | 2.97 × 1010 | 2.74 × 10−11 | 0.985 | 0.173 |
| 0.185 wt% AMF02 | 2.91 × 1010 | 6.4 × 10−11 | 0.987 | 0.141 |
| 0.37 wt% AMF02 | 3.82 × 1010 | 5.8 × 10−11 | 0.992 | 0.106 |
| 1.85 wt% AMF02 | 4.69 × 1010 | 4.98 × 10−11 | 0.977 | 0.087 |
| 3.7 wt% AMF02 | 6.1 × 1010 | 4.17 × 10−11 | 0.973 | 0.068 |
| 11.11 wt% AMF02 | 1.02 × 109 | 1.76 × 10−10 | 0.936 | 3.994 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zyoud, S.H.; Jilani, W.; Bouzidi, A.; AlAbdulaal, T.H.; Harraz, F.A.; Al-Assiri, M.S.; Yahia, I.S.; Zahran, H.Y.; Ibrahim, M.A.; Abdel-wahab, M.S. The Impact of Ammonium Fluoride on Structural, Absorbance Edge, and the Dielectric Properties of Polyvinyl Alcohol Films: Towards a Novel Analysis of the Optical Refractive Index, and CUT-OFF Laser Filters. Crystals 2023, 13, 376. https://doi.org/10.3390/cryst13030376
Zyoud SH, Jilani W, Bouzidi A, AlAbdulaal TH, Harraz FA, Al-Assiri MS, Yahia IS, Zahran HY, Ibrahim MA, Abdel-wahab MS. The Impact of Ammonium Fluoride on Structural, Absorbance Edge, and the Dielectric Properties of Polyvinyl Alcohol Films: Towards a Novel Analysis of the Optical Refractive Index, and CUT-OFF Laser Filters. Crystals. 2023; 13(3):376. https://doi.org/10.3390/cryst13030376
Chicago/Turabian StyleZyoud, Samer H., Wissal Jilani, Abdelfatteh Bouzidi, Thekrayat H. AlAbdulaal, Farid A. Harraz, Mohammad S. Al-Assiri, Ibrahim S. Yahia, Heba Y. Zahran, Medhat A. Ibrahim, and Mohamed Sh. Abdel-wahab. 2023. "The Impact of Ammonium Fluoride on Structural, Absorbance Edge, and the Dielectric Properties of Polyvinyl Alcohol Films: Towards a Novel Analysis of the Optical Refractive Index, and CUT-OFF Laser Filters" Crystals 13, no. 3: 376. https://doi.org/10.3390/cryst13030376
APA StyleZyoud, S. H., Jilani, W., Bouzidi, A., AlAbdulaal, T. H., Harraz, F. A., Al-Assiri, M. S., Yahia, I. S., Zahran, H. Y., Ibrahim, M. A., & Abdel-wahab, M. S. (2023). The Impact of Ammonium Fluoride on Structural, Absorbance Edge, and the Dielectric Properties of Polyvinyl Alcohol Films: Towards a Novel Analysis of the Optical Refractive Index, and CUT-OFF Laser Filters. Crystals, 13(3), 376. https://doi.org/10.3390/cryst13030376






