First-Principle Investigation of the Interface Properties of the Core-Shelled L12-Al3M (M = Sc, Zr, Er, Y) Phase
Abstract
1. Introduction
2. Computational Method
2.1. Calculation Details
2.2. Interface Energy
2.3. Interface Segregation Energy
3. Results and Discussion
3.1. Interface Energy
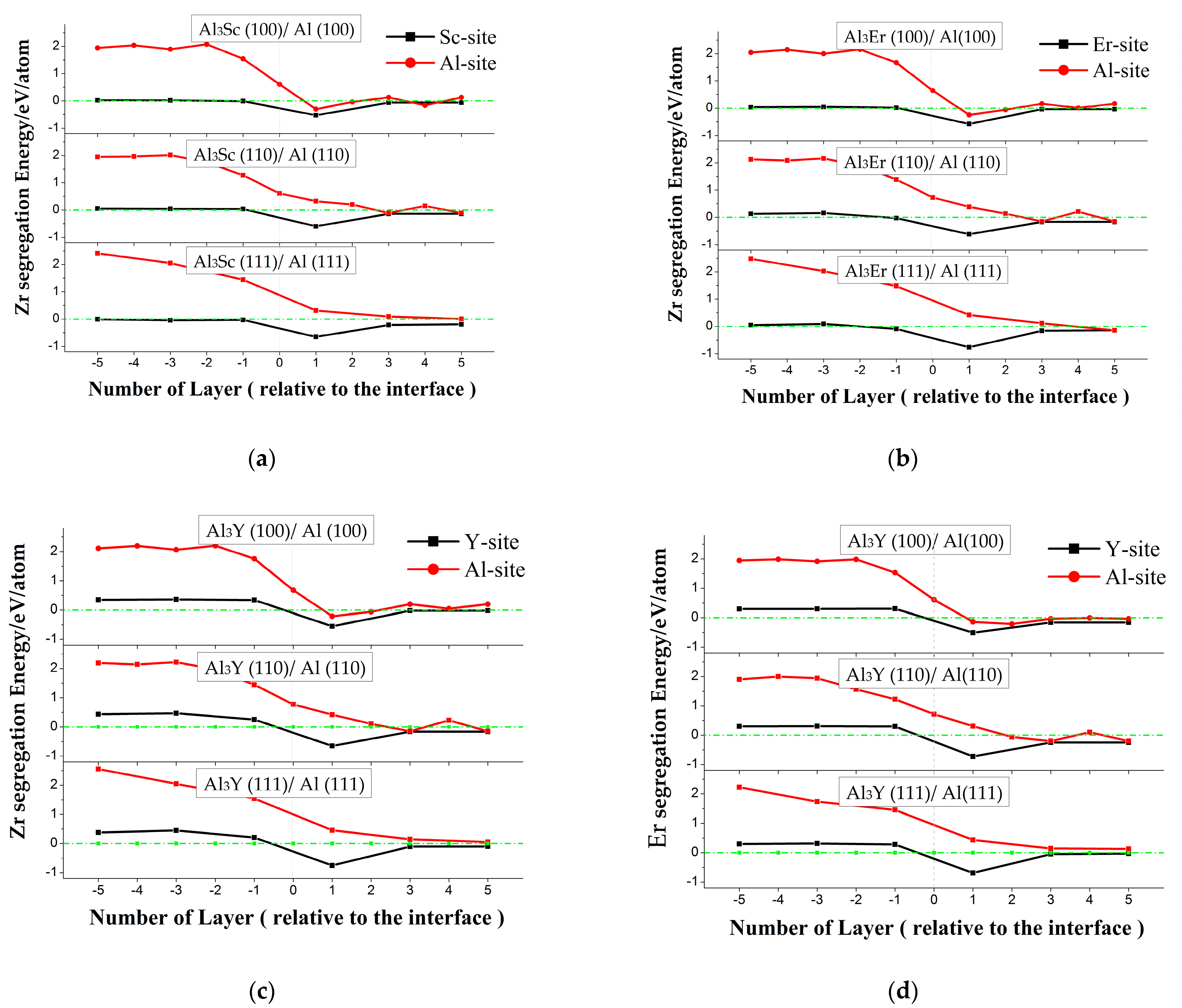
3.2. Segregation Energy
4. Conclusions
- (1)
- The order of the thermodynamically stable interface was Al3Zr/Al > Al3Sc/Al > Al3Er/Al > Al3Y/Al. The interfaces of Al3Sc/Al3Zr, Al3Er/Al3Zr, and Al3Y/Al3Er obtained negative interfacial energies and low coherent strain energies and were favorable to form a clear interface. The high coherent strain energy hindered the formation of the Al3Y/Al3Zr interface.
- (2)
- Zr atoms tended to segregate to the first atomic layer on the Al side of the Al/Al3Sc, Al/Al3Er, and Al/Al3Y interfaces and occupied the Sc, Er, and Y lattice positions on the layer. The driving effect of the Zr atom segregation to the Al3Y was stronger than that of Al3Sc and Al3Er.
- (3)
- Er atoms tended to segregate at the Al/Al3Y interface and accelerated the formation of core-shelled Al3Y/Al3Er. It can obtain double core-shelled Al3Y/Al3Er/Al3Zr microstructure for Al-Y-Er-Zr alloys.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Liu, L.; Xu, G.; Deng, Y.; Yu, Q.; Li, G.; Zhang, L.; Liu, B.; Fu, L.; Pan, Q. Existing form of Sc in metal-inert gas welded Al-0.60 Mg-0.75 Si alloy and its role in welding strength. Mater. Charact. 2023, 197, 112649. [Google Scholar] [CrossRef]
- Ye, J.; Pan, Q.; Liu, B.; Hu, Q.; Qu, L.; Wang, W.; Wang, X. Effects of co-addition of minor Sc and Zr on aging precipitates and mechanical properties of Al-Zn-Mg-Cu alloys. J. Mater. Res. Technol. 2023, 22, 2944–2954. [Google Scholar] [CrossRef]
- Deng, P.; Mo, W.; Ouyang, Z.; Tang, C.; Luo, B.; Bai, Z. Mechanical properties and corrosion behaviors of (Sc, Zr) modified Al-Cu-Mg alloy. Mater. Charact. 2022, 196, 112619. [Google Scholar] [CrossRef]
- Dorin, T.; Babaniaris, S.; Jiang, L.; Cassel, A.; Eggeman, A.; Robson, J. Precipitation sequence in Al-Sc-Zr alloys revisited. Materialia 2022, 26, 101608. [Google Scholar] [CrossRef]
- Ekaputra, C.; Weiss, D.; Mogonye, J.E.; Dunand, D.C. Eutectic, precipitation-strengthened alloy via laser fusion of blends of Al-7Ce-10Mg (wt.%), Zr, and Sc powders. Acta Mater. 2023, 246, 118676. [Google Scholar] [CrossRef]
- Zha, M.; Tian, T.; Jia, H.L.; Zhang, H.M.; Wang, H.Y. Sc/Zr ratio-dependent mechanisms of strength evolution and microstructural thermal stability of multi-scale hetero-structured Al–Mg–Sc–Zr alloys. J. Mater. Sci. Technol. 2023, 140, 67–78. [Google Scholar] [CrossRef]
- Harada, Y.; Dunand, D.C. Microstructure of Al3Sc with ternary transition-metal additions. Mater. Sci. Eng. A 2002, 329, 686–695. [Google Scholar] [CrossRef]
- Seidman, D.N.; Marquis, E.A.; Dunand, D.C. Precipitation strengthening at ambient and elevated temperatures of heat-treatable Al(Sc) alloys. Acta Mater. 2002, 50, 4021–4035. [Google Scholar] [CrossRef]
- Chen, S.; Li, C.; Lian, G.; Guo, C.; Du, Z. Effect of elastic strain energy on the core-shell structures of the precipitates in Al-Sc-Er alloys. J. Rare Earth. 2012, 30, 1276–1280. [Google Scholar] [CrossRef]
- Xue, D.; Wei, W.; Wen, S.; Wu, X.; Shi, W.; Zhou, X.; Gao, K.; Huang, H.; Nie, Z. Microstructural evolution of Al-Mg-Er-Zr alloy by equal channel angular extrusion at room temperature. Mater. Lett. 2022, 334, 133759. [Google Scholar] [CrossRef]
- Peng, G.; Chen, K.; Fang, H.; Chen, S. A study of nanoscale Al3 (Zr, Yb) dispersoids structure and thermal stability in Al–Zr–Yb alloy. Mater. Sci. Eng. A 2012, 535, 311–315. [Google Scholar] [CrossRef]
- Zhang, Y.; Gu, J.; Tian, Y.; Gao, H.; Wang, J.; Sun, B. Microstructural evolution and mechanical property of Al-Zr and Al-Zr-Y alloys. Mater. Sci. Eng. A 2014, 616, 132–140. [Google Scholar] [CrossRef]
- Gao, H.; Feng, W.; Wang, Y.; Gu, J.; Zhang, Y.; Wang, J.; Sun, B. Structural and compositional evolution of Al3(Zr, Y) precipitates in Al-Zr-Y alloy. Mater. Charact. 2016, 121, 195–198. [Google Scholar] [CrossRef]
- Christian, M.; David, C.D. Chemistry and structure of core/double-shell nanoscale precipitates in Al–6.5Li–0.07Sc–0.02Yb (at.%). Acta Mater. 2011, 59, 3398–3409. [Google Scholar]
- Booth-Morrison, C.; Dunand, D.C.; Seidman, D.N. Coarsening resistance at 400 °C of precipitation-strengthened Al–Zr–Sc–Er alloys. Acta Mater. 2011, 59, 7029–7042. [Google Scholar] [CrossRef]
- Van Dalen, M.E.; Dunand, D.C.; Seidman, D.N. Microstructural evolution and creep properties of precipitation-strengthened Al–0.06 Sc–0.02 Gd and Al–0.06 Sc–0.02 Yb (at.%) alloys. Acta Mater. 2011, 59, 5224–5237. [Google Scholar] [CrossRef]
- Zhao, Y.X.; Huang, Y.C.; Liu, Y. Insight into the stacking fault energy, dislocation, and thermodynamic properties of L12-Al3X (X = Sc, Ti, V) intermetallics from first-principles calculations. Mater. Today Commun. 2022, 31, 103684. [Google Scholar] [CrossRef]
- Zhang, C.; Jiang, Y.; Cao, F. Formation of coherent, core-shelled nano-particles in dilute Al-Sc-Zr alloys from the first-principles. J. Mater. Sci. Technol. 2019, 35, 930–938. [Google Scholar] [CrossRef]
- Zhang, C.; Yin, D.; Jiang, Y.; Wang, Y. Precipitation of L12-phase nano-particles in dilute Al-Er-Zr alloys from the first-principles. Comp. Mater. Sci. 2019, 162, 171–177. [Google Scholar] [CrossRef]
- Zhang, C.; Jiang, Y.; Guo, X. Formation and Relative Stabilities of Core-Shelled L12-Phase Nano-structures in Dilute Al–Sc–Er Alloys. Acta Metall. Sin.-Engl. 2020, 33, 1627–1634. [Google Scholar] [CrossRef]
- Zhang, C.M.; Xie, P.; Jiang, Y.; Zhan, S.; Ming, W.Q.; Chen, J.H.; Song, K.; Zhang, H. Double-Shelled L12 nano-structures in quaternary Al-Er-Sc-Zr alloys: Origin and critical significance. Acta Metall. Sin.-Engl. 2021, 34, 1277–1284. [Google Scholar] [CrossRef]
- Li, Y.; Huang, Y.; Zhang, X. Ab-initio studies of the micromechanics and interfacial behavior of Al3Y|fcc-Al. Metals 2022, 12, 1680. [Google Scholar] [CrossRef]
- Nityananda, R.; Hohenberg, P.; Kohn, W. Inhomogeneous electron gas. Resonance 2017, 22, 809–811. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmüller, J. Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set. Comp. Mater. Sci. 1996, 6, 15–50. [Google Scholar] [CrossRef]
- Kresse, G.; Joubert, D. From ultrasoft pseudopotentials to the projector augmented-wave method. Phys. Rev. B 1999, 59, 1758–1775. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1998, 77, 3865–3868. [Google Scholar] [CrossRef]
- Budimir, M.; Damjanovic, D.; Setter, N. Piezoelectric Response and Free Energy Instability in the Perovskite Crystals BaTiO3, PbTiO3 and Pb(Zr, Ti)O3. Phys. Rev. B 2006, 73, 4106. [Google Scholar] [CrossRef]
- Mao, Z.; Chen, W.; Seidman, D.N.; Wolverton, C. First-principles study of the nucleation and stability of ordered precipitates in ternary Al–Sc–Li alloys. Acta Mater. 2011, 59, 3012–3023. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, Z.K.; Chen, L.Q.; Wolverton, C. First-principles calculations of β″-Mg5Si6/α-Al interfaces. Acta Mater. 2007, 55, 5934–5947. [Google Scholar] [CrossRef]
- Li, J.; Zhang, M.; Zhou, Y.; Chen, G. First-principles study of Al/A13Ti heterogeneous nucleation interface. Appl. Surf. Sci. 2014, 307, 593–600. [Google Scholar] [CrossRef]
- Scheiber, D.; Pippan, R.; Puschnig, P.; Romaner, L. Ab initio search for cohesion-enhancing impurity elements at grain boundaries in molybdenum and tungsten. Model. Simul. Mater. Sci. Eng. 2016, 24, 085009. [Google Scholar] [CrossRef]
- Vo, N.Q.; Dunand, D.C.; Seidman, D.N. Improving aging and creep resistance in a dilute Al–Sc alloy by microalloying with Si, Zr and Er. Acta Mater. 2014, 63, 73–85. [Google Scholar] [CrossRef]
- Foley, J.C.; Perepezko, J.H.; Skinner, D.J. Formation of metastable L12-Al3Y through rapid solidification processing. Mater. Sci. Eng. A 1994, 179, 205–209. [Google Scholar] [CrossRef]
- Knipling, K.E.; Dunand, D.C.; Seidman, D.N. Precipitation evolution in Al–Zr and Al–Zr–Ti alloys during aging at 450–600 °C. Acta Mater. 2008, 56, 1182–1195. [Google Scholar] [CrossRef]
- Wang, Y.; Miao, Y.; Peng, P.; Gao, H.; Wang, J.; Sun, B. Ab initio investigation on preferred orientation at the Al/Al3 (Zr, Y) interface in Al–Zr–Y alloy. J. Appl. Phys. 2022, 131, 225111. [Google Scholar] [CrossRef]


| Interface | Lattice Parameter (Å) | Interface Energy γ (J/m2) | Strain Energy GS (meV/Atom) | |||
|---|---|---|---|---|---|---|
| a | b | c | ||||
| Al/Al3Sc | (100) | 4.075 | − | 36.884 | 0.135 | 3.5 |
| (110) | 4.077 | 5.818 | 34.608 | 0.204 | 3.2 | |
| (111) | 5.760 | 10.039 | 35.255 | 0.221 | 1.7 | |
| Al/Al3Zr | (100) | 4.061 | − | 37.182 | 0.078 | 2.3 |
| (110) | 4.063 | 5.824 | 34.693 | 0.109 | 1.3 | |
| (111) | 5.768 | 10.015 | 35.500 | 0.076 | 1.3 | |
| Al/Al3Er | (100) | 4.177 | − | 37.064 | 0.181 | 8.8 |
| (110) | 4.183 | 5.955 | 35.196 | 0.185 | 7.7 | |
| (111) | 5.933 | 10.239 | 35.452 | 0.227 | 9.0 | |
| Al/Al3Y | (100) | 4.201 | − | 37.068 | 0.197 | 9.4 |
| (110) | 4.203 | 5.986 | 35.355 | 0.185 | 9.6 | |
| (111) | 5.964 | 10.286 | 35.513 | 0.231 | 10.8 | |
| Interface | Lattice Parameter (Å) | Interface Energy γ (J/m2) | Strain Energy GS (meV/Atom) | |||
|---|---|---|---|---|---|---|
| a | b | c | ||||
| Al3Sc/Al3Zr | (100) | 4.102 | - | 36.982 | −0.079 | 0.1 |
| (110) | 4.099 | 5.798 | 34.913 | −0.021 | 0.2 | |
| (111) | 5.801 | 10.050 | 35.579 | −0.056 | 0.2 | |
| Al3Er/Al3Zr | (100) | 4.247 | - | 38.218 | −0.087 | 4.6 |
| (110) | 4.249 | 6.028 | 35.868 | −0.047 | 6.5 | |
| (111) | 6.007 | 10.398 | 36.709 | −0.062 | 5.4 | |
| Al3Zr/Al3Y | (100) | 4.171 | - | 37.554 | −0.088 | 6.9 |
| (110) | 4.171 | 5.899 | 35.511 | −0.048 | 9.1 | |
| (111) | 5.895 | 10.216 | 36.112 | −0.056 | 7.9 | |
| Al3Er/Al3Y | (100) | 4.188 | - | 37.673 | −0.018 | 0.1 |
| (110) | 4.187 | 5.922 | 35.644 | −0.004 | 0.2 | |
| (111) | 5.915 | 10.254 | 36.204 | −0.0004 | 0.2 | |
| Solute Atoms | Interface | ΔEAl3M (eV/Atom) | ΔEAl (eV/Atom) | |
|---|---|---|---|---|
| Zr | Al3Sc/Al | (100)/(100) | 0.552 | 0.464 |
| (110)/(110) | 0.653 | 0.459 | ||
| (111)/(111) | 0.644 | 0.457 | ||
| Al3Er/Al | (100)/(100) | 0.615 | 0.540 | |
| (110)/(110) | 0.749 | 0.449 | ||
| (111)/(111) | 0.810 | 0.617 | ||
| Al3Y/Al | (100)/(100) | 0.898 | 0.530 | |
| (110)/(110) | 1.086 | 0.488 | ||
| (111)/(111) | 1.131 | 0.653 | ||
| Er | Al3Y/Al | (100)/(100) | 0.807 | 0.354 |
| (110)/(110) | 1.030 | 0.480 | ||
| (111)/(111) | 0.991 | 0.659 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, Y.; Zhan, S.; Nie, B.; Liu, S.; Qi, H.; Liu, F.; Fan, T.; Chen, D. First-Principle Investigation of the Interface Properties of the Core-Shelled L12-Al3M (M = Sc, Zr, Er, Y) Phase. Crystals 2023, 13, 420. https://doi.org/10.3390/cryst13030420
Song Y, Zhan S, Nie B, Liu S, Qi H, Liu F, Fan T, Chen D. First-Principle Investigation of the Interface Properties of the Core-Shelled L12-Al3M (M = Sc, Zr, Er, Y) Phase. Crystals. 2023; 13(3):420. https://doi.org/10.3390/cryst13030420
Chicago/Turabian StyleSong, Yu, Songtao Zhan, Baohua Nie, Shuai Liu, Haiying Qi, Fangjun Liu, Touwen Fan, and Dongchu Chen. 2023. "First-Principle Investigation of the Interface Properties of the Core-Shelled L12-Al3M (M = Sc, Zr, Er, Y) Phase" Crystals 13, no. 3: 420. https://doi.org/10.3390/cryst13030420
APA StyleSong, Y., Zhan, S., Nie, B., Liu, S., Qi, H., Liu, F., Fan, T., & Chen, D. (2023). First-Principle Investigation of the Interface Properties of the Core-Shelled L12-Al3M (M = Sc, Zr, Er, Y) Phase. Crystals, 13(3), 420. https://doi.org/10.3390/cryst13030420





