Synergistic Electrochemical Properties of Graphene Incorporated LCZ-Oxide Cathode for Low Temperature Solid Oxide Fuel Cell
Abstract
:1. Introduction
2. Experimental Technique
2.1. Synthesis of Cathode Materials
2.2. Characterizations
2.3. Conductivity Measurements
2.4. Fuel Cell Fabrication and Performance
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Winter, U.; Weidner, H. Hydrogen for the mobility of the future results of GM/Opel’s well-to-wheel studies in north America and Europe. Fuel Cells 2003, 3, 76–83. [Google Scholar] [CrossRef]
- Abdalla, A.M.; Hossain, S.; Azad, A.T.; Petra, P.M.I.; Begum, F.; Eriksson, S.G.; Azad, A.K. Nanomaterials for solid oxide fuel cells: A review. Renew. Sustain. Energy Rev. 2018, 82, 353–368. [Google Scholar] [CrossRef]
- Abbas, G.; Raza, R.; Ashfaq, M.; Chaudhry, M.; Khan, A.; Ahmad, I.; Zhu, B. Electrochemical study of nanostructured electrode for low-temperature solid oxide fuel cell (LTSOFC). Int. J. Energy Res. 2014, 38, 518–523. [Google Scholar] [CrossRef]
- Solid Oxide Fuel Cells, Illinois Institute of Technology Notes. Available online: https://mypages.iit.edu/~smart/garrear/fuelcells.htm (accessed on 5 February 2018).
- Mumtaz, S.; Ahmad, M.A.; Raza, R.; Arshad, M.S.; Ahmed, B.; Ashiq, M.N.; Abbas, G. Nano grained Sr and Zr co-doped BaCeO3 electrolytes for intermediate temperature solid oxide fuel cells. Ceram. Int. 2017, 43, 14354–14360. [Google Scholar] [CrossRef]
- Liu, F.; Diercks, D.; Hussain, A.M.; Dale, N.; Furuya, Y.; Miura, Y.; Fukuyama, Y.; Duan, C. Nanocomposite Catalyst for High-Performance and Durable Intermediate-Temperature Methane-Fueled Metal-Supported Solid Oxide Fuel Cells. ACS Appl. Mater. Interfaces 2022, 14, 53840–53849. [Google Scholar] [CrossRef]
- Liu, F.; Duan, C. Direct-Hydrocarbon Proton-Conducting Solid Oxide Fuel Cells. Sustainability 2021, 13, 4736. [Google Scholar] [CrossRef]
- Suda, S.; Itagaki, M.; Node, E.; Takahashi, S.; Kawano, M.; Yoshida, H.; Inagaki, T. Preparation of SOFC anode composites by spray pyrolysis. J. Eur. Ceram. Soc. 2006, 26, 593–597. [Google Scholar] [CrossRef]
- Khan, M.A.; Xu, C.; Song, Z.; Raza, R.; Ahmad, M.A.; Abbas, G.; Zhu, B. Synthesize and characterization of ceria based nano-composite materials for low temperature solid oxide fuel cell. Int. J. Hydrog. Energy 2018, 43, 6310–6317. [Google Scholar] [CrossRef]
- Fang, X.; Zhu, G.; Xia, C.; Liu, X.; Meng, G. Synthesis and properties of Ni–SDC cermets for IT–SOFC anode by co-precipitation. Solid State Ion. 2004, 168, 31–36. [Google Scholar] [CrossRef]
- Yang, C.; Yang, Z.; Jin, C.; Xiao, G.; Chen, F.F.; Han, M. Sulfur-Tolerant redox-reversible anode material for direct hydrocar-bon solid oxide fuel cells. Adv. Mater. 2012, 24, 1439–1443. [Google Scholar] [CrossRef]
- Misono, T.; Murata, K.; Fukui, T.; Chaichanawong, J.; Sato, K.; Abe, H.; Naito, M. Ni-SDC cermet anode fabricated from NiO–SDC composite powder for intermediate temperature SOFC. J. Power Sources 2006, 157, 754–757. [Google Scholar] [CrossRef]
- Wang, W.G.; Mogensen, M.B. High-performance lanthanum-ferrite-based cathode for SOFC. Solid State Ion. 2005, 176, 457–462. [Google Scholar] [CrossRef]
- Tietz, F.; Buchkremer, H.-P.; Stöver, D. Components manufacturing for solid oxide fuel cells. Solid State Ion. 2002, 152, 249–259. [Google Scholar] [CrossRef]
- Huang, K.; Lee, H.; Goodenough, J. Sr-and Ni-doped LaCoO3 and LaFeO3 perovskites new cathode materials for solid-oxide fuel cells. J. Electrochem. Soc. 1998, 145, 3220–3227. [Google Scholar] [CrossRef]
- Jun, A.; Yoo, S.; Gwon, O.; Shin, J.; Kim, G. Thermodynamic and electrical properties of Ba0.5Sr0.5Co0.8Fe0.2O3−δ and La0.6Sr0.4Co0.2Fe0.8O3−δ for intermediate-temperature solid oxide fuel cells. Electrochim. Acta 2013, 89, 372–376. [Google Scholar] [CrossRef]
- Ahmad, K.; Abbas, G.; Ahmad, M.A. Structural and electrical study of nanocomposite Fe0.2Ni00.3Cu0.5 anode with effect of graphene oxide (GO) for low-temperature SOFCs. Int. J. Energy Res. 2022, 46, 16949–16958. [Google Scholar] [CrossRef]
- Zhu, B.; Liu, X.; Zhou, P.; Yang, X.; Zhu, Z.; Zhu, W. Innovative solid carbonate-ceria composite electrolyte fuel cells. Fuel Cells Bull. 2001, 2002, 8–12. [Google Scholar] [CrossRef]
- Raza, R.; Wang, X.; Ma, Y.; Zhu, B. A nanostructure anode (Cu0.2Zn0.8) for low-temperature solid oxide fuel cell at 400–600 °C. J. Power Sources 2010, 195, 8067–8070. [Google Scholar] [CrossRef]
- Zhu, B.; Raza, R.; Abbas, G.; Singh, M. An electrolyte-free fuel cell constructed from one homogenous layer with mixed con-ductivity. Adv. Funct. Mater. 2011, 21, 2465–2469. [Google Scholar] [CrossRef]
- Raza, R.; Abbas, G.; Liu, Q.; Pate, I.; Zhu, B. La0.3Sr0.2Mn0.1Zn0.4 Oxide-Sm0.2Ce0.8O1.9 (LSMZ-SDC) Nanocomposite Cathode for Low Temperature SOFCs. J. Nanosci. Nanotechnol. 2012, 12, 4994–4997. [Google Scholar] [CrossRef]
- Fan, L.; Zhu, B.; Chen, M.; Wang, C.; Raza, R.; Qin, H.; Wang, X.; Wang, X.; Ma, Y. High performance transition metal oxide composite cathode for low temperature solid oxide fuel cells. J. Power Sources 2012, 203, 65–71. [Google Scholar] [CrossRef]
- Hussain, M.; Raza, R.; Ahmad, M.; Ali, A.; Ahmad, I.; Syed, W.; Abbas, G. Electrochemical study of natural gas fueled elec-trodes for low temperature solid oxide fuel cell. Int. J. Mod. Phys. B 2016, 30, 1650161. [Google Scholar] [CrossRef]
- Allen, M.J.; Tung, V.C.; Kaner, R.B. Honeycomb Carbon: A Review of Graphene. Chem. Rev. 2009, 110, 132–145. [Google Scholar] [CrossRef] [PubMed]
- Jee, Y.; Karimaghaloo, A.; Andrade, A.M.; Moon, H.; Li, Y.; Han, J.; Ji, S.; Ishihara, H.; Su, P.; Cha, S.W.; et al. Graphene-based Oxygen Reduction Electrodes for Low Temperature Solid Oxide Fuel Cells. Fuel Cells 2017, 17, 344–352. [Google Scholar] [CrossRef]
- Marinha, D.; Belmonte, M. Mixed-ionic and electronic conduction and stability of YSZ-graphene composites. J. Eur. Ceram. Soc. 2019, 39, 389–395. [Google Scholar] [CrossRef]
- Zheng, B.; Wang, J.; Wang, F.-B.; Xia, X.-H. Low-loading cobalt coupled with nitrogen-doped porous graphene as excellent electrocatalyst for oxygen reduction reaction. J. Mater. Chem. A 2014, 2, 9079–9084. [Google Scholar] [CrossRef]
- Fuchsbichler, B.; Stangl, C.; Kren, H.; Uhlig, F.; Koller, S. High capacity graphite–silicon composite anode material for lithi-um-ion batteries. J. Power Sources 2011, 196, 2889–2892. [Google Scholar] [CrossRef]
- Wu, Z.-S.; Ren, W.; Wen, L.; Gao, L.; Zhao, J.; Chen, Z.; Zhou, G.; Li, F.; Cheng, H.-M. Graphene Anchored with Co3O4 Nanoparticles as Anode of Lithium Ion Batteries with Enhanced Reversible Capacity and Cyclic Performance. ACS Nano 2010, 4, 3187–3194. [Google Scholar] [CrossRef]
- Pollak, E.; Geng, B.; Jeon, K.; Lucas, I.; Richardson, T.; Wang, F.; Kostecki, R. The interaction of Li+ with single-layer and few-layer graphene. Nano Lett. 2010, 10, 3386–3388. [Google Scholar] [CrossRef]
- Aslam, S.; Mustafa, F.; Ahmad, M. Facile synthesis of graphene oxide with significant enhanced properties for optoelec-tronic and energy devices. Ceram. Int. 2018, 44, 6823–6828. [Google Scholar] [CrossRef]
- Zhao, Y.; He, J.; Fan, L.; Ran, W.; Zhang, C.; Gao, D.; Gao, F. Synthesis and characterization of hierarchical porous LiNi-CuZn-oxides as potential electrode materials for low temperature solid oxide fuel cells. Int. J. Hydrog. Energy 2013, 38, 16558–16562. [Google Scholar] [CrossRef]
- Zhang, L.; Xiao, J.; Xie, Y.; Tang, Y.; Liu, J.; Liu, M. Behavior of strontium- and magnesium-doped gallate electrolyte in direct carbon solid oxide fuel cells. J. Alloys Compd. 2014, 608, 272–277. [Google Scholar] [CrossRef]
- Zhang, L.; Lan, R.; Kraft, A.; Tao, S. A stable intermediate temperature fuel cell based on doped-ceria–carbonate composite electrolyte and perovskite cathode. Electrochem. Commun. 2011, 13, 582–585. [Google Scholar] [CrossRef]
- Li, S.; Lü, Z.; Huang, X.; Wei, B.; Su, W. Electrical and thermal properties of (Ba0.5Sr0.5) 1−xSmxCo0.8Fe0.2O3−δ perovskite oxides. Solid State Ion. 2007, 178, 417–422. [Google Scholar] [CrossRef]
- Cai, D.; Song, M. Preparation of fully exfoliated graphite oxide nanoplatelets in organic solvents. J. Mater. Chem. 2007, 17, 3678–3680. [Google Scholar] [CrossRef]
- Yang, Z.; Xu, N.; Han, M.; Chen, F. Performance evaluation of La0.4Sr0.6Co0.2Fe0.7Nb0.1O3−δ as both anode and cathode material in solid oxide fuel cells. Int. J. Hydrog. Energy 2014, 39, 7402–7406. [Google Scholar] [CrossRef]
- Du, Z.; Zhao, H.; Yi, S.; Xia, Q.; Gong, Y.; Zhang, Y.; Świerczek, K. High-performance anode material Sr2FeMo0.65Ni0.35O6−δ with in situ exsolved nanoparticle catalyst. ACS Nano 2016, 10, 8660–8669. [Google Scholar] [CrossRef]
- Esmaeili, Y.; Bidram, E.; Zarrabi, A.; Amini, A.; Cheng, C. Graphene oxide and its derivatives as promising In-vitro bio-imaging platforms. Sci. Rep. 2020, 10, 18052. [Google Scholar] [CrossRef]
- Xiong, H.; Shchukin, D.; Möhwald, H.; Xu, Y.; Xia, Y. Sonochemical synthesis of highly luminescent zinc oxide nano-particles doped with magnesium (II). Angew Chem. Int. Ed. 2009, 48, 2727–2731. [Google Scholar] [CrossRef]
- Umar, A.; Lee, J.-H.; Kumar, R.; Al-Dossary, O.; Ibrahim, A.A.; Baskoutas, S. Development of highly sensitive and selective ethanol sensor based on lance-shaped CuO nanostructures. Mater. Des. 2016, 105, 16–24. [Google Scholar] [CrossRef]
- Korkishko, Y.; Fedorov, V. Ion Exchange in Single Crystals for Integrated Optics and Optoelectronics; Cambridge Int Science Publishing: Great Abington, UK, 1999. [Google Scholar]
- Fan, L.; Wang, C.; Osamudiamen, O.; Raza, R.; Singh, M.; Zhu, B. Mixed ion and electron conductive composites for single component fuel cells: I. Effects of composition and pellet thickness. J. Power Sources 2012, 217, 164–169. [Google Scholar] [CrossRef]
- Memon, S.A.; Shaikh, H.I.; Raza, R.; Mughal, Z.U.N.; Memon, A.A.; Memon, S. Graphene incorporated mesoporous perovskite with excellent conductivity and catalytic activity for low temperature solid oxide fuel cells. New J. Chem. 2022, 46, 12530–12539. [Google Scholar] [CrossRef]
- McIntosh, S.; Gorte, R.J. Direct Hydrocarbon Solid Oxide Fuel Cells. Chem. Rev. 2004, 104, 4845–4866. [Google Scholar] [CrossRef] [PubMed]
- Kong, W.; Zhang, M.; Han, Z.; Zhang, Q. A Theoretical Model for the Triple Phase Boundary of Solid Oxide Fuel Cell Electrospun Electrodes. Appl. Sci. 2019, 9, 493. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Ma, Y.; Raza, R.; Muhammed, M.; Zhu, B. Novel core–shell SDC/amorphous Na2CO3 nanocomposite electrolyte for low-temperature SOFCs. Electrochem. Commun. 2008, 10, 1617–1620. [Google Scholar] [CrossRef]
- Zhang, G.; Li, W.; Huang, W.; Cao, Z.; Shao, K.; Li, F.; Tang, C.; Li, C.; Chuanxin, H.; Zhang, Q.; et al. Strongly coupled Sm0.2Ce0.8O2-Na2CO3 nanocomposite for low temperature solid oxide fuel cells: One-step synthesis and super interfacial proton conduction. J. Power Sources 2018, 386, 56–65. [Google Scholar] [CrossRef]
- Solovyev, A.; Shipilova, A.; Smolyanskiy, E.; Rabotkin, S.; Semenov, V. The properties of intermediate-temperature solid ox-ide fuel cells with thin film gadolinium-doped ceria electrolyte. Membranes 2022, 12, 896. [Google Scholar] [CrossRef]
- Matsui, T.; Kosaka, T.; Inaba, M.; Mineshige, A.; Ogumi, Z. Effects of mixed conduction on the open-circuit voltage of interme-diate temperature SOFCs based on Sm-doped ceria electrolytes. Solid State Ion. 2005, 176, 663–668. [Google Scholar] [CrossRef]
- Zagórski, K.; Wachowski, S.; Szymczewska, D.; Mielewczyk-Gryń, A.; Jasiński, P.; Gazda, M. Performance of a single layer fuel cell based on a mixed proton-electron conducting composite. J. Power Sources 2017, 353, 230–236. [Google Scholar] [CrossRef]
- Xu, N.; Zhu, T.; Yang, Z.; Han, M. Fabrication and optimization of La0.4Sr0.6Co0.2Fe0.7Nb0.1O3−δ electrode for symmetric solid oxide fuel cell with zirconia based electrolyte. J. Mater. Sci. Technol. 2017, 33, 1329–1333. [Google Scholar] [CrossRef]
- Tan, C.; Huang, X.; Zhang, H. Synthesis and applications of graphene-based noble metal nanostructures. Mater. Today 2013, 16, 29–36. [Google Scholar] [CrossRef]
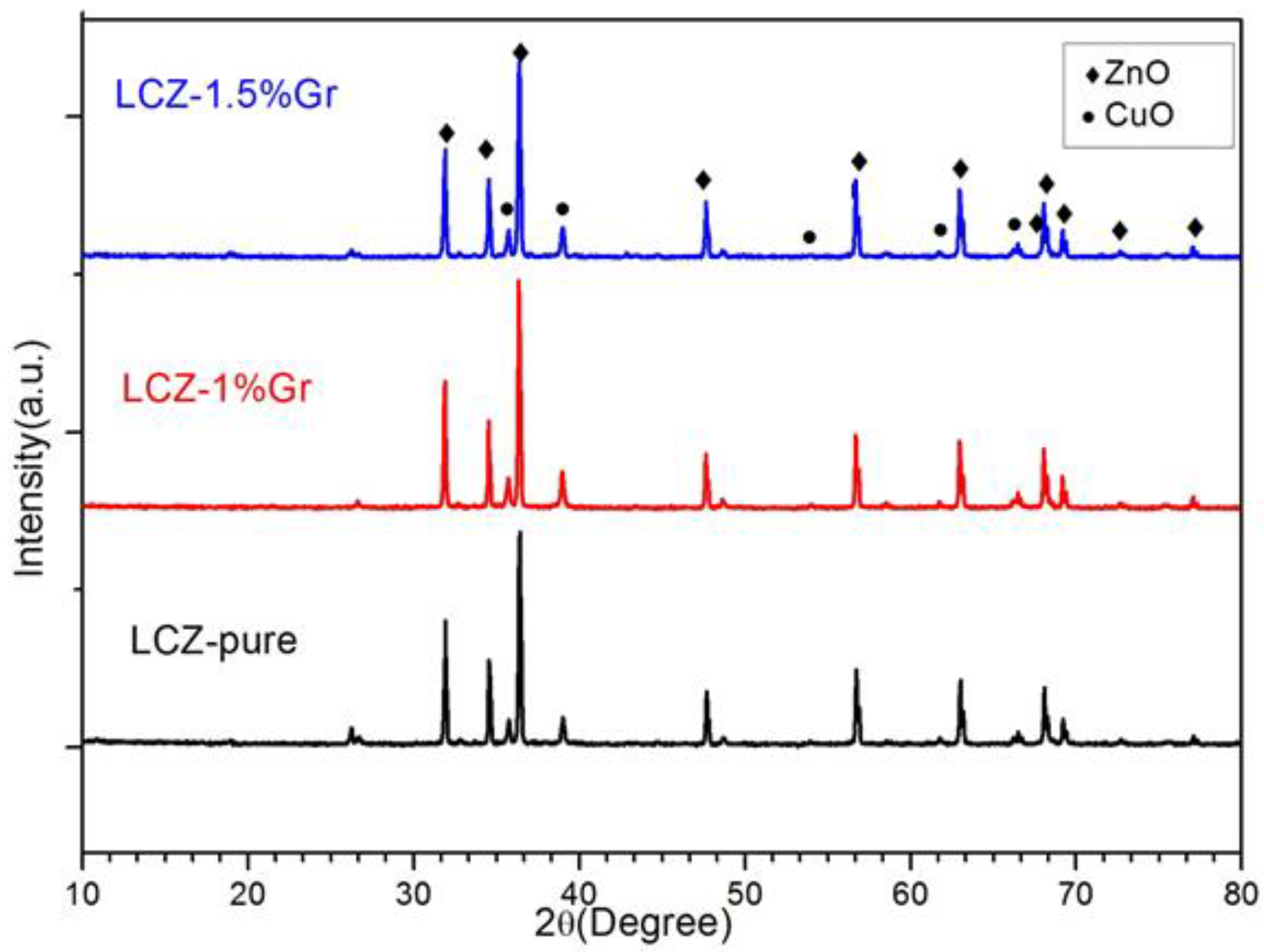
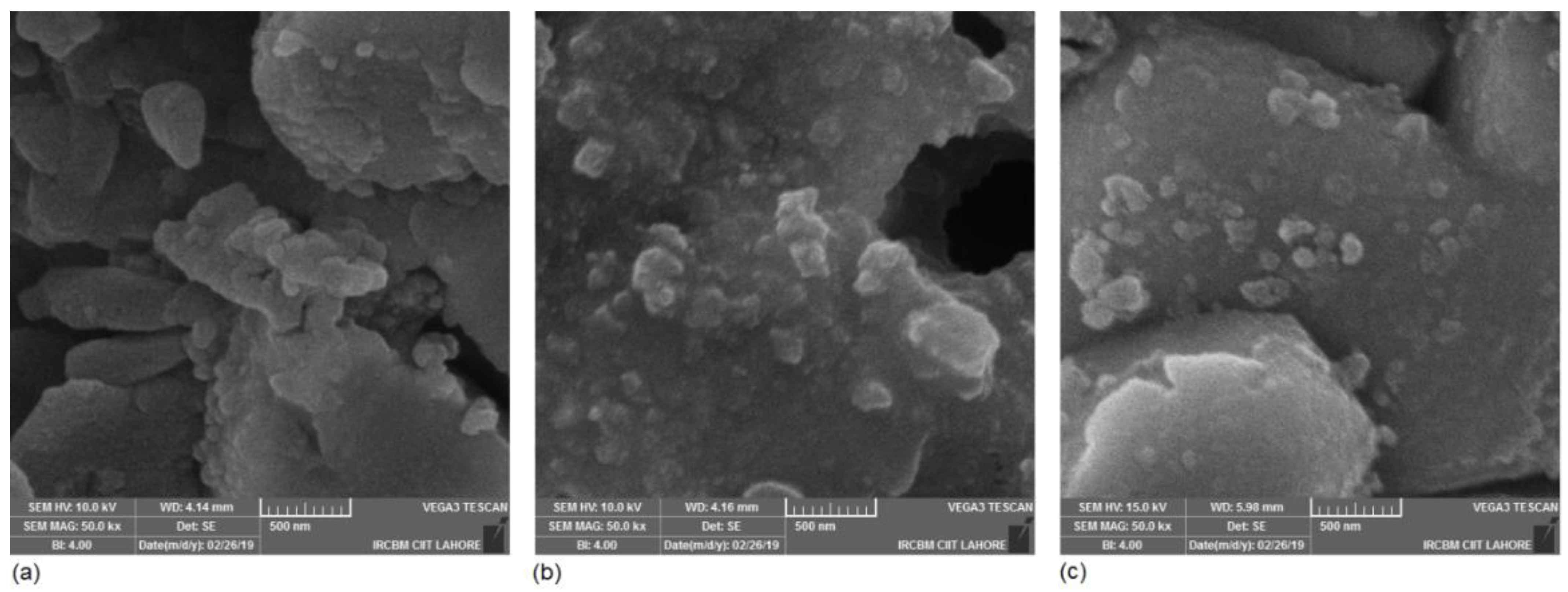




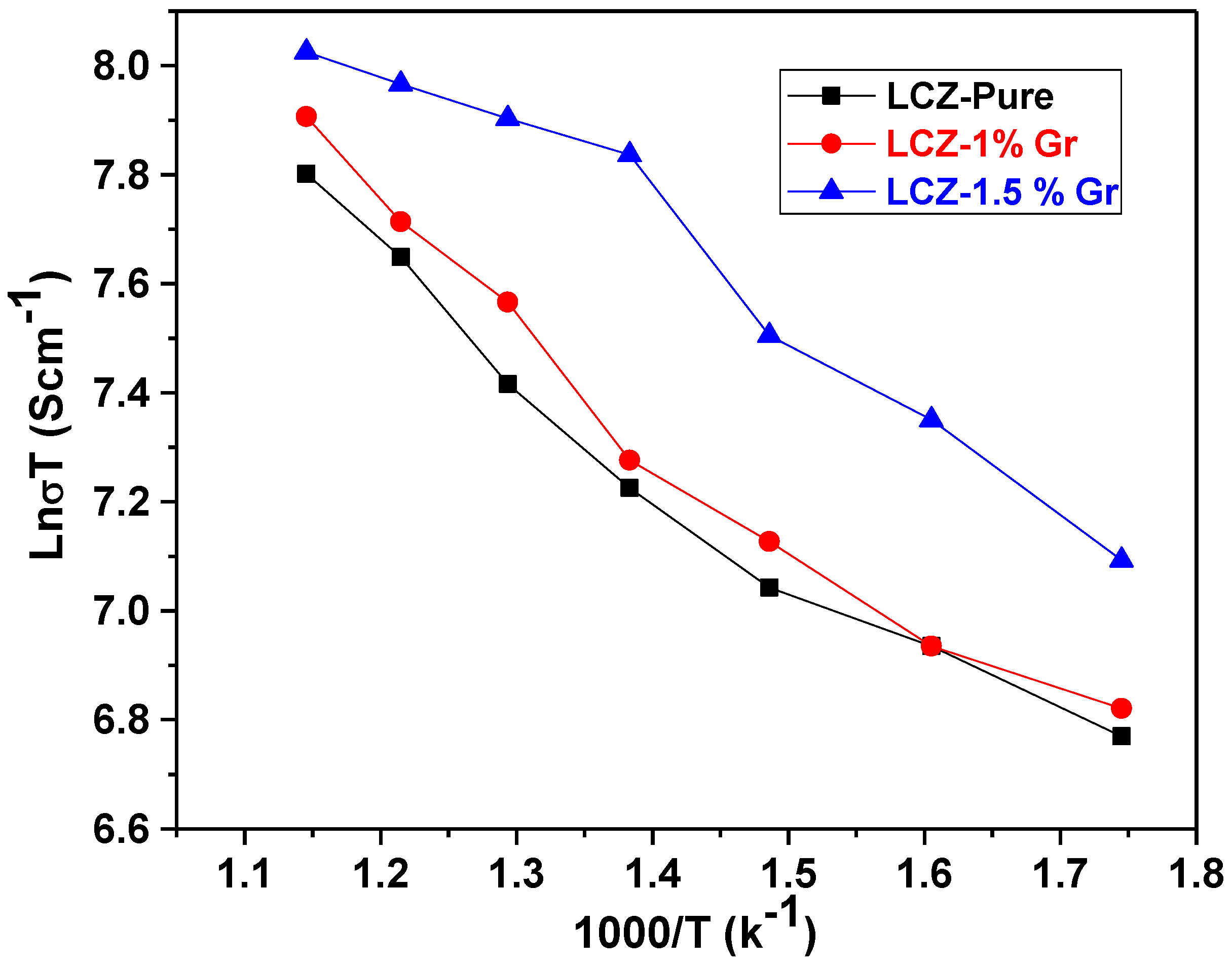
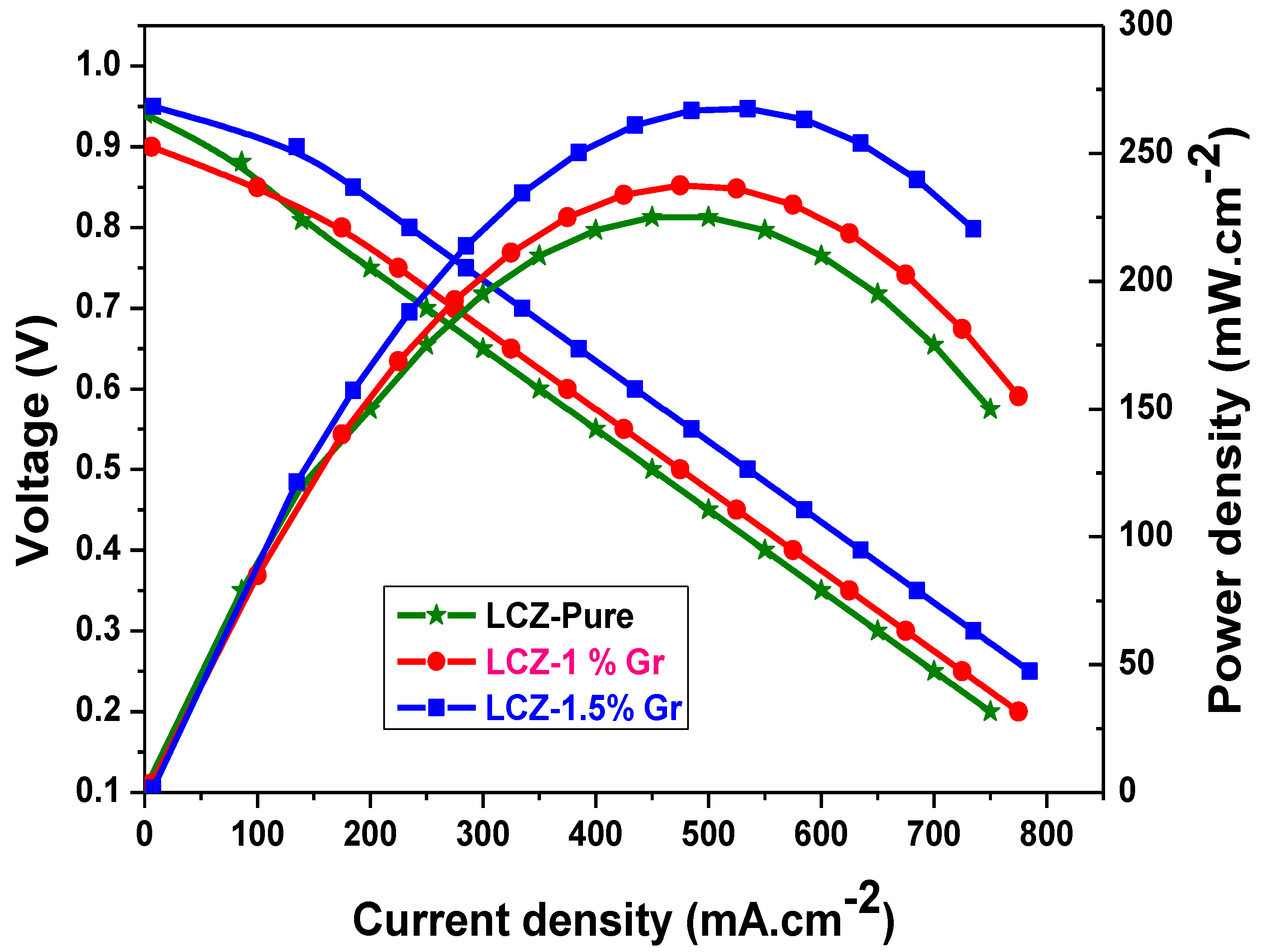
| Sample Name | Material Composition | Average Crystal Size (nm) | Conductivity (Scm−1) at 600 °C | Area-specific Resistance (Ωcm2) at 600 °C |
|---|---|---|---|---|
| LCZ-Pure | Li0.10Cu0.20Zn0.70-Pure | 110 | 2.8 | 0.071 |
| 1 wt.% Gr-LCZ | Li0.10Cu0.20Zn0.70—1 wt%. Graphene | 97 | 3.11 | 0.064 |
| 1.5 wt.% Gr-LCZ | Li0.10Cu0.20Zn0.70—1.5 wt.%. Graphene | 82 | 3.5 | 0.057 |
| Sample Name | Material Composition | Maximum OCV(V) | Current Density (mAcm−2) | Peak Power Density (mWcm−2) | References |
|---|---|---|---|---|---|
| BCZYO | BaCe0.6Zr0.2Y0.2O3−δ | 0.83 | 1505 | 3.86 | 42 |
| LSCFN | La0.4Sr0.6Co0.2Fe0.7Nb0.1O3−δ | 1.1 | 900 | 124 | 43 |
| LCZ-Pure | Li0.10Cu0.20Zn0.70-Pure | 0.9 | 750 | 225 | Our Work |
| LCZ-1%Gr | Li0.10Cu0.20Zn0.70-1 wt.% Graphene | 0.9 | 770 | 237.5 | |
| LCZ-1.5% Gr | Li0.10Cu0.20Zn0.70-1.5 wt.% Graphene | 0.95 | 785 | 267.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahmad, M.A.; Ahmad, K.; Li, H.; Gassoumi, A.; Raza, R.; Saleem, M.; Jafri, S.H.M.; Abbas, G. Synergistic Electrochemical Properties of Graphene Incorporated LCZ-Oxide Cathode for Low Temperature Solid Oxide Fuel Cell. Crystals 2023, 13, 434. https://doi.org/10.3390/cryst13030434
Ahmad MA, Ahmad K, Li H, Gassoumi A, Raza R, Saleem M, Jafri SHM, Abbas G. Synergistic Electrochemical Properties of Graphene Incorporated LCZ-Oxide Cathode for Low Temperature Solid Oxide Fuel Cell. Crystals. 2023; 13(3):434. https://doi.org/10.3390/cryst13030434
Chicago/Turabian StyleAhmad, Muhammad Ashfaq, Khalil Ahmad, Hu Li, Abdelaziz Gassoumi, Rizwan Raza, Muhammad Saleem, Syed Hassan Mujtaba Jafri, and Ghazanfar Abbas. 2023. "Synergistic Electrochemical Properties of Graphene Incorporated LCZ-Oxide Cathode for Low Temperature Solid Oxide Fuel Cell" Crystals 13, no. 3: 434. https://doi.org/10.3390/cryst13030434
APA StyleAhmad, M. A., Ahmad, K., Li, H., Gassoumi, A., Raza, R., Saleem, M., Jafri, S. H. M., & Abbas, G. (2023). Synergistic Electrochemical Properties of Graphene Incorporated LCZ-Oxide Cathode for Low Temperature Solid Oxide Fuel Cell. Crystals, 13(3), 434. https://doi.org/10.3390/cryst13030434









