Investigation of the Influence of High-Pressure Torsion and Solution Treatment on Corrosion and Tribocorrosion Behavior of CoCrMo Alloys for Biomedical Applications
Abstract
1. Introduction
2. Experimental Procedure
2.1. Material
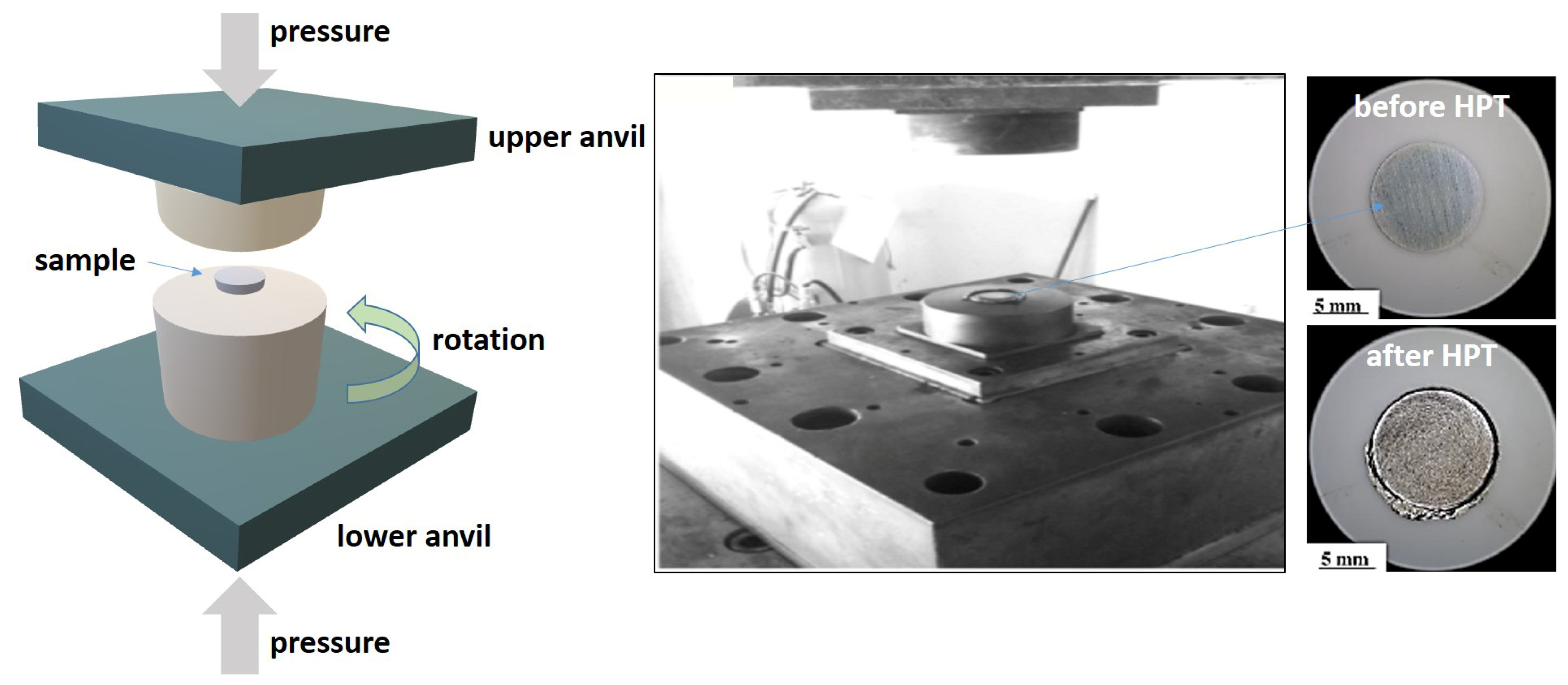
2.2. High-Pressure Torsion (HPT) Processes
2.3. In Vitro Corrosion Tests
2.4. Tribocorrosion Tests
2.5. Characterizations
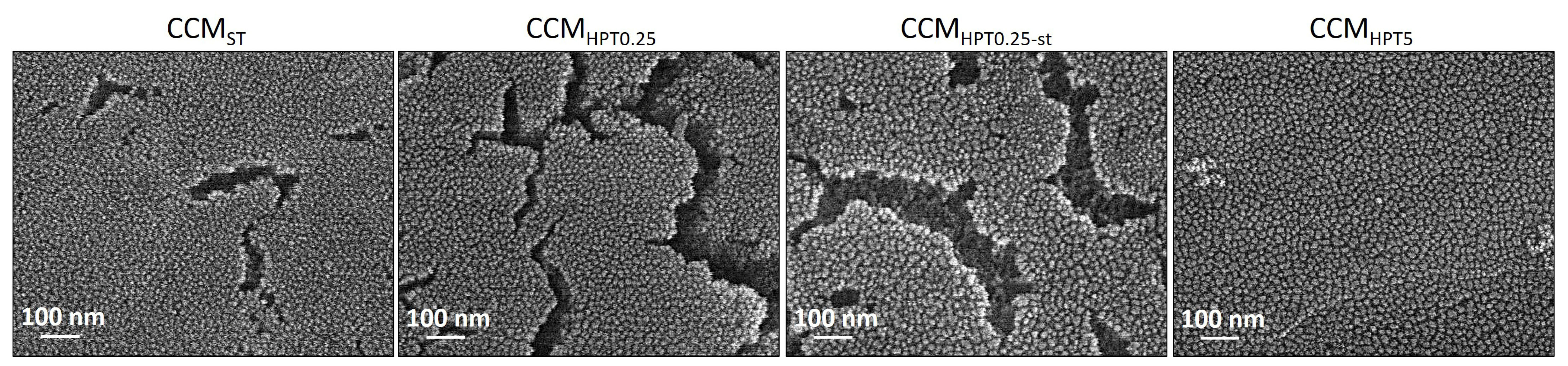
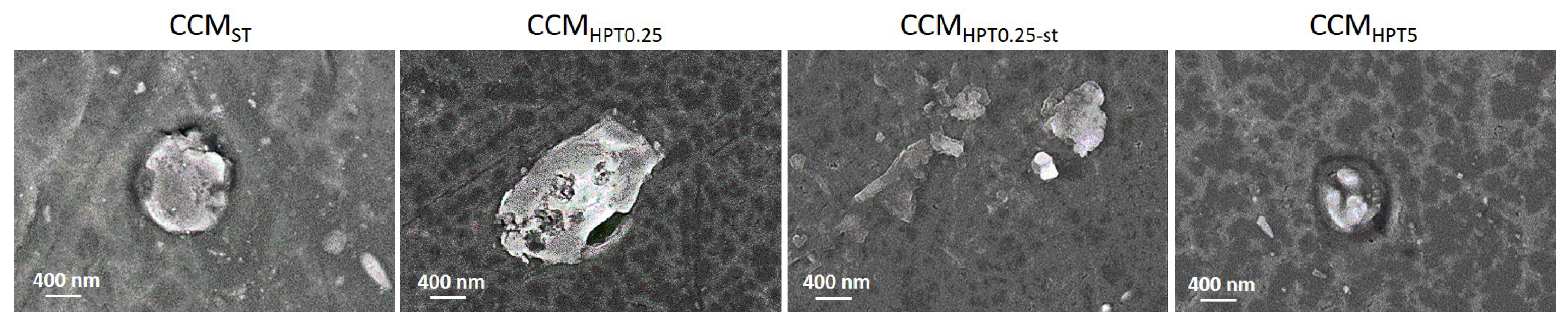
3. Results and Discussion
4. Conclusions
- All samples showed similar polarization curves and corrosion behavior according to PDS tests. There were no significant differences between corroded samples and all CCM samples had a similar passivation plateau.
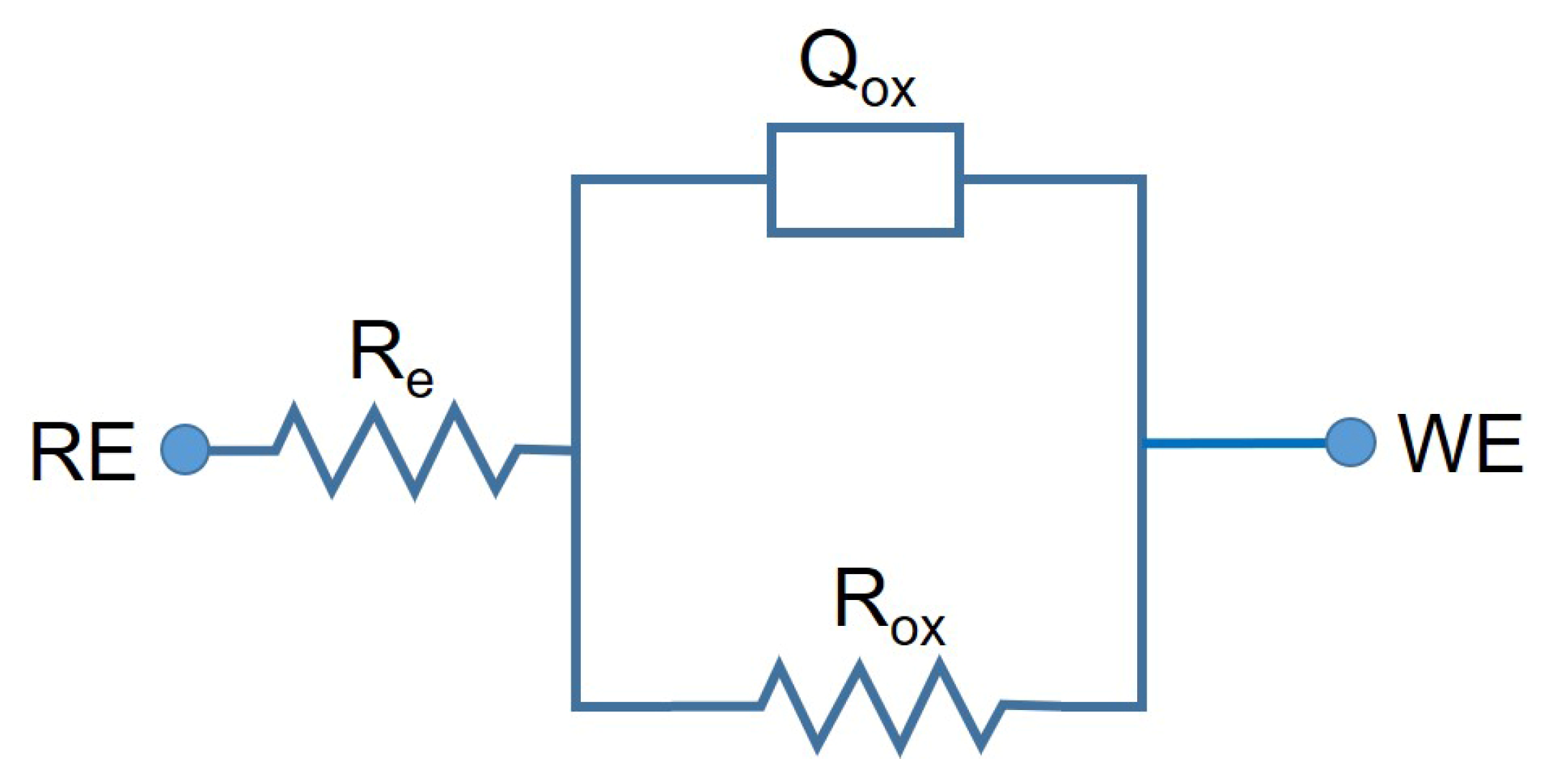
- After the EIS tests, it was determined that the corrosion resistance of CCMHPT0.25-st was slightly better than other test groups by comparing the semi-circular arcs of the samples in Nyquist diagrams. It was concluded that the HPT and ST processes did not significantly affect the corrosion behavior of samples when all corrosion results were considered.
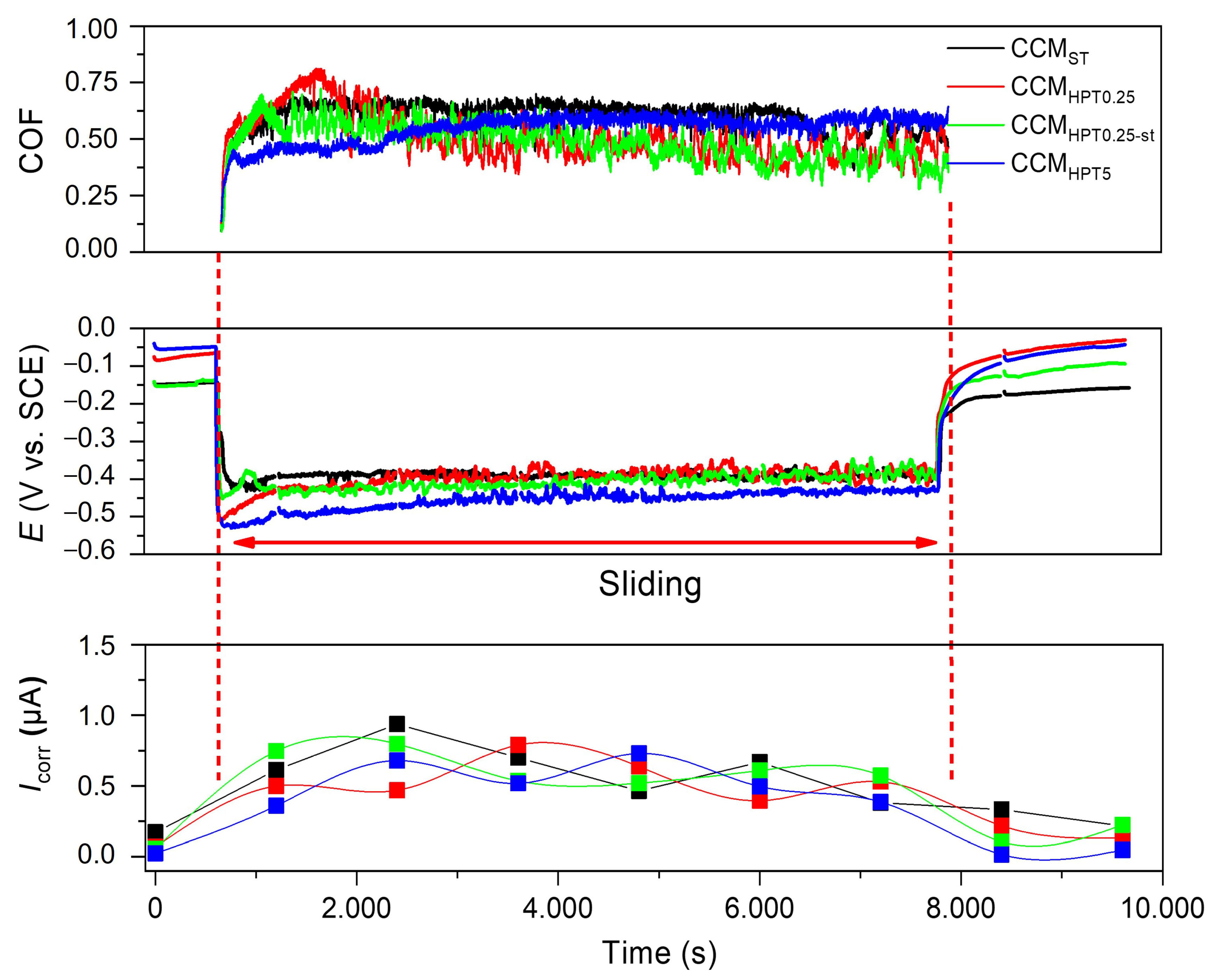
- During the tribocorrosion tests, the COF values of the samples changed between 0.5 and 0.7. The hardness variations on the CCMST and CCMHPT5 due to differences in their microstructure may have caused these oscillations on the COF graphs. During sliding, lower OCP and higher Icorr values were recorded due to the damage given to the passive film.
- The ST samples had a higher material loss during sliding in PBS solution at body temperature. The lowest material losses were obtained in CCMHPT0.25 and CCMHPT5 samples because of their hardness values and mechanical properties.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dikici, B.; Esen, Z.; Duygulu, O.; Koc, S.G. Corrosion of Metallic Biomaterials. In Advances in Metallic Biomaterials: Tissues, Materials and Biological Reactions; Niinomi, M., Narushima, T., Nakai, M., Eds.; Springer Series in Biomaterials Science and Engineering; Springer: Berlin/Heidelberg, Germany, 2015; Volume 3, pp. 275–303. ISBN 978-3-662-46835-7. [Google Scholar]
- Kajzer, W.; Szewczenko, J.; Kajzer, A.; Basiaga, M.; Jaworska, J.; Jelonek, K.; Nowińska, K.; Kaczmarek, M.; Orłowska, A. Physical Properties of Electropolished CoCrMo Alloy Coated with Biodegradable Polymeric Coatings Releasing Heparin after Prolonged Exposure to Artificial Urine. Materials 2021, 14, 2551. [Google Scholar] [CrossRef]
- Dikici, B.; Niinomi, M.; Topuz, M.; Koc, S.G.; Nakai, M. Synthesis of Biphasic Calcium Phosphate (BCP) Coatings on Β–type Titanium Alloys Reinforced with Rutile-TiO2 Compounds: Adhesion Resistance and in-Vitro Corrosion. J. Sol.-Gel. Sci. Technol. 2018, 87, 713–724. [Google Scholar] [CrossRef]
- Dikici, B.; Topuz, M. Production of Annealed Cold-Sprayed 316L Stainless Steel Coatings for Biomedical Applications and Their in-Vitro Corrosion Response. Prot. Met. Phys. Chem. Surf. 2018, 54, 333–339. [Google Scholar] [CrossRef]
- Zarka, M.; Dikici, B.; Niinomi, M.; Ezirmik, K.V.; Nakai, M.; Yilmazer, H. A Systematic Study of β-Type Ti-Based PVD Coatings on Magnesium for Biomedical Application. Vacuum 2021, 183, 109850. [Google Scholar] [CrossRef]
- Kopova, I.; Kronek, J.; Bacakova, L.; Fencl, J. A Cytotoxicity and Wear Analysis of Trapeziometacarpal Total Joint Replacement Implant Consisting of DLC-Coated Co-Cr-Mo Alloy with the Use of Titanium Gradient Interlayer. Diam. Relat. Mater. 2019, 97, 107456. [Google Scholar] [CrossRef]
- Guo, Z.; Pang, X.; Yan, Y.; Gao, K.; Volinsky, A.A.; Zhang, T.-Y. CoCrMo Alloy for Orthopedic Implant Application Enhanced Corrosion and Tribocorrosion Properties by Nitrogen Ion Implantation. Appl. Surf. Sci. 2015, 347, 23–34. [Google Scholar] [CrossRef]
- Milošev, I. CoCrMo Alloy for Biomedical Applications. In Biomedical Applications; Djokić, S., Ed.; Springer: Boston, MA, USA, 2012; pp. 1–72. [Google Scholar]
- Niinomi, M. Recent Metallic Materials for Biomedical Applications. Metall. Mater. Trans. A 2002, 33, 477–486. [Google Scholar] [CrossRef]
- Tunthawiroon, P.; Li, Y.; Chiba, A. Influences of Alloyed Si on the Corrosion Resistance of Co–Cr–Mo Alloy to Molten Al by Iso-Thermal Oxidation in Air. Corros. Sci. 2015, 100, 428–434. [Google Scholar] [CrossRef]
- Davim, J.P. Wear of Advanced Materials; John Wiley & Sons: Hoboken, NJ, USA, 2013; ISBN 9781848213524. [Google Scholar]
- Kajima, Y.; Takaichi, A.; Kittikundecha, N.; Nakamoto, T.; Kimura, T.; Nomura, N.; Kawasaki, A.; Hanawa, T.; Takahashi, H.; Wakabayashi, N. Effect of Heat-Treatment Temperature on Microstructures and Mechanical Properties of Co–Cr–Mo Alloys Fabricated by Selective Laser Melting. Mater. Sci. Eng. A 2018, 726, 21–31. [Google Scholar] [CrossRef]
- Varano, R.; Bobyn, J.D.; Medley, J.B.; Yue, S. Effect of Microstructure on the Dry Sliding Friction Behavior of CoCrMo Alloys Used in Metal-on-Metal Hip Implants. J. Biomed. Mater. Res. Part B Appl. Biomater. 2006, 76, 281–286. [Google Scholar] [CrossRef]
- Wu, K.; Li, B.; Guo, J. Fatigue Crack Growth and Fracture of Internal Fixation Materials in In Vivo Environments—A Review. Materials 2021, 14, 176. [Google Scholar] [CrossRef]
- Naerheim, Y.; Kendig, M.W. The Influence of Electrochemical Potential on Wear. Wear 1985, 104, 139–150. [Google Scholar] [CrossRef]
- Mischler, S.; Spiegel, A.; Landolt, D. The Role of Passive Oxide Films on the Degradation of Steel in Tribocorrosion Systems. Wear 1999, 225–229, 1078–1087. [Google Scholar] [CrossRef]
- García, I.; Drees, D.; Celis, J.P. Corrosion-Wear of Passivating Materials in Sliding Contacts Based on a Concept of Active Wear Track Area. Wear 2001, 249, 452–460. [Google Scholar] [CrossRef]
- Mischler, S.; Muñoz, A.I. Wear of CoCrMo Alloys Used in Metal-on-Metal Hip Joints: A Tribocorrosion Appraisal. Wear 2013, 297, 1081–1094. [Google Scholar] [CrossRef]
- Ren, F.; Zhu, W.; Chu, K. Fabrication, Tribological and Corrosion Behaviors of Ultra-Fine Grained Co-28Cr-6Mo Alloy for Biomedical Applications. J. Mech. Behav. Biomed. Mater. 2016, 60, 139–147. [Google Scholar] [CrossRef]
- Doni, Z.; Alves, A.C.; Toptan, F.; Gomes, J.R.; Ramalho, A.; Buciumeanu, M.; Palaghian, L.; Silva, F.S. Dry Sliding and Tribocorrosion Behaviour of Hot Pressed CoCrMo Biomedical Alloy as Compared with the Cast CoCrMo and Ti6Al4V Alloys. Mater. Des. 2013, 52, 47–57. [Google Scholar] [CrossRef]
- Ocran, E.K.; Guenther, L.E.; Brandt, J.-M.; Wyss, U.; Ojo, O.A. Corrosion and Fretting Corrosion Studies of Medical Grade CoCrMo Alloy in a Clinically Relevant Simulated Body Fluid Environment. Metall. Mater. Trans. A Phys. Metall. Mater. Sci. 2015, 46, 2696–2709. [Google Scholar] [CrossRef]
- Espallargas, N.; Torres, C.; Muñoz, A.I. A Metal Ion Release Study of CoCrMo Exposed to Corrosion and Tribocorrosion Conditions in Simulated Body Fluids. Wear 2015, 332–333, 669–678. [Google Scholar] [CrossRef]
- Igual Muñoz, A.; Casabán Julián, L. Influence of Electrochemical Potential on the Tribocorrosion Behaviour of High Carbon CoCrMo Biomedical Alloy in Simulated Body Fluids by Electrochemical Impedance Spectroscopy. Electrochim. Acta 2010, 55, 5428–5439. [Google Scholar] [CrossRef]
- Sadiq, K.; Black, R.A.; Stack, M.M. Bio-Tribocorrosion Mechanisms in Orthopaedic Devices: Mapping the Micro-Abrasion-Corrosion Behaviour of a Simulated CoCrMo Hip Replacement in Calf Serum Solution. Wear 2014, 316, 58–69. [Google Scholar] [CrossRef]
- Ribeiro, A.M.; Alves, A.C.; Rocha, L.A.; Silva, F.S.; Toptan, F. Synergism between Corrosion and Wear on CoCrMo-Al2O3 Biocomposites in a Physiological Solution. Tribol. Int. 2014, 91, 198–205. [Google Scholar] [CrossRef]
- Akahori, T.; Niinomi, M.; Otani, M. Notch Fatigue Properties of a Ti–29Nb–13Ta–4.6Zr Alloy for Biomedical Applications. J. Jpn. Inst. Light Met. 2005, 55, 575–581. [Google Scholar] [CrossRef]
- Isik, M.; Niinomi, M.; Liu, H.; Cho, K.; Nakai, M.; Horita, Z.; Narushima, T.; Ueda, K. Optimization of Microstructure and Mechanical Properties of Co–Cr–Mo Alloys by High-Pressure Torsion and Subsequent Short Annealing. Mater. Trans. 2016, 57, 1887–1896. [Google Scholar] [CrossRef]
- Yilmazer, H.; Niinomi, M.; Nakai, M.; Cho, K.; Hieda, J.; Todaka, Y.; Miyazaki, T. Mechanical Properties of a Medical β-Type Titanium Alloy with Specific Microstructural Evolution through High-Pressure Torsion. Mater. Sci. Eng. C 2013, 33, 2499–2507. [Google Scholar] [CrossRef]
- Valiev, R.Z.; Islamgaliev, R.K.; Alexandrov, I.V. Bulk Nanostructured Materials from Severe Plastic Deformation. Prog. Mater. Sci. 2000, 45, 103–189. [Google Scholar] [CrossRef]
- Mohan Agarwal, K.; Tyagi, R.K.; Chaubey, V.K.; Dixit, A. Comparison of Different Methods of Severe Plastic Deformation for Grain Refinement. IOP Conf. Ser. Mater. Sci. Eng. 2019, 691, 012074. [Google Scholar] [CrossRef]
- Azushima, A.; Kopp, R.; Korhonen, A.; Yang, D.Y.; Micari, F.; Lahoti, G.D.; Groche, P.; Yanagimoto, J.; Tsuji, N.; Rosochowski, A.; et al. Severe Plastic Deformation (SPD) Processes for Metals. CIRP Ann. 2008, 57, 716–735. [Google Scholar] [CrossRef]
- Smirnova, N.A.; Levit, V.I.; Pilyugin, V.I.; Kuznetsov, R.I.; Davydova, L.S.; Sazonova, V.A. Evolution of Structure of Fcc Single Crystals upon Large Plastic Deformations. Phys. Met. Metallogr. 1986, 61, 127–134. [Google Scholar]
- Hebesberger, T.; Stüwe, H.P.; Vorhauer, A.; Wetscher, F.; Pippan, R. Structure of Cu Deformed by High Pressure Torsion. Acta Mater. 2005, 53, 393–402. [Google Scholar] [CrossRef]
- Stolyarov, V.; Valiev, R. Metastable Nanostructured Alloys Processed by Severe Plastic Deformation. In Ultrafine Grained Mater II; Wiley: Hoboken, NJ, USA, 2002; pp. 209–218. [Google Scholar] [CrossRef]
- Rentenberger, C.; Waitz, T.; Karnthaler, H.P. HRTEM Analysis of Nanostructured Alloys Processed by Severe Plastic Deformation. Scr. Mater. 2004, 51, 789–794. [Google Scholar] [CrossRef]
- Namus, R.; Rainforth, W.M.; Huang, Y.; Langdon, T.G. Effect of Grain Size and Crystallographic Structure on the Corrosion and Tribocorrosion Behaviour of a CoCrMo Biomedical Grade Alloy in Simulated Body Fluid. Wear 2021, 478–479, 203884. [Google Scholar] [CrossRef]
- Isik, M.; Niinomi, M.; Cho, K.; Nakai, M.; Liu, H.; Yilmazer, H.; Horita, Z.; Sato, S.; Narushima, T. Microstructural Evolution and Mechanical Properties of Biomedical Co-Cr-Mo Alloy Subjected to High-Pressure Torsion. J. Mech. Behav. Biomed. Mater. 2016, 59, 226–235. [Google Scholar] [CrossRef] [PubMed]
- Isik, M.; Niinomi, M.; Cho, K.; Nakai, M.; Hieda, J.; Yilmazer, H.; Horita, Z.J.; Narushima, T. Microstructural Analysis of Biomedical Co-Cr-Mo Alloy Subjected to High-Pressure Torsion Processing. Key Eng. Mater. 2014, 616, 263–269. [Google Scholar] [CrossRef]
- Isik, M.; Niinomi, M.; Liu, H.; Cho, K.; Nakai, M.; Horita, Z.; Sato, S.; Narushima, T.; Yilmazer, H.; Nagasako, M. Grain Refinement Mechanism and Evolution of Dislocation Structure of Co-Cr-Mo Alloy Subjected to High-Pressure Torsion. Mater. Trans. 2016, 57, 1109–1118. [Google Scholar] [CrossRef]
- Figueiredo, R.B.; Pereira, P.H.R.; Aguilar, M.T.P.; Cetlin, P.R.; Langdon, T.G. Using Finite Element Modeling to Examine the Temperature Distribution in Quasi-Constrained High-Pressure Torsion. Acta Mater. 2012, 60, 3190–3198. [Google Scholar] [CrossRef]
- Hansen, N. Preface to the Viewpoint Set: “Metals and Alloys with a Structural Scale from the Micrometre to the Atomic Dimensions”. Scr. Mater. 2004, 51, 751–753. [Google Scholar] [CrossRef]
- Zheng, G.P.; Wang, Y.M.; Li, M. Atomistic Simulation Studies on Deformation Mechanism of Nanocrystalline Cobalt. Acta Mater. 2005, 53, 3893–3901. [Google Scholar] [CrossRef]
- Edalati, K.; Toh, S.; Arita, M.; Watanabe, M.; Horita, Z. High-Pressure Torsion of Pure Cobalt: Hcp-Fcc Phase Transformations and Twinning during Severe Plastic Deformation. Appl. Phys. Lett. 2013, 102, 181902. [Google Scholar] [CrossRef]
- Huang, J.Y.; Wu, Y.K.; Ye, H.Q.; Lu, K. Allotropic Transformation of Cobalt Induced by Ball Milling. Nanostruct. Mater. 1995, 6, 723–726. [Google Scholar] [CrossRef]
- Yilmazer, H.; Niinomi, M.; Cho, K.; Nakai, M.; Hieda, J.; Sato, S.; Todaka, Y. Microstructural Evolution of Precipitation-Hardened β-Type Titanium Alloy through High-Pressure Torsion. Acta Mater. 2014, 80, 172–182. [Google Scholar] [CrossRef]
- Zhilyaev, A.; Lee, S.; Nurislamova, G.; Valiev, R.; Langdon, T. Microhardness and Microstructural Evolution in Pure Nickel during High-Pressure Torsion. Scr. Mater. 2001, 44, 2753–2758. [Google Scholar] [CrossRef]
- Gong, X.; Li, Y.; Nie, Y.; Huang, Z.; Liu, F.; Huang, L.; Jiang, L.; Mei, H. Corrosion Behaviour of CoCrMo Alloy Fabricated by Electron Beam Melting. Corros. Sci. 2018, 139, 68–75. [Google Scholar] [CrossRef]
- Vidal, C.V.; Mu, A.I. Electrochimica Acta Effect of Thermal Treatment and Applied Potential on the Electrochemical Behaviour of CoCrMo Biomedical Alloy. Electrochim. Acta 2009, 54, 1798–1809. [Google Scholar] [CrossRef]
- Lewis, A.C.; Heard, P.J. The Effects of Calcium Phosphate Deposition upon Corrosion of CoCr Alloys and the Potential for Implant Failure. J. Biomed. Mater. Res.-Part A 2005, 75, 365–373. [Google Scholar] [CrossRef]
- Silva, J.I.; Alves, A.C.; Pinto, A.M.; Toptan, F. Corrosion and Tribocorrosion Behavior of Ti–TiB–TiNxin-Situ Hybrid Composite Synthesized by Reactive Hot Pressing. J. Mech. Behav. Biomed. Mater. 2017, 74, 195–203. [Google Scholar] [CrossRef]
- Lin, C.H.; Duh, J.G. Electrochemical Impedance Spectroscopy (EIS) Study on Corrosion Performance of CrAlSiN Coated Steels in 3.5 Wt.% NaCl Solution. Surf. Coat. Technol. 2009, 204, 784–787. [Google Scholar] [CrossRef]






| Co | Cr | Mo | Ni | Mn | Si | C | N | Fe |
|---|---|---|---|---|---|---|---|---|
| bal. | 27.7 | 5.87 | <0.01 | 0.58 | 0.50 | 0.045 | 0.14 | <0.1 |
| ID | Eocp (V) | Ecorr (V) | ipass (μA·cm−2) | Ebd (V) |
|---|---|---|---|---|
| CCMST | −0.23 (±0.10) | −0.25 (±0.11) | 1.57 (±0.48) | 0.43 (±0.01) |
| CCMHPT0.25 | −0.28 (±0.06) | −0.31 (±0.07) | 1.87 (±0.28) | 0.42 (±0.02) |
| CCMHPT0.25-st | −0.29 (±0.05) | −0.32 (±0.06) | 1.64 (±0.06) | 0.42 (±0.01) |
| CCMHPT5 | −0.30 (±0.05) | −0.33 (±0.07) | 1.41 (±0.21) | 0.42 (±0.00) |
| ID | Rox (MΩ·cm2) | Cox (µF·cm−2) |
|---|---|---|
| CCMST | 1.37 (±0.65) | 3.19 (±0.98) |
| CCMHPT0.25 | 1.78 (±1.22) | 3.36 (±1.51) |
| CCMHPT0.25-st | 1.97 (±1.06) | 2.48 (±0.81) |
| CCMHPT5 | 2.10 (±1.35) | 2.60 (±0.20) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yilmazer, H.; Caha, I.; Dikici, B.; Toptan, F.; Isik, M.; Niinomi, M.; Nakai, M.; Alves, A.C. Investigation of the Influence of High-Pressure Torsion and Solution Treatment on Corrosion and Tribocorrosion Behavior of CoCrMo Alloys for Biomedical Applications. Crystals 2023, 13, 590. https://doi.org/10.3390/cryst13040590
Yilmazer H, Caha I, Dikici B, Toptan F, Isik M, Niinomi M, Nakai M, Alves AC. Investigation of the Influence of High-Pressure Torsion and Solution Treatment on Corrosion and Tribocorrosion Behavior of CoCrMo Alloys for Biomedical Applications. Crystals. 2023; 13(4):590. https://doi.org/10.3390/cryst13040590
Chicago/Turabian StyleYilmazer, Hakan, Ihsan Caha, Burak Dikici, Fatih Toptan, Murat Isik, Mitsuo Niinomi, Masaaki Nakai, and Alexandra Cruz Alves. 2023. "Investigation of the Influence of High-Pressure Torsion and Solution Treatment on Corrosion and Tribocorrosion Behavior of CoCrMo Alloys for Biomedical Applications" Crystals 13, no. 4: 590. https://doi.org/10.3390/cryst13040590
APA StyleYilmazer, H., Caha, I., Dikici, B., Toptan, F., Isik, M., Niinomi, M., Nakai, M., & Alves, A. C. (2023). Investigation of the Influence of High-Pressure Torsion and Solution Treatment on Corrosion and Tribocorrosion Behavior of CoCrMo Alloys for Biomedical Applications. Crystals, 13(4), 590. https://doi.org/10.3390/cryst13040590










