Abstract
Sesquioxides of lanthanides, yttrium, and scandium are promising hosts for laser and scintillation materials; however, the crystallization of such compounds is complicated by very high melting temperatures, as well as polymorph transitions. This work reports for the first time the growth of Y2O3 and Y2−xScxO3 crystals by the Vertical Gradient Freezing method from tungsten crucibles, proposing an alternative to extremely expensive rhenium and iridium crucibles. Translucent Y2O3 samples are obtained, and their luminescent and scintillation parameters are evaluated. The main issues of Y2O3 crystallization under the proposed conditions are discussed, as well as ways of enhancing the crystal quality. Finally, polymorph transitions are avoided by decreasing the average radius of the rare earth cation by Y3+/Sc3+ substitution, providing transparent Y2−xScxO3 crystals with a cubic structure.
1. Introduction
Sesquioxides are attractive hosts for applications in optics, lasers, and scintillators. In particular, a fast scintillation response was reported in undoped Y2O3 and Sc2O3 [1], while Y2O3 and Lu2O3 doped with other rare earths were proposed as alpha detectors and luminophores [2,3,4], as well as laser elements for technical and medical applications [5,6]. In particular, the reported light yields of Sc2O3, Y2O3, and Lu2O3 were 16,200, 2600, and 200 ph/5.5 MeV-alpha, respectively. Under 137Cs gamma-ray excitation, the light yields of Sc2O3, Y2O3, and Lu2O3 were 11,500, 3200, and 500 ph/MeV, respectively [1]. The scintillation decay times of these sesquioxides comprised several tens of nanoseconds [1]. Therefore, sesquioxides of lanthanides, scandium, and yttrium possess low-to-moderate light yield and fast scintillation decay. These properties in combination with an extremely high density of up to 9.42 g/cm3 and effective atomic number of 68.8 in Lu2O3 [6] should provide an efficient registration of ionizing particles.
Lanthanide, scandium, and yttrium sesquioxides are compounds with extremely high melting temperatures of up to 2500 °C [7,8]. The technologies of their crystallization are complicated and energy consuming. Furthermore, a very limited range of materials may be used as crucibles for the crystallization of these compounds from melts. Some of the alternatives are a ceramic synthesis at temperatures well below the melting point [5,6,9,10,11,12,13], spark plasma sintering [1,14], and sol-gel synthesis [4]. Meanwhile, sesquioxide crystals can be obtained by direct crystallization from melts by crucible-free methods, such as laser heating pedestal growth [14], or from rhenium crucibles by micro pulling down, edge-defined film-fed growth [15,16,17,18]; however, crystal size is limited in these methods to a few millimeters in the cross-section. The largest ingots of up to 40 mm in diameter and 30 mm in length were obtained by the HEM method [19,20], but crystals stuck to crucibles after the growth process, thus increasing the risk of destruction of crystals and expensive crucibles.
Recent works [21,22] reported a successful growth of mixed of Y and Sc sesquioxides by the Czochralski method. Y3+/Sc3+ substitution decreases the averaged cationic radius in the composition, as well as reduces the crystallization temperature down to 2150 °C, making possible the usage of Ir crucibles. Furthermore, unlike in Y2O3, there are no polymorph transitions between H-, B(monoclinic)-, and C (cubic) phases in Y2−xScxO3 at a large enough x that ensures a direct crystallization by the Czochralski and other methods of growth from the melt. However, currently iridium is even more expensive than rhenium, and the lifetime of Ir crucibles at such temperatures is short, making this approach not economically feasible. At the same time, cheap tungsten crucibles with a melting temperature of ~3400 °C are also potentially applicable for the crystallization of sesquioxides from melts. For instance, Sc2O3:Pr,Ho was crystallized from W crucibles in [23], but no details of the process were provided. Following our experience [24,25,26], high-melting-point oxide crystals may be grown from W crucibles under reducing atmosphere conditions, but the process is complicated by tungsten oxidation and its interaction with melts.
This work explores possibilities of using W crucibles for the crystallization of sesquioxides on the example of Y2O3 and mixed Y2−xScxO3. The Vertical Gradient Freezing (VGF) method was chosen due to the possibility of protecting the melt from interaction with the reducing atmosphere in the growth chamber and good thermal insulation of the crystallizer in the absence of crystal/crucible pulling and any moving parts. Furthermore, this method should be scalable relatively easily. We report the methods to prevent the melt from interaction with the growth atmosphere and carbidization during the process of crystal growth, as well as structure, optical, and scintillation properties of the obtained crystals.
2. Materials and Methods
Powder of Y2O3 99.999% (Nexconn, Shenzhen, China) and Sc2O3 99.99% (Stanford Materials, Lake Forest, CA, USA) were used as starting materials. Bulk densities of raw material powders were around thrice lower as compared to single crystals. The powders were pressed into an Mo tube and sintered at temperatures from 1500 to 2250 °C to ensure the density of >95% with respect to the Y2O3 crystal. The largest density of >95% was achieved when the calcination temperature was increased up to 2250 °C. The sintered rods were then loaded into tungsten growth crucibles, which comprised tungsten rods of 10 mm in diameter with axially drilled holes of 6–9 mm in diameter. The crucibles of 20–40 mm height were installed inside the radiofrequency-heated (8 kHz) graphite insulation. Temperature was controlled with a WRe-thermocouple and a Raytek Marathon MM2MH pyrometer.
Crystals were grown by the VGF method with radiofrequency-heated graphite heaters and gradual cooling of the crucible starting from its bottom. The grown crystals were heat treated in air using a standard tube furnace at 1200 °C during a 12 h period. The heating/cooling rate was 200 °C/h. Then, plates of different thicknesses were fabricated for tests of luminescent and scintillation performance.
The powder diffraction measurements were carried out by Siemens D500 diffractometer, CuKα radiation, Bragg–Brentano geometry, curved graphite monochromator on the counter arm, 5 < 2θ < 100°, and ∆2θ = 0.02°. The phases were identified using JCPDS PDF-1 card files and EVA retrieval system included in the diffractometer software.
Optical absorption spectra were measured using a Specord40 spectrophotometer operating in the 190–1100 nm wavelength range (Analytik Jena AG, Jena, Germany).
X-ray luminescence spectra were measured in the reflection mode under a steady-state X-ray excitation (Ag anode, 40 kV, 40 μA). The emitted light was dispersed by a monochromator with 1200 grooves/mm grating and registered by the PMT Hamamatsu R1926A. The obtained spectra were not corrected for the spectral sensitivity of the detection system. Cathodoluminescence (CL) spectra were registered at RT using an e-beam in a SEM JEOL JSM-820 electron microscope equipped with a Stellar Net spectrometer and a TE-cooled CCD detector working in the 200–925 nm range. The light yield of CL was evaluated in comparison with etalon of Bi4Ge3O12 (BGO) scintillator. BGO is used quite often as a reference for determination of light yield in scintillators, because this crystal possesses around the same light yield of around 8000–9000 phot/MeV independent of the producer [27]. The etalon used in the present work was produced at the Institute for Scintillation Materials NAS of Ukraine and possesses a light yield of 8600 phot/MeV [28].
Luminescence decay times were determined under γ-rays (137Cs), α-particles (239Pu), and β-particles (207Bi). Scintillation pulses were registered by Hamamatsu R1307 PMT, the signal of which was transferred to Rigol DC1302CA digital oscillograph used as analog–digital converter. The obtained data were acquired by PC; 10,000 pulses were registered in each case.
All the measurements were conducted at room temperature (25 °C).
3. Results and Discussion
3.1. Preventing Melt from Carbidization
The crystal growth atmosphere of Ar+CO was formed by the interaction of residual oxygen in the growth chamber with the graphite heater and heat insulation at high temperatures by Reaction (1) proceeding at temperatures over 950 °C [29]:
2C + O2 = 2CO,
Carbon dioxide may reduce or carbidize the Y2O3 melt by Reactions (2)–(4) [30]:
Y2O3 + CO → YxCy + O2 ↑
Y2O3 + CO → YxOCy + O2 ↑
Y2O3 + CO → Y + CO2,
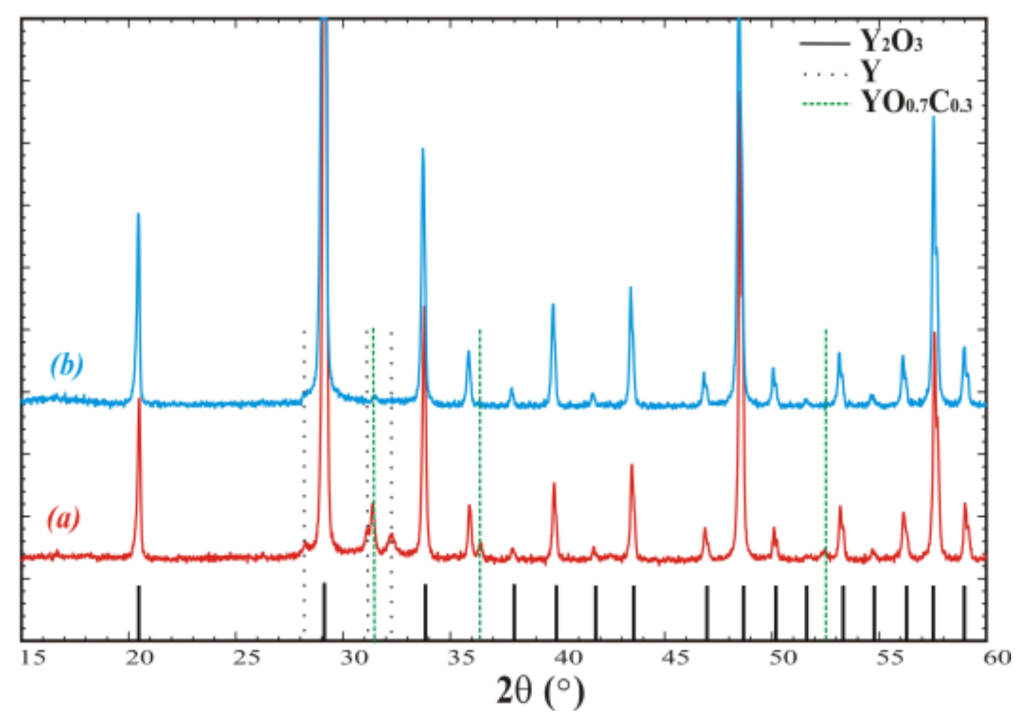
The extent of the negative influence of these reactions on Y2O3 melt purity was evaluated. The crucible was loaded with raw material, heated until melting, held for 24 h, and slowly cooled to room temperature. A phase analysis of the resulting material without any additional treatment was performed (Figure 1a). The XRD data indicate that the main phase was cubic Y2O3 (JCPDS 25-1200, Sp.gr. Ia-3, a = 10.607Å), whereas remarkable quantities of metallic yttrium (JCPDS 12-0702, Sp.gr. P63/mmc, a = 3.647, c = 5.730) and yttrium oxycarbide YO0.7C0.3 (JCPDS 38-1114, Sp.gr. Fm3m, a = 4.930) were detected as well (Figure 1b).
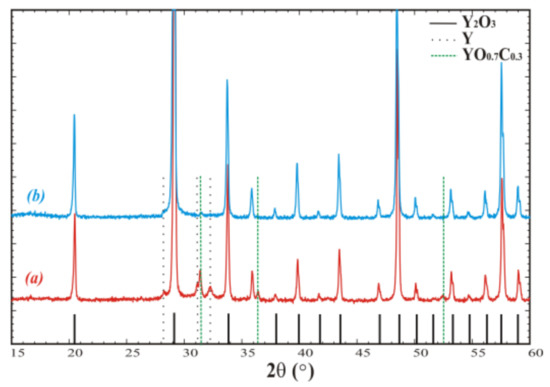
Figure 1.
Powder diffraction patterns of crystallized Y2O3 (a) in open crucible and (b) in crucible covered with lid and additional screening. Peaks corresponding to the admixtures are denoted by the dashed and dotted vertical lines.
Then, the crucible was covered with a W lid and a set of protective W screens was installed to reduce gas diffusion between the graphite and melt and minimize the melt’s interaction with carbon oxide. As the result, the contents of the YO0.7C0.3 admixture phase and metallic yttrium were reduced (Figure 1b).
3.2. VFG Growth
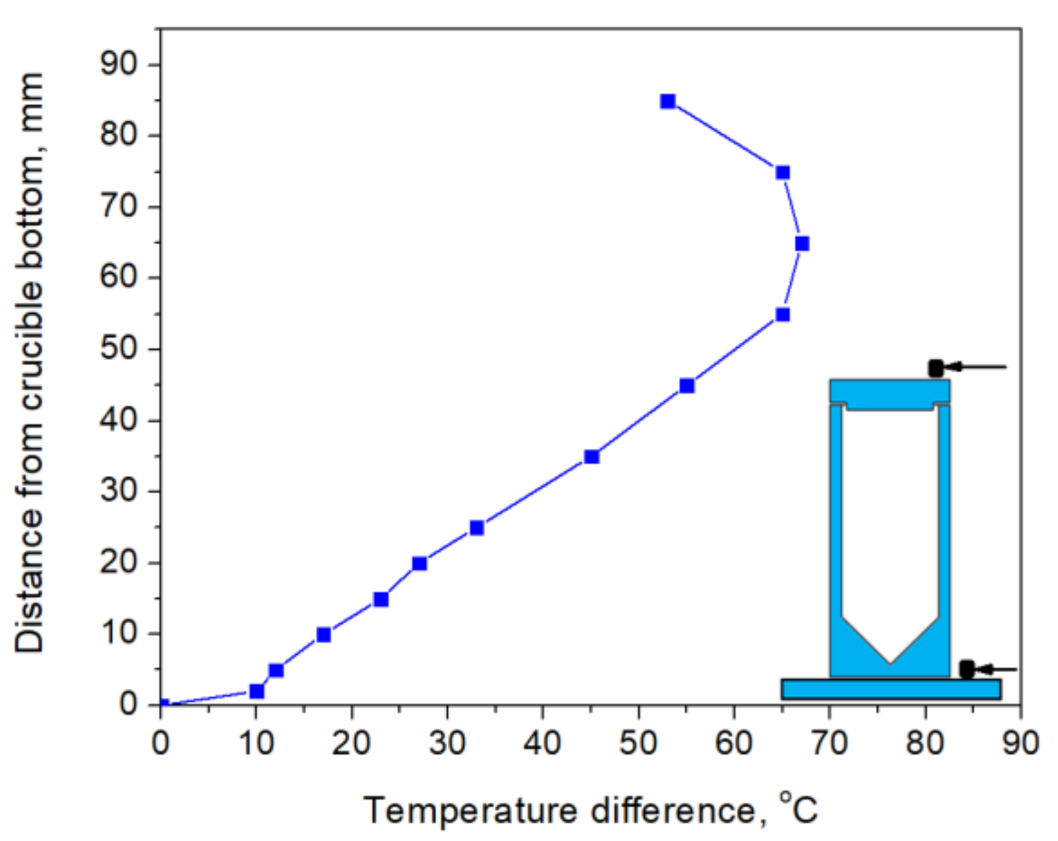
The VGF method was chosen for crystal growth, as it does not require crucible/crystal translation and ensures better heat insulation, which is of special importance at very high temperatures. The thermal assembly design provided axial temperature distribution, illustrated in Figure 2. The crucible with raw material was installed so that its bottom corresponds to 0 mm in Figure 2. The graphite heaters were arranged in such a way to create a higher temperature in the upper part of the crucible as compared to the lower part, providing crystallization from the bottom upwards when decreasing the heating power. For example, for a crucible of 50 mm length, the temperature difference between the top and bottom was 60 °C. As the crystallization process was not controlled visually, the power regimes were adjusted by observing the melting of Y2O3 etalons located at the top and bottom of the crucible in the places indicated by arrows in Figure 2. The melting/crystallization of etalons supposedly corresponded to the simultaneous phase transitions in Y2O3 loaded into the crucible. The growth rate was approximately 0.2–1 mm/h.
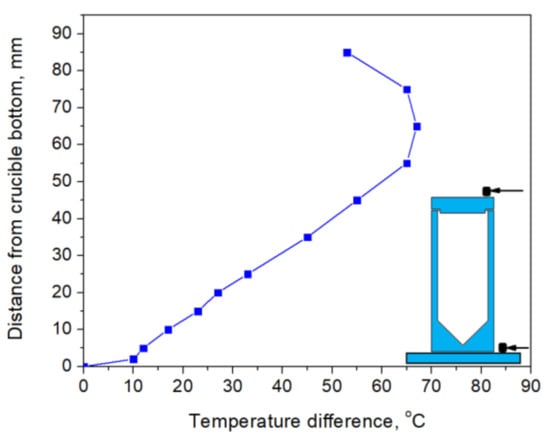
Figure 2.
Axial temperature distribution in heat assembly without the crucible. The positions of Y2O3 etalons for controlling melting/crystallization inside the crucible are depicted by the arrows in the inset (the crucible drawing is presented for visualization purpose; its dimensions do not correspond to the temperature distribution).
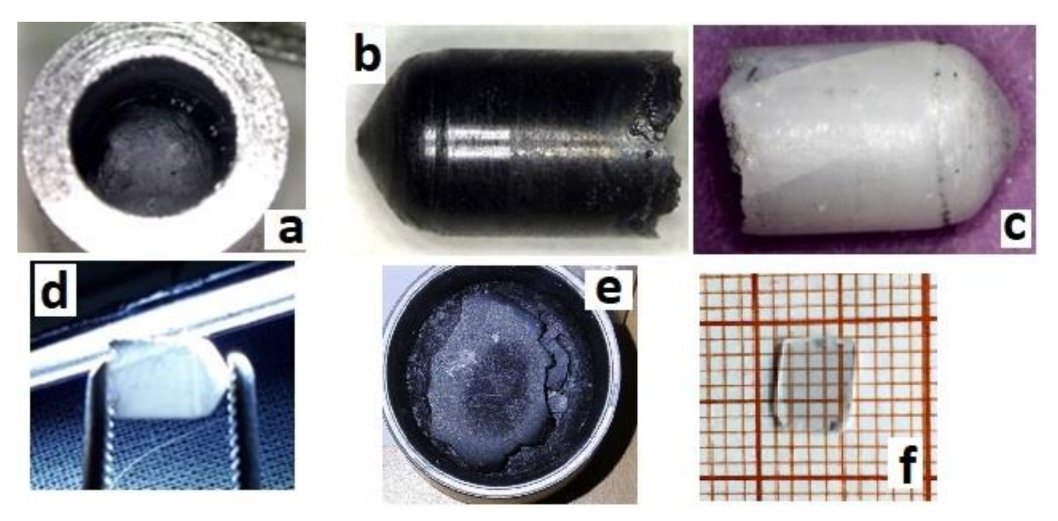
After cooling down the crucible to room temperature (Figure 3a), black ingots were extracted (Figure 3b). It was established that crystals grown for several hours were easily extracted from crucibles, whereas ingots grown for ≥24 h stuck to the crucible. In the latter case, ingots could not be extracted without destruction of the crucible.
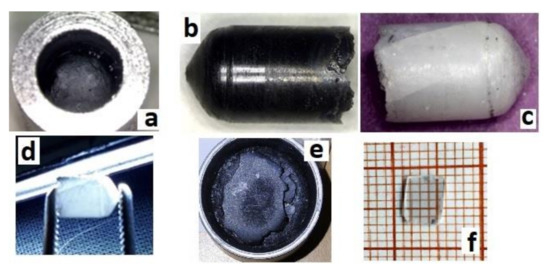
Figure 3.
Obtained sesquioxide crystals: (a) Y2O3 with the diameter 6 mm as grown in crucible, (b) Y2O3 as grown extracted from the crucible, (c,d) Y2O3 after annealing in air at 1200 °C, (e) as-grown Y1.1Sc0.9O3, and (f) polished sample with a thickness of 0.5 mm cut from the same Y1.1Sc0.9O3 ingot after the annealing in air at 1200 °C.
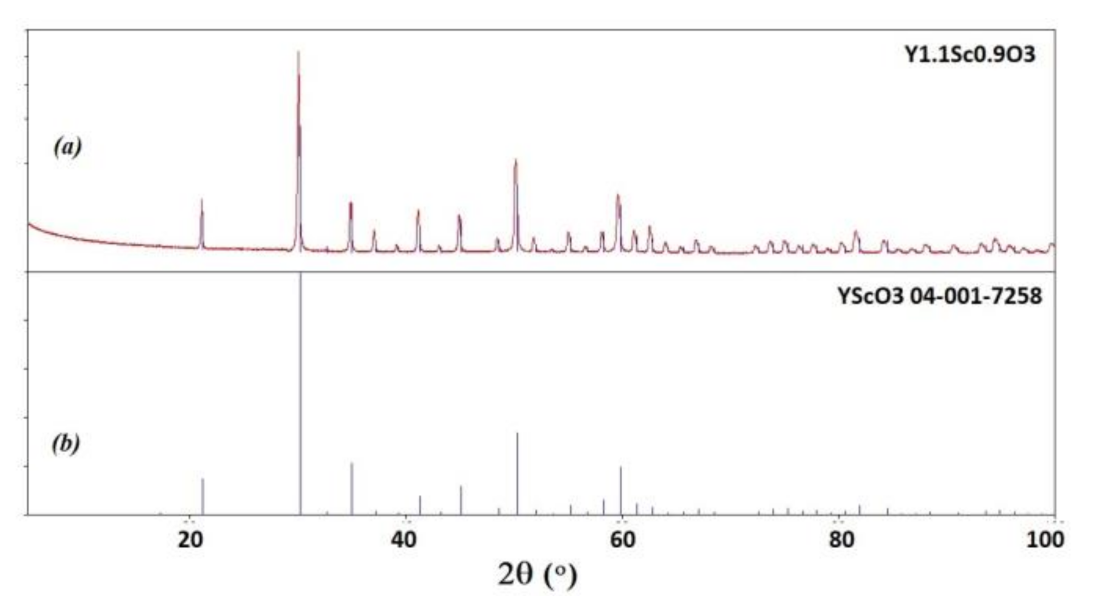
Y2O3 crystals were annealed at 1200 °C for 12 h to reduce the number of defects caused by the growth in oxygen-deficient conditions. The annealed ingots were bleached but translucent, with visible cracks (Figure 3c,d). Basically, the main obstacle in optimizing the crystal performance is the presence of polymorph transitions during the crystallization of Y2O3 from the melt. The cracks were eventually formed due to polymorphic phase transitions between H-, B (monoclinic), and C (cubic) phases occurring due to direct crystallization from the melt of Y2O3 and sesquioxides of lanthanides with ionic radii larger than approximately 0.085 nm [31]. This issue may be overcome by decreasing the average size of the rare earth cation in solid solutions of Y2O3 with Lu2O3 or Sc2O3 oxides, providing direct crystallization of the cubic phase from the melt. Analogous with [21], the mixed Y1.1Sc0.9O3 oxide crystal was grown by the same procedure as described above for Y2O3. The only difference was the sintering temperature, which was decreased to 2000 °C, because the melting temperature of the mixed composition is lower. The as-grown ingot was completely opaque (Figure 3e), however, the one annealed in air provided complete discoloration of the crystals. Transparent plates of Y1.1Sc0.9O3 were obtained after cutting and polishing (Figure 3f). The crystal possesses a cubic structure (Sp.gr. Ia-3, a = 10.2766 Å). The lattice parameter of Y1.11Sc0.9O3 (Figure 4a) is larger than 10.240 Å in the YScO3 reference (Figure 4b) due to the higher content of yttrium. We evaluated the composition of this crystal assuming linearity between the lattice parameters and Y/Sc ratio. The calculated composition is Y1.1Sc0.9O3, which completely corresponds to the melt composition, so there is no remarkable segregation of cations during the crystallization of this solid solution.
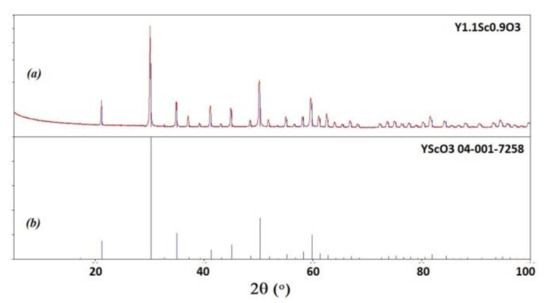
Figure 4.
Powder diffraction patterns of Y1.1Sc0.9O3 (a) in comparison with PDF 04-001-7258 YScO3 reference (b).
3.3. Optical and Scintillation Characterization
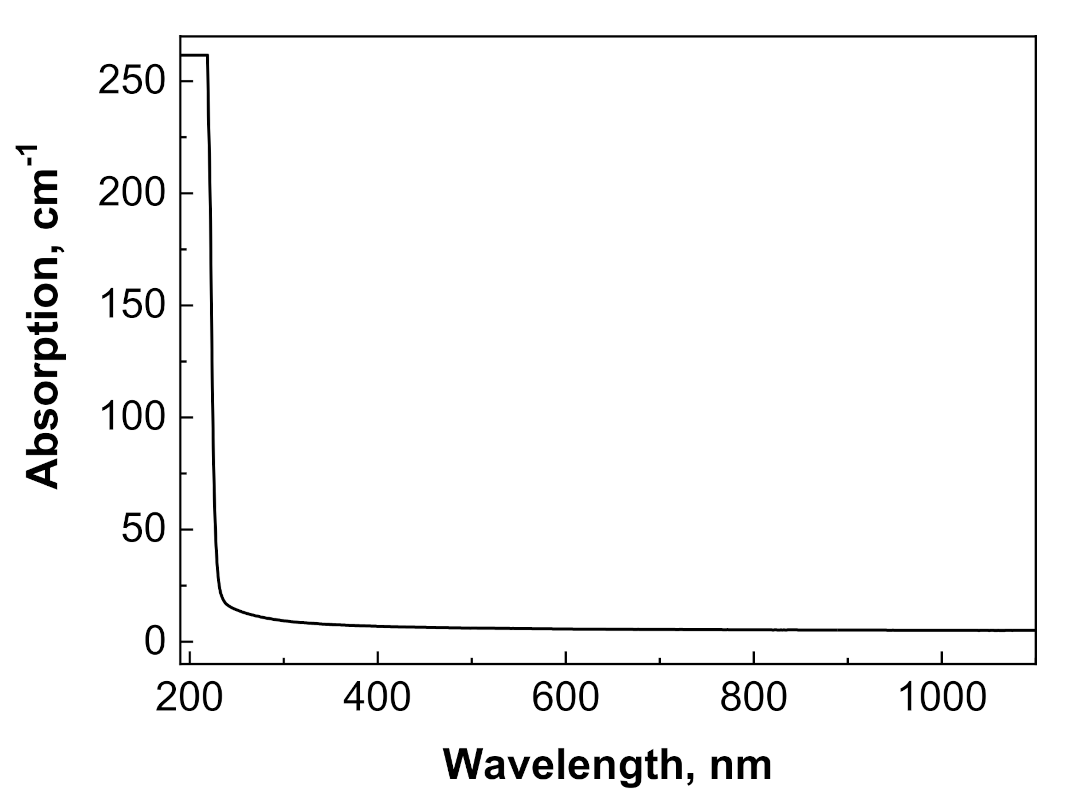
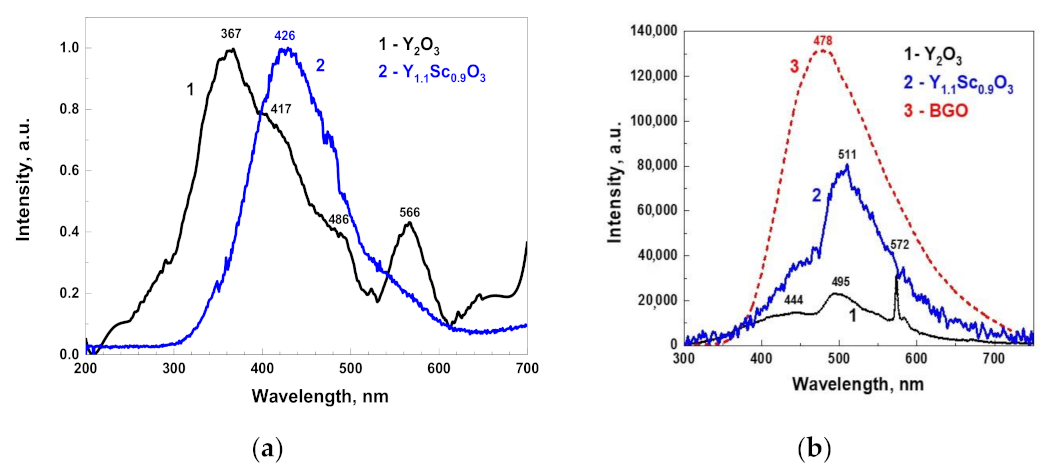
Optical transmission of translucent Y2O3 could not be measured using the standard spectrometer at our disposal. Meanwhile, Y1.1Sc0.9O3 mixed crystals (Figure 5) were transparent in the 230–1100 nm range, without any notable absorption peaks. The fundamental absorption edge at <230 nm corresponded to the literature values [32] of the band gap in sesquioxides of around 5–6 eV. The X-ray emission spectrum of the Y2O3 crystal (Figure 6a) had a weak intensity and contained a wide complex band in the 300–500 nm range with resolved sub-bands that peaked around 367, 417, and 486 nm and separate bands that peaked at 566 nm. The CL spectrum of this crystal possesses much stronger intensity (Figure 6b) and contains two dominant bands that peaked at 444 and 495 nm, with a bump around 370 nm and a narrow peak at 572 nm.
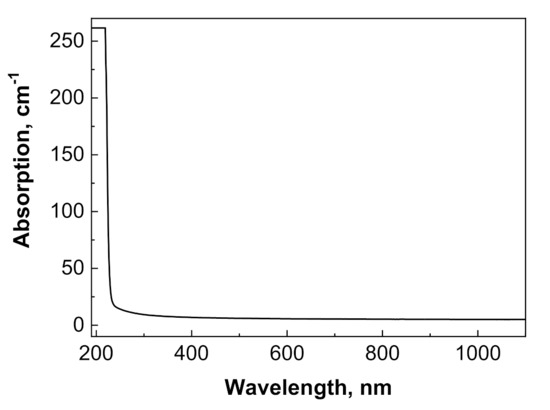
Figure 5.
Optical absorption spectrum of Y1.1Sc0.9O3 sample with a thickness of 0.5 mm.
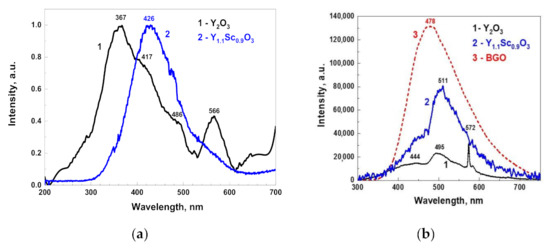
Figure 6.
Normalized X-ray luminescence spectra of Y2O3 and Y1.1Sc0.9O3 (a); cathodoluminescence spectra (b) of Y2O3 and Y1.1Sc0.9O3 in comparison with the BGO reference crystal.
As the Y2O3 crystals in our experiments were grown in oxygen-poor conditions, the nature of the observed wide bands is probably associated with charged oxygen vacancies and their aggregates [33,34,35,36,37]. The nature of emission centers in Y2O3 crystals may be analyzed by comparing the XRL and CL spectra (Figure 6). The band that peaked around 370 nm dominates in the XRL spectra, while the CL spectrum consists of two broad emissions centered around 444 and 495 nm. Most likely, the mentioned bands are connected with the luminescence of F+ and F centers and their aggregates with other defects of Y2O3 crystals [35,36,37]. We assumed that CL and XRL emission spectra also contain the components related to Cr3+ and Mn4+ trace impurities, because such dopants are typically presented in Y2O3 raw materials with 4N–5N purity.
However, it is important to note that the CL spectrum is much more intense in the visible range as compared to that observed under X-rays, as well as that reported in other publications [2,38]. This can be due to the quite different excitation conditions (much stronger in the CL) and the different shaping time of the luminescence registration at respective setups (μs in XRL and ms in CL), as well as a deviation in the spectral sensitivities of experimental setups used for measurements of X-ray excited emission and cathodoluminescence spectra.
The light yield of Y2O3 was evaluated by comparing the squares under its CL spectrum with that of a standard BGO crystal taken in the same experimental conditions (Figure 6b). Assuming a BGO light yield as 8600 phot/MeV [28], the area under the Y2O3 peak is 19.5% of BGO, i.e., around 1700 phot/MeV. The X-ray luminescence spectrum of Y1.1Sc0.9O3 (Figure 6c) was more intense as compared to that in Y2O3 and was represented by a wide band from 300 to 600 nm that peaked at 440 nm with a shoulder at 570 nm. The spectral band was remarkably red-shifted as compared to the Y2O3 XRL spectrum. The CL intensity of Y1.1Sc0.9O3 was approximately 61.3% of BGO, corresponding to a light yield of approximately 5300 phot/MeV. The values for the mixed crystal are approximately in the middle between the light yield of 11,500–16,200 phot/MeV for Sc2O3 and 2600–3200 phot/MeV for Y2O3, if assuming a monotonous increase in light yield from Y2O3 to Sc2O3. Comparing the light yield with decay curves and luminescence spectra, one may link the increase in light yield with the slower emission of long-wavelength spectral components.
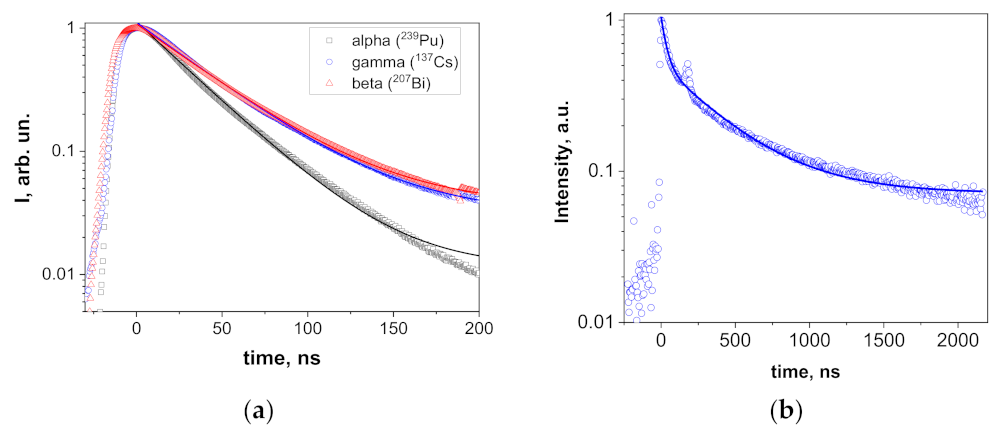
The scintillation decay curves of Y2O3 were fitted well (R2 > 0.998) by single exponential decay functions, and the registered scintillation decay times were 33.2, 42.7, and 42.0 ns at excitation by α- and β-particles and γ-rays, respectively (Figure 7a). Such decay times are typical for the scintillation of Y2O3; for instance, the decay time of 34 ns under γ-rays was reported in [1] for ceramics. The nature of the differences in decay times under different types of excitations is a topic for a separate work. Here, we may just note that similar behavior (faster decay under α-particles and nearly similar kinetics under β-particles and γ-rays) was registered in garnet film scintillators (see for example [39,40]). The scintillation decay in the mixed Y1.1Sc0.9O3 crystal at excitation by γ-rays was fitted rather well (R2 = 0.988) by a two-exponential function with the decay constants of 36.7 and 403.2 ns (Figure 7b) with the contributions of 10.8 and 89.2%, respectively, to the overall signal. The decay constant of the fast component is similar to that in Y2O3 (42 ns), whereas the slow component may be tentatively attributed to Sc-related defects.
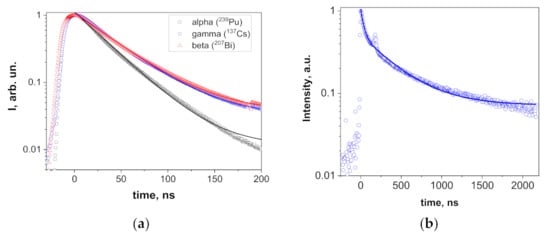
Figure 7.
Scintillation decay in Y2O3 (a) at excitations with α- and β-particles and γ-rays and Y1.1Sc0.9O3 (b) at excitation with γ-rays. The splash around 180 ns in (b) is an experimental artefact.
4. Conclusions
Y2O3 and Y1.1Sc0.9O3 crystals were obtained for the first time by the VGF method in tungsten crucibles. The conducted experiments demonstrated that melt carbidization and polymorph transitions are the main obstacles for obtaining high-quality crystals. Carbidization may be effectively reduced by sealing the melt in the crucible from the CO-containing atmosphere by tungsten lid and screens, diminishing interactions between CO gas and Y2O3 melt. While Y2O3 crystals were translucent and contained numerous cracks due to the polymorph transitions during the crystallization from the melt, transparent Y1.1Sc0.9O3 crystals with crack-free parts were successfully obtained. The issue of polymorph transitions was resolved by decreasing the average radius of the rare earth cation by switching to Sc2O3–Y2O3 solid solutions.
Luminescent and scintillation parameters of the obtained crystals were studied. Typical luminescence bands attributed to defects in Y2O3 were registered, as well as luminescence decay times of 33–43 ns depending on the type of ionizing radiation. The light yield of cathodoluminescence was around 1700 phot/MeV. Y1.1Sc0.9O3 are transparent from 230 nm, possess more intense luminescence with a light yield of approximately 5300 phot/MeV, and the second slow decay component of ~400 ns, which may be tentatively attributed to Sc-related defects.
The obtained results demonstrate the reliability of the VGF method and W crucibles for the growth of single crystals of lanthanides, yttrium, and scandium sesquioxides. Furthermore, they open a perspective of obtaining these sesquioxides in W crucibles by Bridgman and Czochralski methods, although the problem of melt insulation from the growth atmosphere may be critical for the Czochralski growth process.
Author Contributions
Investigation, V.G., D.K., S.T., P.A., S.S., V.A., A.S., I.B. and V.N.; data curation, I.G.; writing—original draft preparation, E.G. and S.W.-Ł.; measurements, data curation, editing, writing—review and editing, Y.Z.; writing—review and editing, O.S. All authors have read and agreed to the published version of the manuscript.
Funding
This work was carried out in the frame of the National Academy of Science of Ukraine Projects KORZE (reg. no. 0121U108986), “Carbon-2” (reg. no. 0122U002637). S.S. and O.S. acknowledge the support from the “ENSEMBLE3” project (GA no. MAB/2020/14) carried out within the International Research Agendas program of the Foundation for Polish Science and Teaming for Excellence project (GA no. 857543). S.W.L. and Y.Z. also acknowledge the support from the Polish NCN UMO-2018/31/B/ST8/03390 project.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Futami, Y.; Yanagida, T.; Fujimoto, Y.; Pejchal, J.; Sugiyama, M.; Kurosawa, S.; Yokota, Y.; Ito, A.; Yoshikawa, A.; Goto, T. Optical and scintillation properties of Sc2O3, Y2O3 and Lu2O3 transparent ceramics synthesized by SPS method. Radiat. Meas. 2013, 55, 136–140. [Google Scholar] [CrossRef]
- Fukabori, A.; Chani, V.; Kamada, K.; Moretti FYoshikawa, A. Growth of Tm3+-doped Y2O3, Sc2O3, and Lu2O3 crystals by the micropulling down technique and their optical and scintillation characteristics. Cryst. Growth Des. 2011, 11, 2404–2411. [Google Scholar] [CrossRef]
- Thoř, T.; Rubešová, K.; Jakeš, V.; Cajzl, J.; Nádherný, L.; Mikolášová, D.; Kučerková, R.; Nikl, M. Lanthanide-doped Lu2O3 phosphors and scintillators with green-to-red emission. J. Lumin. 2019, 215, 116647. [Google Scholar] [CrossRef]
- Thoř, T.; Rubešová, K.; Jakeš, V.; Cajzl, J.; Nádherný, L.; Mikolášová, D.; Beitlerová, A.; Nikl, M. Europium-doped Lu2O3 phosphors prepared by a sol-gel method. IOP Conf. Ser. Mater. Sci. Eng. 2018, 465, 012009. [Google Scholar] [CrossRef]
- Pirri, A.; Toci, G.; Patrizi, B.; Vannini, M. An overview on Yb-Doped transparent polycrystalline sesquioxide laser ceramics. IEEE J. Sel. Top. Quantum Electron. 2018, 24, 1–8. [Google Scholar] [CrossRef]
- Liu, Z.; Toci, G.; Pirri, A.; Patrizi, B.; Feng, Y.; Chen, X.; Hu, D.; Feng, T.; Lexiang, W.; Matteo, V.; et al. Fabrication and Optical Property of Nd:Lu2O3 Transparent Ceramics for Solid-state Laser Applications. Inorg. Mater. 2020, 36, 210–216. [Google Scholar] [CrossRef]
- Hlavac, J. Melting temperatures of refractory oxides: Part I. Pure Appl. Chem. 1982, 54, 681–688. [Google Scholar] [CrossRef]
- Coutures, J.P.; Rand, M.H. Melting temperatures of refractory oxides-Part II: Lanthanoid sesquioxides. Pure Appl. Chem. 1989, 61, 1461–1482. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, H.; Wang, J.; Wang, D.; Han, D.; Zhang, J.; Wang, S. Preparation of (Tb1−xLux)2O3 transparent ceramics by solid solution for magneto-optical application. J. Eur. Ceram. Soc. 2021, 41, 2818–2825. [Google Scholar] [CrossRef]
- Südmeyer, T.; Kränkel, C.; Baer, C.R.E.; Heckl, O.H.; Saraceno, C.J.; Golling, M.; Peters, R.; Petermann, K.; Huber, G.; Keller, U. High-power ultrafast thin disk laser oscillators and their potential for sub-100-femtosecond pulse generation. Appl. Phys. B 2009, 97, 281–295. [Google Scholar] [CrossRef]
- Kränkel, C. Rare-earth-doped sesquioxides for diode-pumped high-power lasers in the 1-, 2-, and 3-μm spectral range. IEEE J. Sel. Top. Quantum. Electron. 2014, 21, 250–262. [Google Scholar] [CrossRef]
- Boulesteix, R.; Epherre, R.; Noyau, S.; Vandenhende, M.; Maitre, A.; Sallé, C.; Alombert-Goget, G.; Guyot, Y.; Brenier, A. Highly transparent Nd: Lu2O3 ceramics obtained by coupling slip-casting and spark plasma sintering. Scr. Mater. 2014, 75, 54–57. [Google Scholar] [CrossRef]
- Kryzhanovska, O.S.; Baumer, V.N.; Parkhomenko, S.V.; Doroshenko, A.G.; Yavetskiy, R.P.; Balabanov, A.E.; Tolmachev, A.V.; Skorik, S.N.; Li, J.; Kuncser, A. Formation peculiarities and optical properties of highly-doped (Y0.86La0.09Yb0.05)2O3 transparent ceramics. Ceram. Int. 2019, 45, 16002–16007. [Google Scholar] [CrossRef]
- Kim, K.J.; Kamada, K.; Murakami, R.; Horiai, T.; Ishikawa, S.; Kochurikhin, V.V.; Yoshino, M.; Yamaji, A.; Shoji, Y.; Kurosawa, S.; et al. Growth of Lu2O3 and HfO2 Based High Melting Temperature Single Crystals by Indirect Heating Method Using Arc Plasma. Crystals 2020, 10, 619. [Google Scholar] [CrossRef]
- Zhang, N.; Yin, Y.; Zhang, J.; Wang, T.; Wang, S.; Yin, Y.; Fu, X.; Jia, Z.; Tao, X. Optimized growth of high length-to-diameter ratio Lu2O3 single crystal fibers by the LHPG method. CrystEngComm 2021, 23, 1657–1662. [Google Scholar] [CrossRef]
- Hou, W.; Zhao, H.; Li, N.; Xue, Y.; Shi, J.; Xu, X.; Xu, J. Growth and spectroscopic properties of Er: Lu2O3 crystal grown by floating zone method. Mater. Res. Express 2019, 6, 066203. [Google Scholar] [CrossRef]
- Guzik, M.; Pejchal, J.; Yoshikawa, A.; Ito, A.; Goto, T.; Siczek, M.; Lis, T.; Boulon, G. Structural investigations of Lu2O3 as single crystal and polycrystalline transparent ceramic. Cryst. Growth Des. 2014, 14, 3327–3334. [Google Scholar] [CrossRef]
- Yin, Y.; Wang, G.; Jia, Z.; Mu, W.; Fu, X.; Zhang, J.; Tao, X. Controllable and directional growth of Er: Lu2O3 single crystals by the edge-defined film-fed technique. CrystEngComm 2020, 22, 6569–6573. [Google Scholar] [CrossRef]
- Peters, R.; Kränkel, C.; Petermann, K.; Huber, G. Crystal growth by the heat exchanger method, spectroscopic characterization and laser operation of high-purity Yb:Lu2O3. J. Cryst. Growth 2008, 310, 1934–1938. [Google Scholar] [CrossRef]
- Hu, K.; Zheng, L.; Zhang, H. Control of interface shape during high melting sesquioxide crystal growth by HEM technique. J. Cryst. Growth 2018, 483, 175–182. [Google Scholar] [CrossRef]
- Kränkel, C.; Uvarova, A.; Haurat, É.; Hülshoff, L.; Brützam, M.; Guguschev, C.; Kalusniak, S.; Klimm, D. Czochralski growth of mixed cubic sesquioxide crystals in the ternary system Lu2O3–Sc2O3–Y2O3. Acta. Crystallogr. B. Struct. Sci. Cryst. Eng. Mater. 2021, 77, 550–558. [Google Scholar] [CrossRef]
- Suzuki, A.; Kalusniak, S.; Tanaka, H.; Brützam, M.; Ganschow, S.; Tokurakawa, M.; Kränkel, C. Spectroscopy and 2.1 µm laser operation of Czochralski-grown Tm3+:YScO3 crystals. Opt. Express 2022, 30, 42762–42771. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.; Zhao, H.; Cao, X.; Wang, W.; Xu, X.; Wu, F.; Luo, P.; Wang, Q.; Xue, Y.; Xu, J. Crystal growth and spectroscopic analysis of Ho, Pr:Sc2O3 crystal for 2.9 μm mid-IR laser. Opt. Mater. Express 2021, 11, 2539–2546. [Google Scholar] [CrossRef]
- Sidletskiy, O.; Gerasymov, I.; Boyaryntseva, Y.; Arhipov, P.; Tkachenko, S.; Zelenskaya, O.; Bryleva, K.; Belikov, K.; Lebbou, K.; Dujardin, C.; et al. Impact of Carbon Co-Doping on the Optical and Scintillation Properties of a YAG: Ce Scintillator. Cryst. Growth Des. 2021, 21, 3063–3070. [Google Scholar] [CrossRef]
- Tkachenko, S.; Arhipov, P.; Gerasymov, I.; Galenin, E.; Shaposhnyk, A.; Hryshyna, O.; Mateychenko, P.; Boyaryntseva, Y.; Zelenskaya, O.; Lebbou, K.; et al. The crystal growth of ortho-and pyrosilicates from W and Mo crucibles. CrystEngComm 2021, 23, 360–367. [Google Scholar] [CrossRef]
- Gerasymov, I.; Hubenko, K.; Viagin, O.; Boyarintseva, Y.; Shaposhnyk, A.; Baumer, V.; Gorbacheva, T.; Zelenskaya, O.; Galenin, E.; Kurtsev, D.; et al. Characterization of LaGPS:Ce scintillation crystals obtained under a reducing atmosphere. CrystEngComm 2022, 24, 7066–7072. [Google Scholar] [CrossRef]
- Scintillation Properties—Lawrence Berkeley National Laboratory. Available online: Scintillator.lbl.gov (accessed on 13 March 2023).
- Sidletskiy, O.; Arhipov, P.; Tkachenko, S.; Zelenskaya, O.; Vasyukov, S.; Moretti, F.; Dujardin, C. Drastic Scintillation Yield Enhancement of YAG:Ce with Carbon Doping. Phys. Status Solidi A 2018, 215, 1800122. [Google Scholar] [CrossRef]
- Holleman, A.F.; Wiberg, E.; Wiberg, N. Inorganic Chemistry; Academic Press: Cambridge, MA, USA, 2001. [Google Scholar]
- Nizhankovsky, S.; Krivonosov, E.; Baranov, V.; Budnikov, A.; Kanishchev, V.; Grin, L.; Adonkin, G. Optical homogeneity of Ti:sapphire crystals grown by horizontal directional solidification. Inorg. Mater. 2012, 48, 1111–1114. [Google Scholar] [CrossRef]
- Adachi, G.; Imanaka, N. The binary rare earth oxides. Chem. Rev. 1998, 98, 1479–1514. [Google Scholar] [CrossRef]
- Badehian, H.A.; Salehi, H.; Ghoohestani, M. First-principles study of elastic, structural, electronic, thermodynamical, and optical properties of Yttria (Y2O3) ceramic in cubic phase. J. Am. Ceram. Soc. 2013, 96, 1832–1840. [Google Scholar] [CrossRef]
- Lushchik, A.; Kirm, M.; Lushchik Ch Martinson, I.; Zimmerer, G. Luminescence of free and self-trapped excitons in wide-gap oxides. J. Lumin. 2000, 87–89, 232–234. [Google Scholar] [CrossRef]
- Zheng, J.X.; Ceder, G.; Maxisch, T.; Chim, W.K.; Choi, W.K. Native point defects in yttria and relevance to its use as a high-dielectric-constant gate oxide material: First-principles study. Phys. Rev. B 2006, 73, 104101. [Google Scholar] [CrossRef]
- Nambu, K.; Hayasaka, H.; Yamamoto, T.; Yoshida, H. Photoluminescence properties of undoped and Si4+-doped polycrystalline Y2O3 phosphors prepared by flash-sintering. Appl. Phys. Express 2019, 12, 075504. [Google Scholar] [CrossRef]
- Huang, H.; Sun, X.; Wang, S.; Liu, Y.; Li, X.; Liu, J.; Kang, Z.; Lee, S.-T. Strong red emission of pure Y2O3 nanoparticles from oxygen related defects. Dalton Trans. 2011, 40, 11362–11366. [Google Scholar] [CrossRef]
- Zorenko, Y.; Zorenko, T.; Voznyak, T.; Mandowski, A.; Xia, Q.; Batentschuk, M.; Fridrich, J. Luminescence of F+ and F centers in Al2O3-Y2O3 oxide compounds. IOP Conf. Ser. Mater. Sci. Eng. 2010, 15, 012060. [Google Scholar] [CrossRef]
- Kumar, Y.; Pal, M.; Herrera, M.; Mathew, X. Effect of Eu ion incorporation on the emission behavior of Y2O3 nanophosphors: A detailed study of structural and optical properties. Opt. Mater. 2016, 60, 159–168. [Google Scholar] [CrossRef]
- Witkiewicz-Lukaszek, S.; Gorbenko, V.; Zorenko, T.; Syrotych, Y.; Mares, J.A.; Nikl, M.; Sidletskiy, O.; Bilski, P.; Yoshikawa, A.; Zorenko, Y. Composite Detectors Based on Single-Crystalline Films and Single Crystals of Garnet Compounds. Materials 2022, 15, 1249. [Google Scholar] [CrossRef]
- Sidletskiy, O.; Gorbenko, V.; Zorenko, T.; Syrotych, Y.; Witkiwicz-Łukaszek, S.; Mares, J.A.; Kucerkova, R.; Nikl, M.; Gerasymov, I.; Kurtsev, D.; et al. Composition Engineering of (Lu,Gd,Tb)3(Al,Ga)5O12:Ce Film/Gd3(Al,Ga)5O12:Ce Substrate Scintillators. Crystals 2022, 12, 1366. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).