Recyclable Carbon-Based Hybrid Adsorbents Functionalized with Alumina Nanoparticles for Water Remediation
Abstract
1. Introduction
2. Materials and Methods
2.1. Fabrication of Alumina Nanoparticles Anchored with Recyclable Carbon-Based Hybrid Adsorbents
2.2. Application Studies for Adsorption of Methylene Blue onto Alumina Nanoparticles Anchored with Recyclable Carbon-Based Hybrid Adsorbents
3. Results and Discussion
3.1. Characteristics of Alumina Nanoparticles Anchored with Recyclable Carbon-Based Hybrid Adsorbents
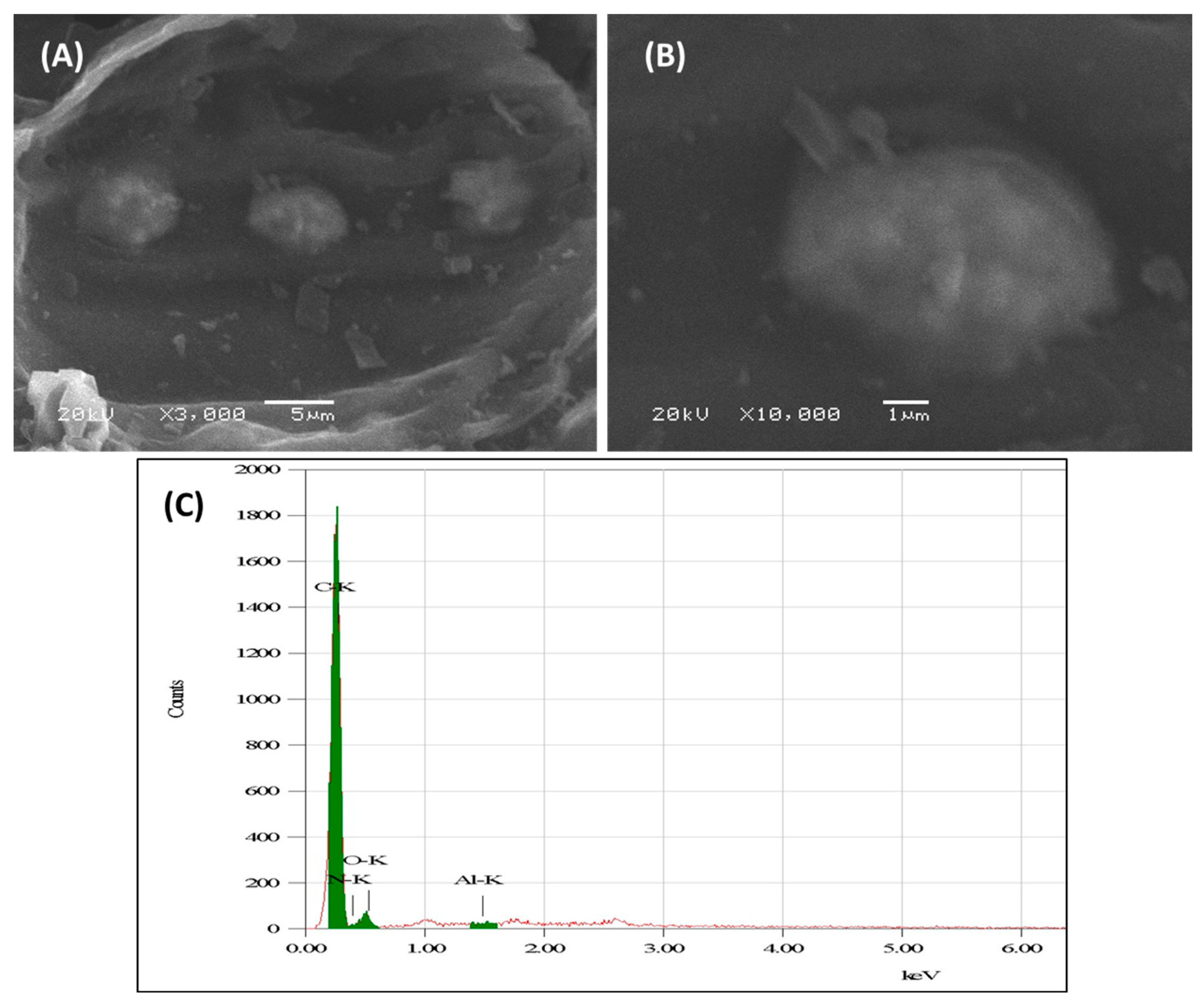
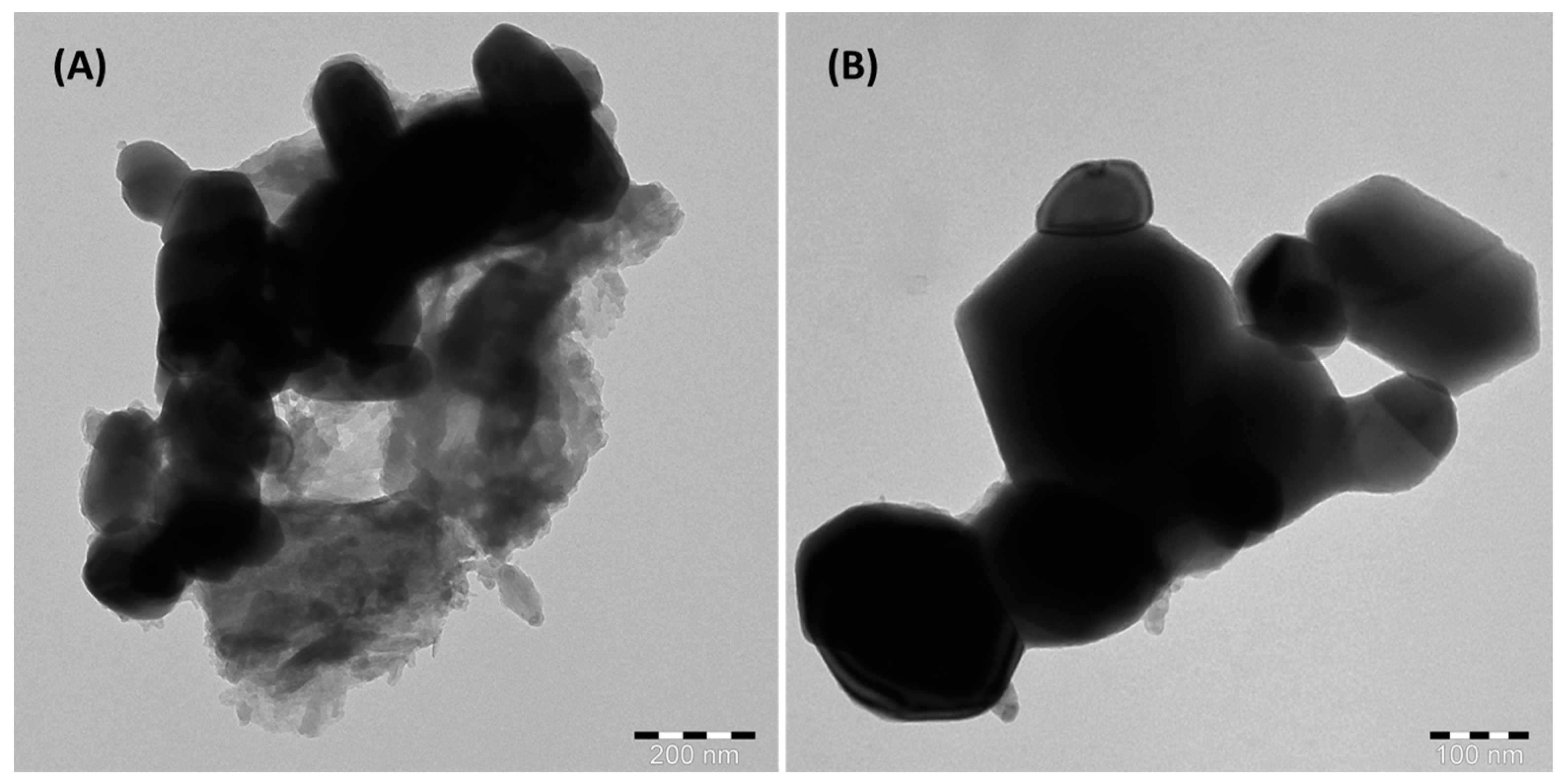
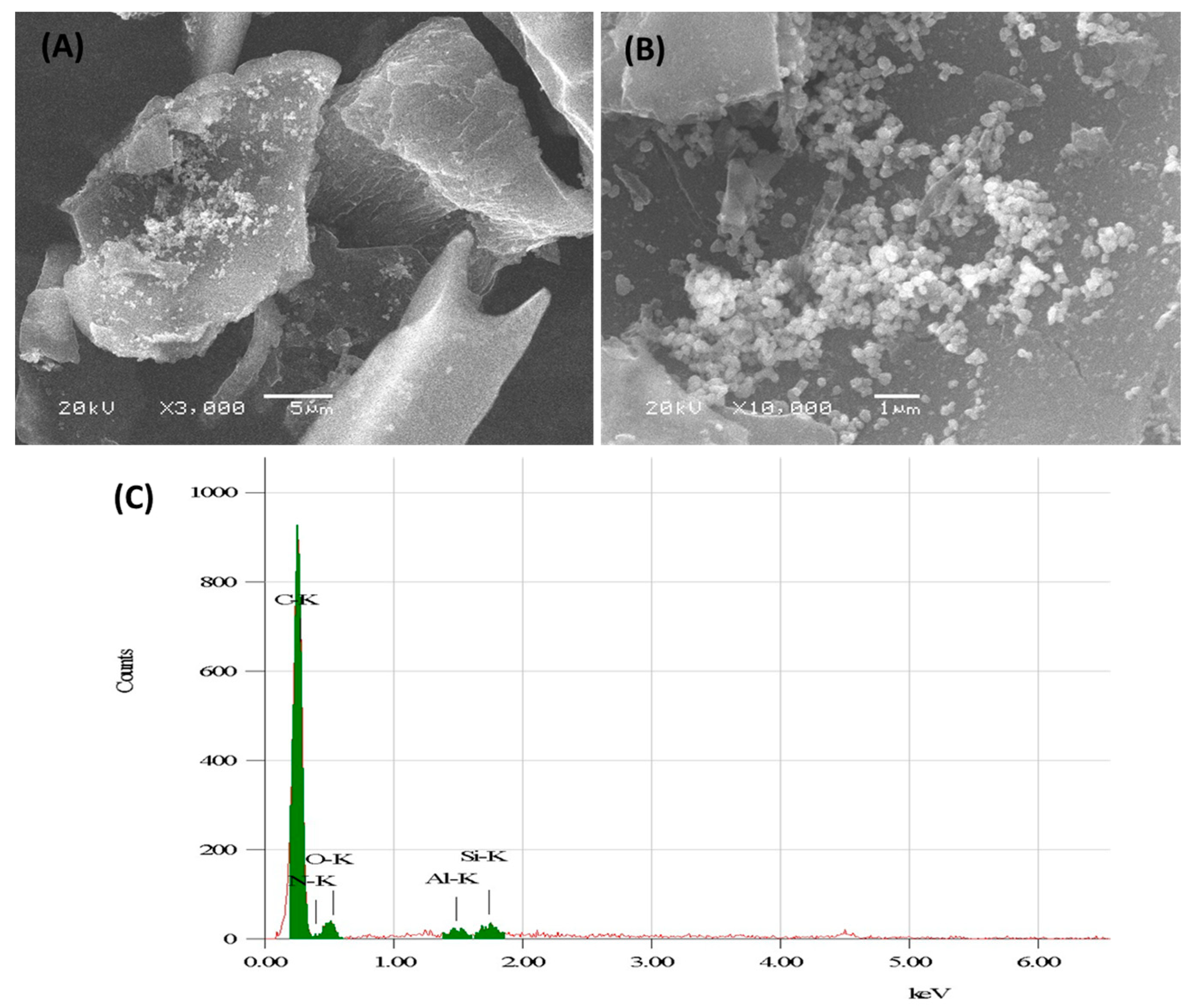
3.1.1. Microscopic Characterization of Alumina Nanoparticles Anchored with Recyclable Carbon-Based Hybrid Adsorbents
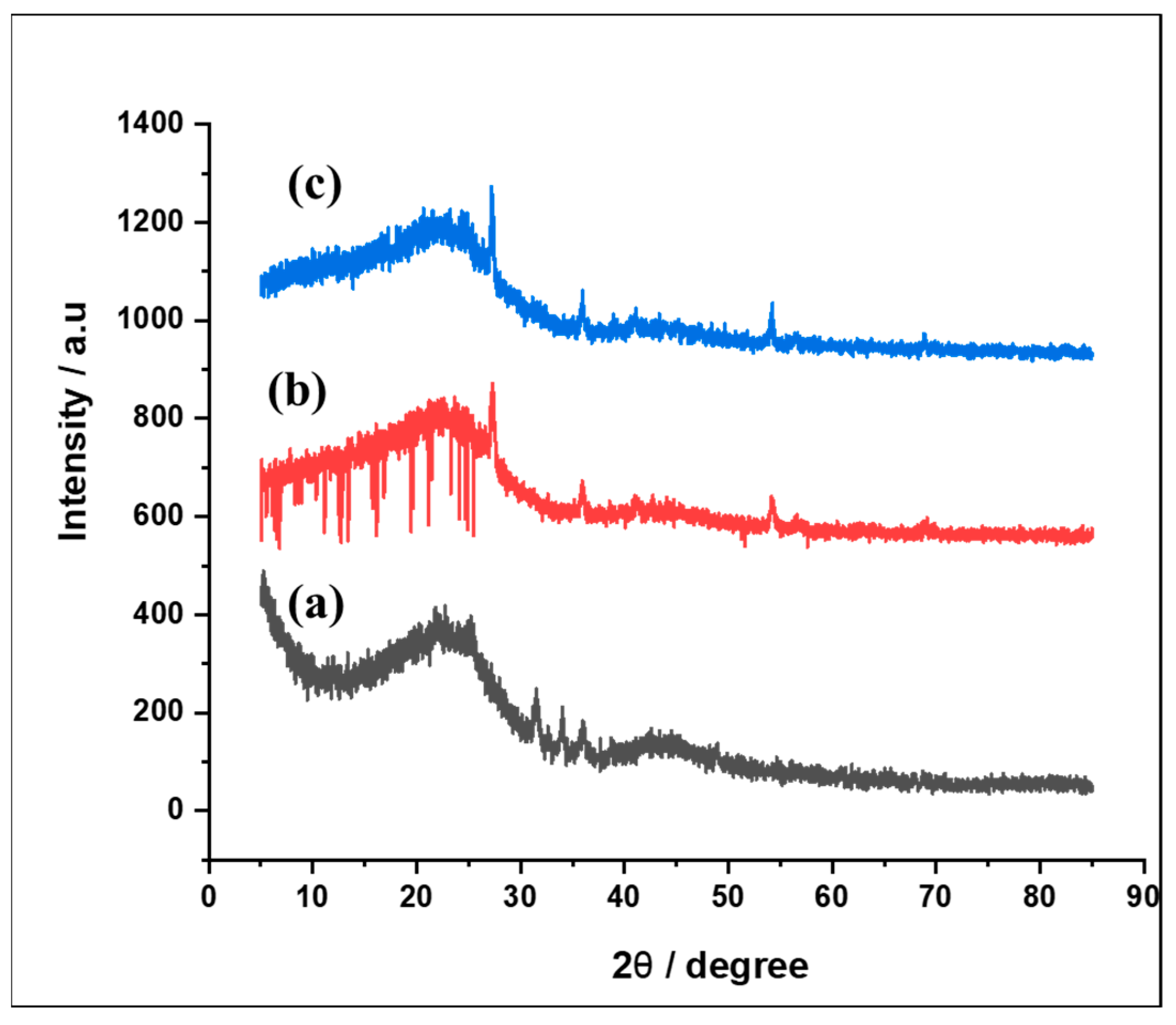
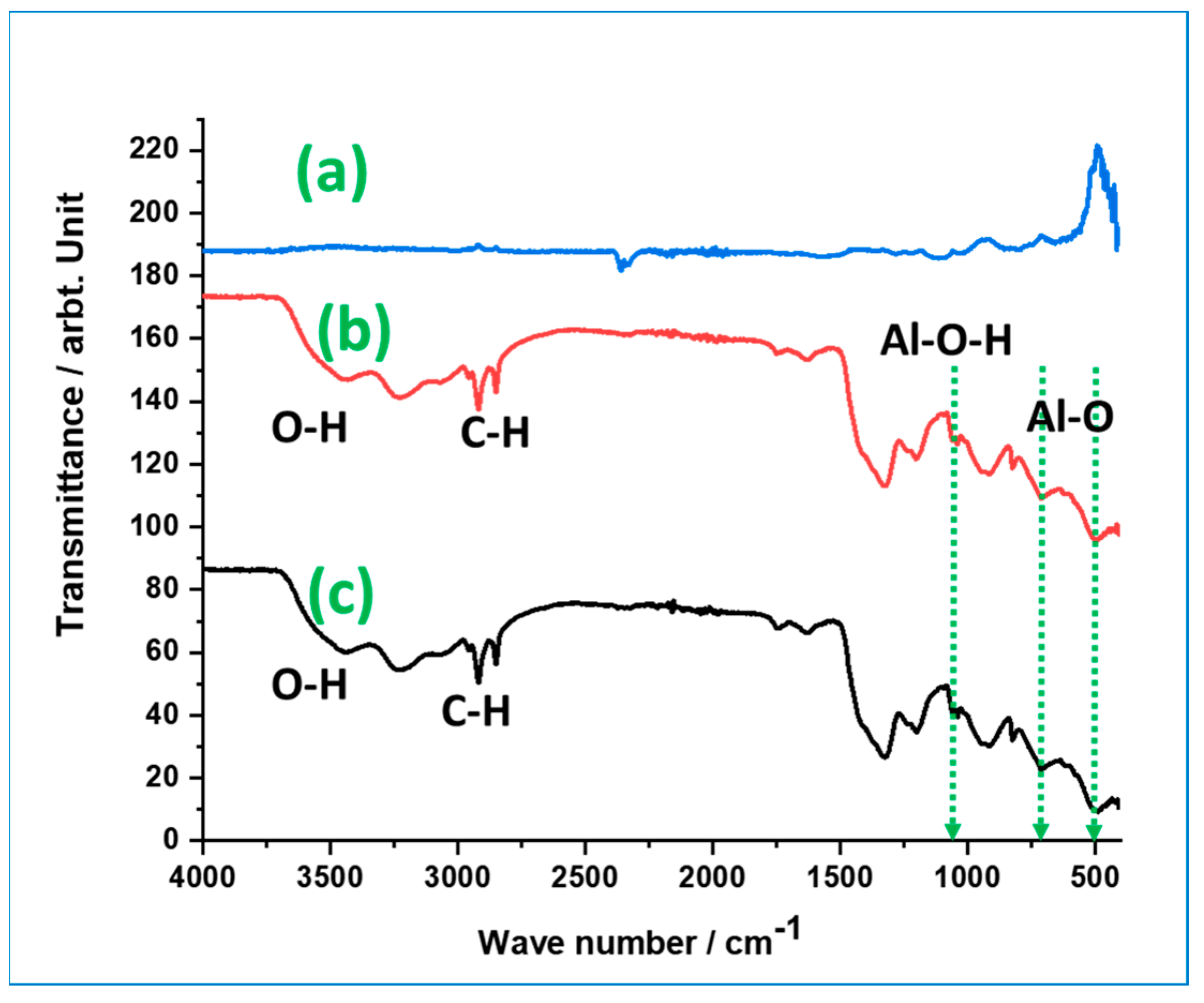
3.1.2. Structural Characterization of Alumina Nanoparticles Anchored with Recyclable Carbon-Based Hybrid Adsorbents
3.2. Adsorption Studies of Methylene Blue
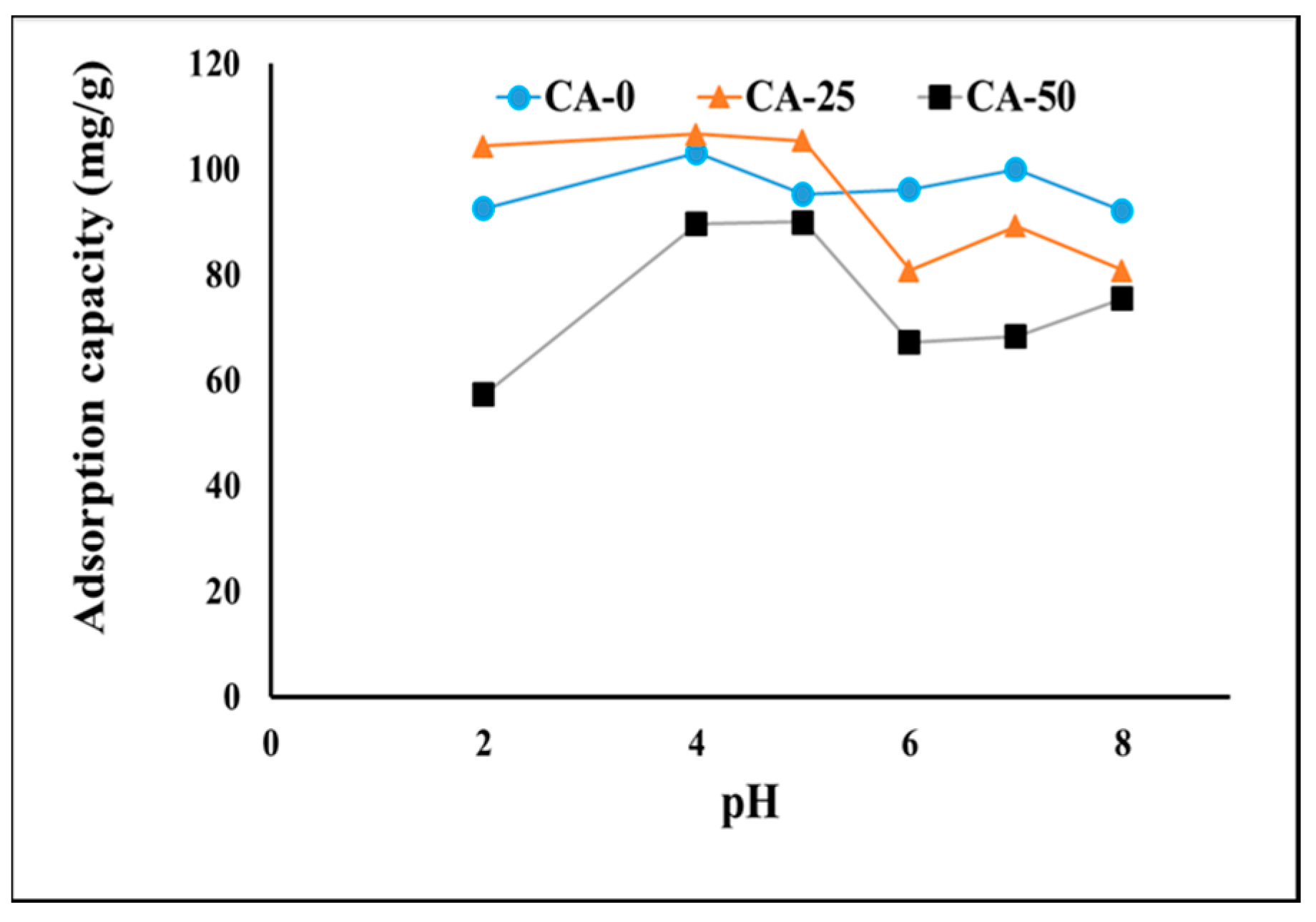
3.2.1. Investigation of pH Effect
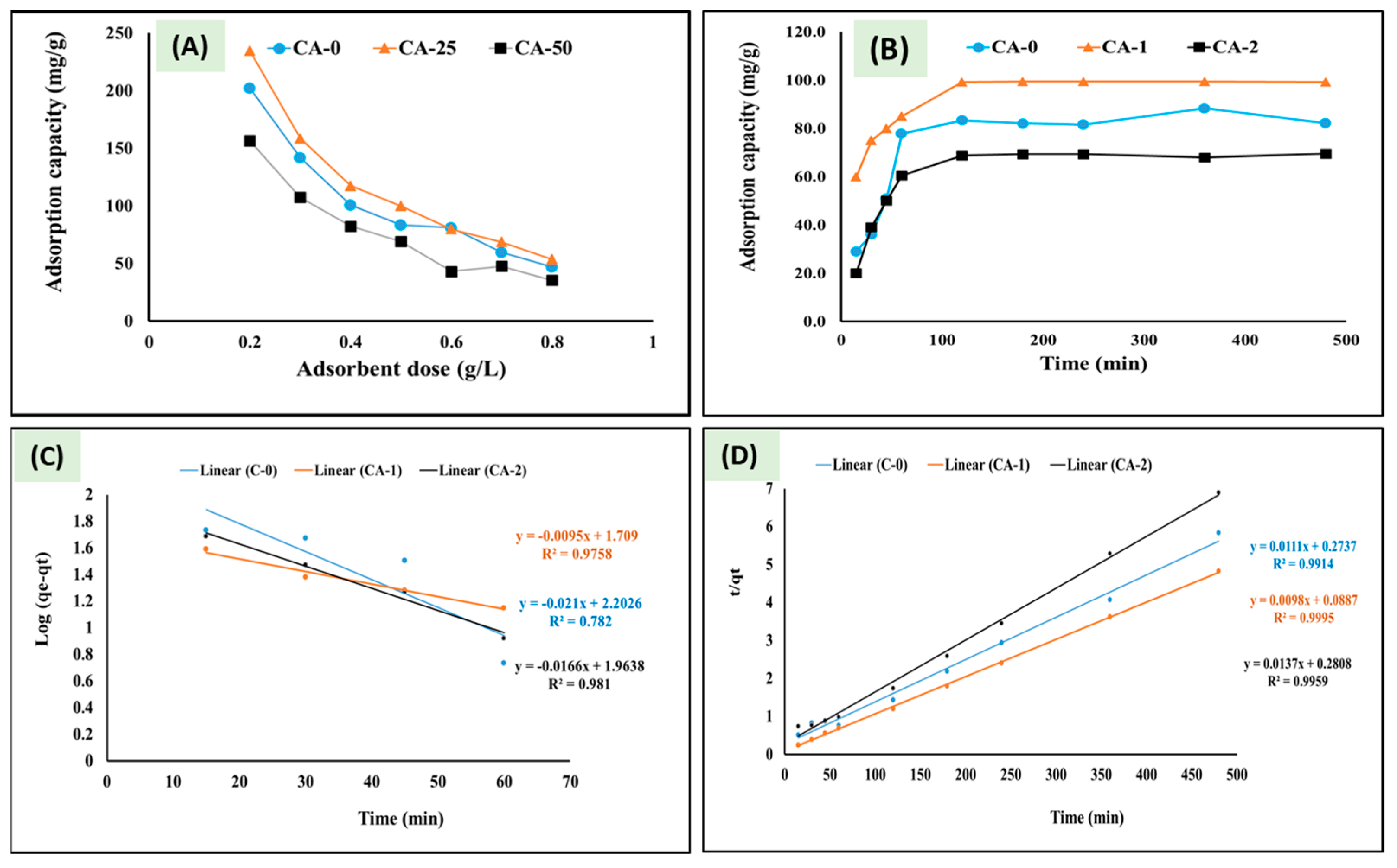
3.2.2. Effect of Adsorbent Dose, Contact Time, and Kinetic Model Evaluation
3.2.3. Isotherm Studies
- Langmuir isotherm [75]
- Freundlich isotherm [81]
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bhatia, D.; Sharma, N.R.; Singh, J.; Kanwar, R.S. Biological methods for textile dye removal from wastewater: A review. Crit. Rev. Environ. Sci. Technol. 2017, 47, 1836–1876. [Google Scholar] [CrossRef]
- Laing, I.G. The impact of effluent regulations on the dyeing industry. Rev. Prog. Color. Relat. Top. 2008, 21, 56–71. [Google Scholar] [CrossRef]
- Forgacs, E.; Cserháti, T.; Oros, G. Removal of synthetic dyes from wastewaters: A review. Environ. Int. 2004, 30, 953–971. [Google Scholar] [CrossRef]
- Ali, H. Biodegradation of synthetic dyes—A review. Water Air Soil Pollut. 2010, 213, 251–273. [Google Scholar] [CrossRef]
- Mishra, S.; Cheng, L.; Maiti, A. The Utilization of agro-biomass/byproducts for effective bio-removal of dyes from dyeing wastewater: A comprehensive review. J. Environ. Chem. Eng. 2021, 9, 104901. [Google Scholar] [CrossRef]
- Chandrasekhar, S.; Pramada, P.N. Rice husk ash as an adsorbent for methylene blue-effect of ashing temperature. Adsorption 2006, 12, 27–43. [Google Scholar] [CrossRef]
- Feddal, I.; Ramdani, A.; Taleb, S.; Gaigneaux, E.M.; Batis, N.; Ghaffour, N. Adsorption capacity of methylene blue, an organic pollutant, by montmorillonite clay. Desalin. Water Treat. 2014, 52, 2654–2661. [Google Scholar] [CrossRef]
- Hakami, A.A.H.; Wabaidur, S.M.; Khan, M.A.; AlOthman, Z.A.; Siddiqui, M.R. Extraction procedures and analytical methods for the determination of methylene blue, rhodamine B and crystal violet—An overview. Curr. Anal. Chem. 2020, 17, 708–728. [Google Scholar] [CrossRef]
- Vutskits, L.; Briner, A.; Klauser, P.; Gascon, E.; Dayer, A.G.; Kiss, J.Z.; Muller, D.; Licker, M.J.; Morel, D.R. Adverse effects of methylene blue on the central nervous system. Anesthesiology 2008, 108, 684–692. [Google Scholar] [CrossRef]
- Auerbach, S.S.; Bristol, D.W.; Peckham, J.C.; Travlos, G.S.; Hébert, C.D.; Chhabra, R.S. Toxicity and carcinogenicity studies of methylene blue trihydrate in F344N rats and B6C3F1 mice. Food Chem. Toxicol. 2010, 48, 169–177. [Google Scholar] [CrossRef]
- Eslami, H.; Sedighi Khavidak, S.; Salehi, F.; Khosravi, R.; Fallahzadeh, R.A.; Peirovi, R.; Sadeghi, S. Biodegradation of Methylene Blue from Aqueous Solution by Bacteria Isolated from Contaminated Soil; Kurdistan University of Medical Sciences: Sanandaj, Iran, 2016; Volume 5. [Google Scholar]
- Contreras, M.; Grande-Tovar, C.D.; Vallejo, W.; Chaves-López, C. Bio-removal of methylene blue from aqueous solution by galactomyces geotrichum KL20A. Water 2019, 11, 282. [Google Scholar] [CrossRef]
- Mubarak, N.M.; Fo, Y.T.; Al-Salim, H.S.; Sahu, J.N.; Abdullah, E.C.; Nizamuddin, S.; Jayakumar, N.S.; Ganesan, P. Removal of methylene blue and orange-G from waste water using magnetic biochar. Int. J. Nanosci. 2015, 14, 1550009. [Google Scholar] [CrossRef]
- Ong, S.A.; Toorisaka, E.; Hirata, M.; Hano, T. Biodegradation of redox dye methylene blue by up-flow anaerobic sludge blanket reactor. J. Hazard. Mater. 2005, 124, 88–94. [Google Scholar] [CrossRef]
- El-Moselhy, M.M.; Kamal, S.M. Selective removal and preconcentration of methylene blue from polluted water using cation exchange polymeric material. Groundw. Sustain. Dev. 2018, 6, 6–13. [Google Scholar] [CrossRef]
- Alkaykh, S.; Mbarek, A.; Ali-Shattle, E.E. Photocatalytic degradation of methylene blue dye in aqueous solution by MnTiO3 nanoparticles under sunlight irradiation. Heliyon 2020, 6, e03663. [Google Scholar] [CrossRef]
- Habila, M.A.; Sheikh Moshab, M.; Mohamed El-Toni, A.; Al-Awadi, A.S.; ALOthman, Z.A. Facile Strategy for Fabricating an Organosilica-Modified Fe3O4 (OS/Fe3O4) Hetero-Nanocore and OS/Fe3O4@SiO2 Core–Shell Structure for Wastewater Treatment with Promising Recyclable Efficiency. ACS Omega 2023, 8, 7626–7638. [Google Scholar] [CrossRef] [PubMed]
- Taha, K.K.; al Zoman, M.; al Outeibi, M.; Alhussain, S.; Modwi, A.; Bagabas, A.A. Green and sonogreen synthesis of zinc oxide nanoparticles for the photocatalytic degradation of methylene blue in water. Nanotechnol. Environ. Eng. 2019, 4, 10. [Google Scholar] [CrossRef]
- El-Ashtoukhy, E.S.Z.; Fouad, Y.O. Liquid-liquid extraction of methylene blue dye from aqueous solutions using sodium dodecylbenzenesulfonate as an extractant. Alex. Eng. J. 2015, 54, 77–81. [Google Scholar] [CrossRef]
- Rafatullah, M.; Sulaiman, O.; Hashim, R.; Ahmad, A. Adsorption of methylene blue on low-cost adsorbents: A review. J. Hazard. Mater. 2010, 177, 70–80. [Google Scholar] [CrossRef]
- Tong, D.S.; Wu, C.W.; Adebajo, M.O.; Jin, G.C.; Yu, W.H.; Ji, S.F.; Zhou, C.H. Adsorption of methylene blue from aqueous solution onto porous cellulose-derived carbon/montmorillonite nanocomposites. Appl. Clay Sci. 2018, 161, 256–264. [Google Scholar] [CrossRef]
- Ghaedi, M.; Nasab, A.G.; Khodadoust, S.; Rajabi, M.; Azizian, S. Application of activated carbon as adsorbents for efficient removal of methylene blue: Kinetics and equilibrium study. J. Ind. Eng. Chem. 2014, 20, 2317–2324. [Google Scholar] [CrossRef]
- Spagnoli, A.A.; Giannakoudakis, D.A.; Bashkova, S. Adsorption of methylene blue on cashew nut shell based carbons activated with zinc chloride: The role of surface and structural parameters. J. Mol. Liq. 2017, 229, 465–471. [Google Scholar] [CrossRef]
- Fito, J.; Abrham, S.; Angassa, K. Adsorption of methylene blue from textile industrial wastewater onto activated carbon of parthenium hysterophorus. Int. J. Environ. Res. 2020, 14, 501–511. [Google Scholar] [CrossRef]
- Kempson, I.M.; Barnes, T.J.; Prestidge, C.A. Use of TOF-SIMS to study adsorption and loading behavior of methylene blue and papain in a nano-porous silicon layer. J. Am. Soc. Mass Spectrom. 2010, 21, 254–260. [Google Scholar] [CrossRef] [PubMed]
- Abdelfatah, A.M.; Fawzy, M.; Eltaweil, A.S.; El-Khouly, M.E. Green synthesis of nano-zero-valent iron using ricinus communis seeds extract: Characterization and application in the treatment of methylene blue-polluted water. ACS Omega 2021, 6, 25397–25411. [Google Scholar] [CrossRef] [PubMed]
- Gunture; Kaushik, J.; Garg, A.K.; Saini, D.; Khare, P.; Sonkar, S.K. Pollutant diesel soot derived onion-like nanocarbons for the adsorption of organic dyes and environmental assessment of treated wastewater. Ind. Eng. Chem. Res. 2020, 59, 12065–12074. [Google Scholar] [CrossRef]
- Maruthapandi, M.; Kumar, V.B.; Gedanken, A. Carbon dot initiated synthesis of poly(4,4′-diaminodiphenylmethane) and its methylene blue adsorption. ACS Omega 2018, 3, 7061–7068. [Google Scholar] [CrossRef] [PubMed]
- Hu, N.; Chen, C.; Tan, J.; Wang, W.; Wang, C.; Fan, H.; Wang, J.; Müller-Buschbaum, P.; Zhong, Q. Enhanced adsorption of methylene blue triggered by the phase transition of thermoresponsive polymers in hybrid interpenetrating polymer network hydrogels. ACS Appl. Polym. Mater. 2020, 2, 3674–3684. [Google Scholar] [CrossRef]
- Dhar, L.; Hossain, S.; Rahman, M.S.; Quraishi, S.B.; Saha, K.; Rahman, F.; Rahman, M.T. Adsorption mechanism of methylene blue by graphene oxide-shielded Mg-Al-layered double hydroxide from synthetic wastewater. J. Phys. Chem. A 2021, 125, 954–965. [Google Scholar] [CrossRef]
- Harris, J.; Silk, R.; Smith, M.; Dong, Y.; Chen, W.T.; Waterhouse, G.I.N. Hierarchical TiO2 Nanoflower photocatalysts with remarkable activity for aqueous methylene blue photo-oxidation. ACS Omega 2020, 5, 18919–18934. [Google Scholar] [CrossRef]
- Vijayalaks, G.; Ramkumar, B.; Mohan, S.C. Isotherm and kinetic studies of methylene blue adsorption using activated carbon prepared from teak wood waste biomass. J. Appl. Sci. 2019, 19, 827–836. [Google Scholar] [CrossRef]
- Heidarinejad, Z.; Rahmanian, O.; Fazlzadeh, M.; Heidari, M. Enhancement of methylene blue adsorption onto activated carbon prepared from date press cake by low frequency ultrasound. J. Mol. Liq. 2018, 264, 591–599. [Google Scholar] [CrossRef]
- Xue, H.; Wang, X.; Xu, Q.; Dhaouadi, F.; Sellaoui, L.; Seliem, M.K.; ben Lamine, A.; Belmabrouk, H.; Bajahzar, A.; Bonilla-Petriciolet, A.; et al. Adsorption of methylene blue from aqueous solution on activated carbons and composite prepared from an agricultural waste biomass: A comparative study by experimental and advanced modeling analysis. Chem. Eng. J. 2022, 430, 132801. [Google Scholar] [CrossRef]
- Habib Dakhil, I.; Ali, A.H. Adsorption of methylene blue dye from industrial wastewater using activated carbon prepared from agriculture wastes. Desalin. Water Treat. 2021, 216, 372–378. [Google Scholar] [CrossRef]
- Pathania, D.; Sharma, S.; Singh, P. Removal of methylene blue by adsorption onto activated carbon developed from ficus carica bast. Arab. J. Chem. 2017, 10, S1445–S1451. [Google Scholar] [CrossRef]
- Siddiqui, M.F.; Khan, S.A.; Hussain, D.; Tabrez, U.; Ahamad, I.; Fatma, T.; Khan, T.A. A sugarcane bagasse carbon-based composite material to decolor and reduce bacterial loads in waste water from textile industry. Ind. Crops Prod. 2022, 176, 114301. [Google Scholar] [CrossRef]
- Primo, A.; Atienzar, P.; Sanchez, E.; Delgado, J.M.; García, H. From biomass wastes to large-area, high-quality, N-doped graphene: Catalyst-free carbonization of chitosan coatings on arbitrary substrates. Chem. Commun. 2012, 48, 9254–9256. [Google Scholar] [CrossRef]
- Ilyas, M.; Ahmad, W.; Khan, H. Utilization of activated carbon derived from waste plastic for decontamination of polycyclic aromatic hydrocarbons laden wastewater. Water Sci. Technol. 2021, 84, 609–631. [Google Scholar] [CrossRef]
- Karagoz, S.; Zengin, A.; Akalin, M.K.; Tekin, K.; Erdem, M.; Tay, T. Preparation and characterization of activated carbons from waste melamine coated chipboard by naoh activation. Ekoloji 2012, 21, 123–128. [Google Scholar] [CrossRef]
- Zięzio, M.; Charmas, B.; Jedynak, K.; Hawryluk, M.; Kucio, K. Preparation and characterization of activated carbons obtained from the waste materials impregnated with phosphoric acid(V). Appl. Nanosci. 2020, 10, 4703–4716. [Google Scholar] [CrossRef]
- Yu, J.; Zhang, D.; Zhu, S.; Chen, P.; Zhu, G.T.; Jiang, X.; Di, S. Eco-friendly and facile one-step synthesis of a three dimensional net-like magnetic mesoporous carbon derived from wastepaper as a renewable adsorbent. RSC Adv. 2019, 9, 12419–12427. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Chen, R.; Du, H.; Zhang, J.; Shi, L.; Qin, Y.; Yue, L.; Wang, J. Synthesis of activated carbon from peanut shell as dye adsorbents for wastewater treatment. Adsorpt. Sci. Technol. 2019, 37, 34–48. [Google Scholar] [CrossRef]
- Zhang, Y.S.; Zhu, H.L.; Yao, D.; Williams, P.T.; Wu, C.; Xu, D.; Hu, Q.; Manos, G.; Yu, L.; Zhao, M.; et al. Thermo-Chemical Conversion of carbonaceous wastes for CNT and hydrogen production: A review. Sustain. Energy Fuels 2021, 5, 4173–4208. [Google Scholar] [CrossRef]
- Li, L.Y.; Gong, X.D.; Abida, O. Waste-to-resources: Exploratory surface modification of sludge-based activated carbon by nitric acid for heavy metal adsorption. Waste Manag. 2019, 87, 375–386. [Google Scholar] [CrossRef]
- Rastegar, A.; Farzadkia, M.; Jonoidi Jafari, A.; Esrafili, A. Preparation of carbon-alumina (C/Al2O3) aerogel nanocomposite for benzene adsorption from flow gas in fixed bed reactor. MethodsX 2019, 6, 2476–2483. [Google Scholar] [CrossRef]
- Dao, T.H.; Vu, T.Q.M.; Nguyen, N.T.; Pham, T.T.; Nguyen, T.L.; Yusa, S.I.; Pham, T.D. Adsorption characteristics of synthesized polyelectrolytes onto alumina nanoparticles and their application in antibiotic removal. Langmuir 2020, 36, 13001–13011. [Google Scholar] [CrossRef]
- Chen, A.S.C.; Snoeyink, V.L.; Fiessinger, F. Activated alumina adsorption of dissolved organic compounds before and after ozonation. Environ. Sci. Technol. 1987, 21, 83–90. [Google Scholar] [CrossRef]
- Rahmani, A.; Mousavi, H.Z.; Fazli, M. Effect of nanostructure alumina on adsorption of heavy metals. Desalination 2010, 253, 94–100. [Google Scholar] [CrossRef]
- Adesina, A.O.; Elvis, O.A.; Mohallem, N.D.S.; Olusegun, S.J. Adsorption of Methylene Blue and Congo Red from Aqueous Solution Using Synthesized Alumina–Zirconia Composite. Environ. Technol. 2019, 42, 1061–1070. [Google Scholar] [CrossRef]
- Nédez, C.; Ray, J.L. Efficient removal of polymerization inhibitors by adsorption on the surface of an optimized alumina. Langmuir 1999, 15, 5932–5936. [Google Scholar] [CrossRef]
- Mckinney, C.M.; Hopkins, R.L. Alumina-adsorption analysis of petroleum aromatics in 420° to 600° F. Range. Anal. Chem. 1954, 26, 1460–1465. [Google Scholar] [CrossRef]
- Yobanny Reyes-López, S.; Arely Aguirre-Terrazas, K.; Torres-Pérez, J.; Nahúm, M.-C.; de Jesús Ruíz-Baltazar, Á. Review of alumina in adsorption processes for emerging pollutants. Int. J. Res. GRANTHAALAYAH 2021, 9, 435–453. [Google Scholar] [CrossRef]
- Dambies, L. Existing and prospective sorption technologies for the removal of arsenic in water. Sep. Sci. Technol. 2005, 39, 603–627. [Google Scholar] [CrossRef]
- Mohammadifar, E.; Shemirani, F.; Majidi, B.; Ezoddin, M. Application of Modified Nano-γ-Alumina as an Efficient Adsorbent for Removing Malachite Green (MG) from Aqueous Solution. New Pub Balaban 2014, 54, 758–768. [Google Scholar] [CrossRef]
- Weidner, E.; Ciesielczyk, F. Removal of hazardous oxyanions from the environment using metal-oxide-based materials. Materials 2019, 12, 927. [Google Scholar] [CrossRef] [PubMed]
- Habila, M.A.; Alothman, Z.A.; Ali, R.; Ghafar, A.A.; Hassouna, M.S.E.-D. Removal of tartrazine dye onto mixed-waste activated carbon: Kinetic and thermodynamic studies. Clean 2014, 42, 1824–1831. [Google Scholar] [CrossRef]
- Al-Gaashani, R.; Almasri, D.; Shomar, B.; Kochkodan, V. Preparation and properties of novel activated carbon doped with aluminum oxide and silver for water treatment. J. Alloys Compd. 2021, 858, 158372. [Google Scholar] [CrossRef]
- Ihsanullah; Asmaly, H.A.; Saleh, T.A.; Laoui, T.; Gupta, V.K.; Atieh, M.A. Enhanced adsorption of phenols from liquids by aluminum oxide/carbon nanotubes: Comprehensive study from synthesis to surface properties. J. Mol. Liq. 2015, 206, 176–182. [Google Scholar] [CrossRef]
- Fatima, S.S.; Borhan, A.; Ayoub, M.; Ghani, N.A. CO2 adsorption performance on surface-functionalized activated carbon impregnated with pyrrolidinium-based ionic liquid. Processes 2022, 10, 2372. [Google Scholar] [CrossRef]
- Yusuf, N.Y.M.; Masdar, M.S.; Isahak, W.N.R.W.; Nordin, D.; Husaini, T.; Majlan, E.H.; Wu, S.Y.; Rejab, S.A.M.; Lye, C.C. Impregnated carbon–ionic liquid as innovative adsorbent for H2/CO2 separation from biohydrogen. Int. J. Hydrogen Energy 2019, 44, 3414–3424. [Google Scholar] [CrossRef]
- Habila, M.A.; ALOthman, Z.A.; ALOthman, M.R.; Hassouna, M.S.E.D. Benzothiophene adsorptive desulfurization onto TrihexYl(tetradecyl)phosphonium dicyanamide ionic-liquid-modified renewable carbon: Kinetic, equilibrium and UV spectroscopy investigations. Molecules 2023, 28, 298. [Google Scholar] [CrossRef] [PubMed]
- Habila, M.; AlOthman, Z.; Ghfar, A.; Al-Zaben, M.; Alothman, A.; Abdeltawab, A.; El-Marghany, A.; Sheikh, M. Phosphonium-based ionic liquid modified activated carbon from mixed recyclable waste for mercury(II) uptake. Molecules 2019, 24, 570. [Google Scholar] [CrossRef] [PubMed]
- Borrell, A.; Rocha, V.G.; Torrecillas, R.; Fernández, A. Surface coating on carbon nanofibers with alumina precursor by different synthesis routes. Compos. Sci. Technol. 2011, 71, 18–22. [Google Scholar] [CrossRef]
- Romero Toledo, R.; Ruiz Santoyo, V.; Moncada Sánchez, C.D.; Martínes Rosales, M. Effect of aluminum precursor on physicochemical properties of Al2O3 by hydrolysis/precipitation method. Nova Sci. 2018, 10, 83–99. [Google Scholar] [CrossRef]
- Prashanth, P.A.; Raveendra, R.S.; Hari Krishna, R.; Ananda, S.; Bhagya, N.P.; Nagabhushana, B.M.; Lingaraju, K.; Raja Naika, H. Synthesis, characterizations, antibacterial and photoluminescence studies of solution combustion-derived α-Al 2 O 3 nanoparticles. J. Asian Ceram. Soc. 2015, 3, 345–351. [Google Scholar] [CrossRef]
- Liu, C.; Shih, K.; Gao, Y.; Li, F.; Wei, L. Dechlorinating transformation of propachlor through nucleophilic substitution by dithionite on the surface of alumina. J. Soils Sediments 2012, 12, 724–733. [Google Scholar] [CrossRef]
- Al-Marghany, A.; Badjah Hadj Ahmed, A.Y.; Alothman, Z.A.; Sheikh, M.; Ghfar, A.A.; Habila, M. Fabrication of schiff’s base-functionalized porous carbon materials for the effective removal of toxic metals from wastewater. Arab. J. Geosci. 2021, 14, 336. [Google Scholar] [CrossRef]
- AlOthman, Z.A.; Habila, M.A.; Ali, R.; Abdel Ghafar, A.; El-din Hassouna, M.S. Valorization of two waste streams into activated carbon and studying its adsorption kinetics, equilibrium isotherms and thermodynamics for methylene blue removal. Arab. J. Chem. 2014, 7, 1148–1158. [Google Scholar] [CrossRef]
- Wu, F.C.; Tseng, R.L.; Juang, R.S. Kinetic modeling of liquid-phase adsorption of reactive dyes and metal ions on chitosan. Water Res. 2001, 35, 613–618. [Google Scholar] [CrossRef]
- Bhatti, H.N.; Safa, Y.; Yakout, S.M.; Shair, O.H.; Iqbal, M.; Nazir, A. Efficient removal of dyes using carboxymethyl cellulose/alginate/polyvinyl alcohol/rice husk composite: Adsorption/desorption, kinetics and recycling studies. Int. J. Biol. Macromol. 2020, 150, 861–870. [Google Scholar] [CrossRef]
- Elmoubarki, R.; Mahjoubi, F.Z.; Tounsadi, H.; Moustadraf, J.; Abdennouri, M.; Zouhri, A.; el Albani, A.; Barka, N. Adsorption of textile dyes on raw and decanted moroccan clays: Kinetics, equilibrium and thermodynamics. Water Resour. Ind. 2015, 9, 16–29. [Google Scholar] [CrossRef]
- Hosseini, F.; Sadighian, S.; Hosseini-Monfared, H.; Mahmoodi, N.M. Dye removal and kinetics of adsorption by magnetic chitosan nanoparticles. Desalin. Water Treat. 2016, 57, 24378–24386. [Google Scholar] [CrossRef]
- Mohamed El-Toni, A.; Habila, M.A.; Abbas Ibrahim, M.; Puzon Labis, J.; ALOthman, Z.A. Simple and facile synthesis of amino functionalized hollow Core-mesoporous shell silica spheres using anionic surfactant for Pb(II), Cd(II), and Zn(II) adsorption and recovery. Chem. Eng. J. 2014, 251, 441–451. [Google Scholar] [CrossRef]
- Langmuir, I. The constitution and fundamental properties of solids and liquids. Part I. Solids. J. Am. Chem. Soc. 1916, 38, 2221–2295. [Google Scholar] [CrossRef]
- Ayawei, N.; Ebelegi, A.N.; Wankasi, D. Modelling and interpretation of adsorption isotherms. J. Chem. 2017, 2017, 3039817. [Google Scholar] [CrossRef]
- Saleh, T.A.; Al-Absi, A.A. Kinetics, isotherms and thermodynamic evaluation of amine functionalized magnetic carbon for methyl red removal from aqueous solutions. J. Mol. Liq. 2017, 248, 577–585. [Google Scholar] [CrossRef]
- Myneni, V.R.; Kanidarapu, N.R.; Vangalapati, M. Methylene blue adsorption by magnesium oxide nanoparticles immobilized with chitosan (CS-MgONP): Response surface methodology, isotherm, kinetics and thermodynamic studies. Iran. J. Chem. Chem. Eng. 2020, 39, 29–42. [Google Scholar]
- Ghate, E.; Ganjidoust, H.; Ayati, B. The thermodynamics, kinetics, and isotherms of sulfamethoxazole adsorption using magnetic activated carbon nanocomposite and its reusability potential. Nanotechnol. Environ. Eng. 2021, 6, 32. [Google Scholar] [CrossRef]
- Wang, S.; Zhai, Y.Y.; Gao, Q.; Luo, W.J.; Xia, H.; Zhou, C.G. Highly efficient removal of acid red 18 from aqueous solution by magnetically retrievable chitosan/carbon nanotube: Batch study, isotherms, kinetics, and thermodynamics. J. Chem. Eng. Data 2014, 59, 39–51. [Google Scholar] [CrossRef]
- Freundlich, H.M.F. Over the adsorption in solution. J. Phys. Chem. A 1906, 57, 385–470. [Google Scholar]




| Adsorbent Materials | BET Surface Area (m2/g) | Adsorption Capacity (mg/g) |
|---|---|---|
| C-A-0 | 568.1 | 201.8 |
| C-A-25 | 249.7 | 234.4 |
| C-A-50 | 247.0 | 156.4 |
| Pseudo-First-Order | Pseudo-Second-Order | ||||||
|---|---|---|---|---|---|---|---|
| Adsorbent Materials | qe,exp (mg/g) | K1 (min−1) | qe,cal (mg/g) | R2 | k2 (g/mg·min) | qe,cal (mg/g) | R2 |
| C-0 | 83.3 | 0.048 | 159.4 | 0.782 | 4.5 × 0−4 | 90.09 | 0.991 |
| CA-1 | 99.2 | 0.021 | 51.1 | 0.975 | 1.08 × 10−3 | 102.04 | 0.999 |
| CA-2 | 68.8 | 0.038 | 92 | 0.981 | 6.68 × 10−4 | 72.99 | 0.995 |
| Langmuir Constants | Freundlich Constants | ||||||
|---|---|---|---|---|---|---|---|
| KL | b | Q max. | R2 | KF | n | R2 | |
| C-0 | 60.86 | 7.8 | 181.8 | 0.9928 | 35.45 | 2.41 | 0.2471 |
| CA-1 | 161.29 | 1.01 | 158.7 | 0.9796 | 102.63 | 9.84 | 0.0847 |
| CA-2 | 4.37 | 0.04 | 192.3 | 0.9388 | 49.26 | 0.63 | 0.7415 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Habila, M.A.; ALOthman, Z.A.; Hakami, H.M.; ALOthman, M.R.; Sheikh, M. Recyclable Carbon-Based Hybrid Adsorbents Functionalized with Alumina Nanoparticles for Water Remediation. Crystals 2023, 13, 598. https://doi.org/10.3390/cryst13040598
Habila MA, ALOthman ZA, Hakami HM, ALOthman MR, Sheikh M. Recyclable Carbon-Based Hybrid Adsorbents Functionalized with Alumina Nanoparticles for Water Remediation. Crystals. 2023; 13(4):598. https://doi.org/10.3390/cryst13040598
Chicago/Turabian StyleHabila, Mohamed A., Zeid A. ALOthman, Hussam Musaad Hakami, Monerah R. ALOthman, and Mohamed Sheikh. 2023. "Recyclable Carbon-Based Hybrid Adsorbents Functionalized with Alumina Nanoparticles for Water Remediation" Crystals 13, no. 4: 598. https://doi.org/10.3390/cryst13040598
APA StyleHabila, M. A., ALOthman, Z. A., Hakami, H. M., ALOthman, M. R., & Sheikh, M. (2023). Recyclable Carbon-Based Hybrid Adsorbents Functionalized with Alumina Nanoparticles for Water Remediation. Crystals, 13(4), 598. https://doi.org/10.3390/cryst13040598







