Fe-Doped CuO/MWCNT as a Sensing Material for Electrochemical Detection of Nitrite
Abstract
:1. Introduction
2. Materials and Methods
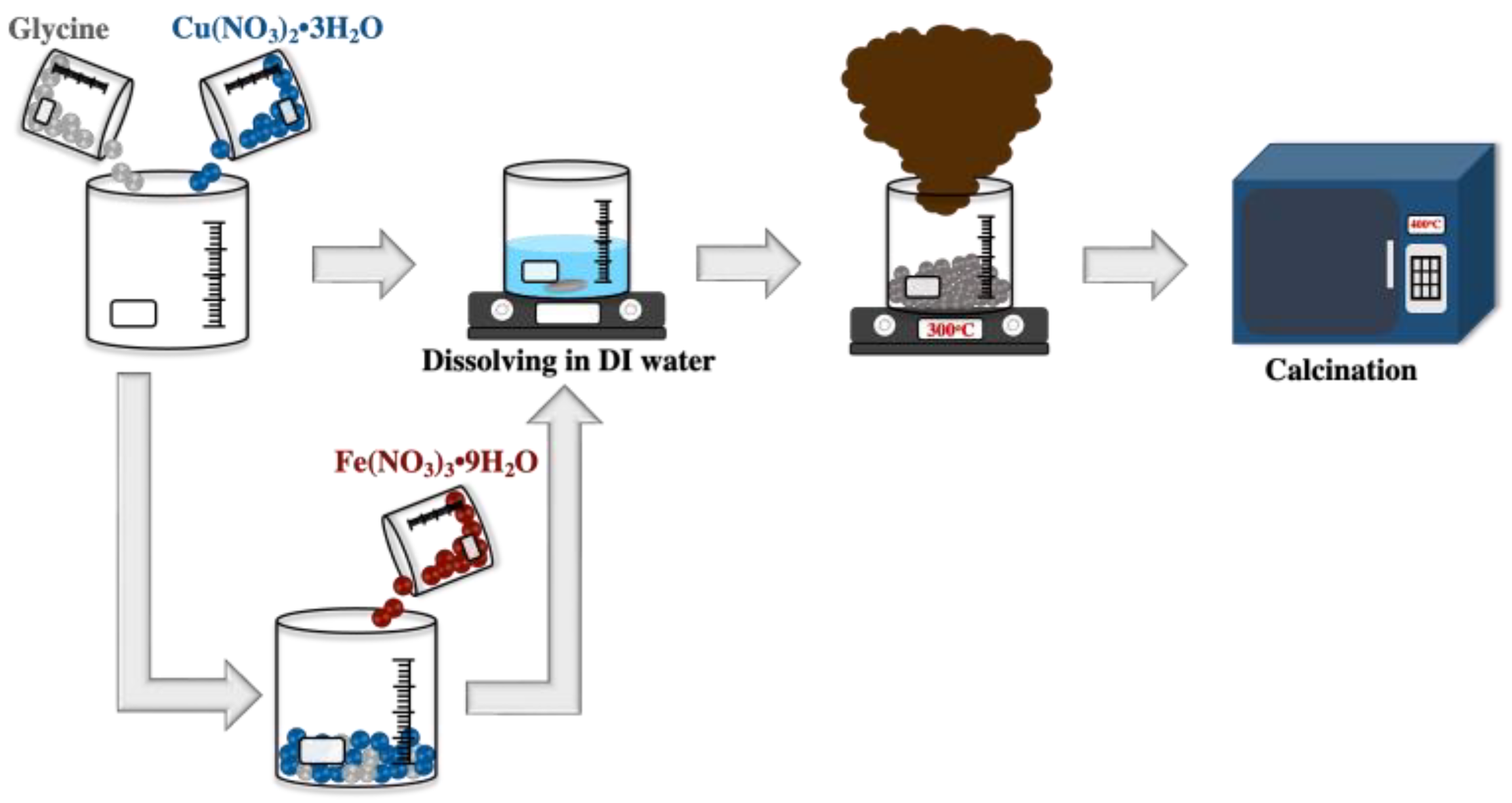
2.1. Synthesis of CuO and 3 mol% Fe-Doped CuO Powders
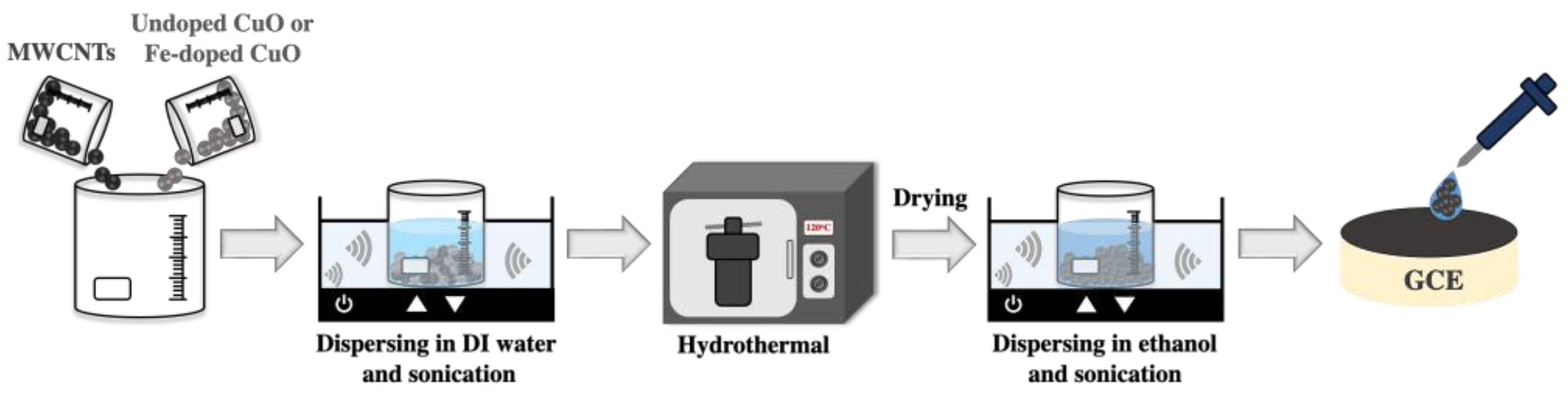
2.2. Preparation of CuO/MWCNT and 3 mol% Fe-Doped CuO/MWCNT Working Electrodes
2.3. Characterization
3. Results
3.1. Chemical Composition and Structural Identification
3.2. Microstructural Examination
3.3. Specific Surface Area
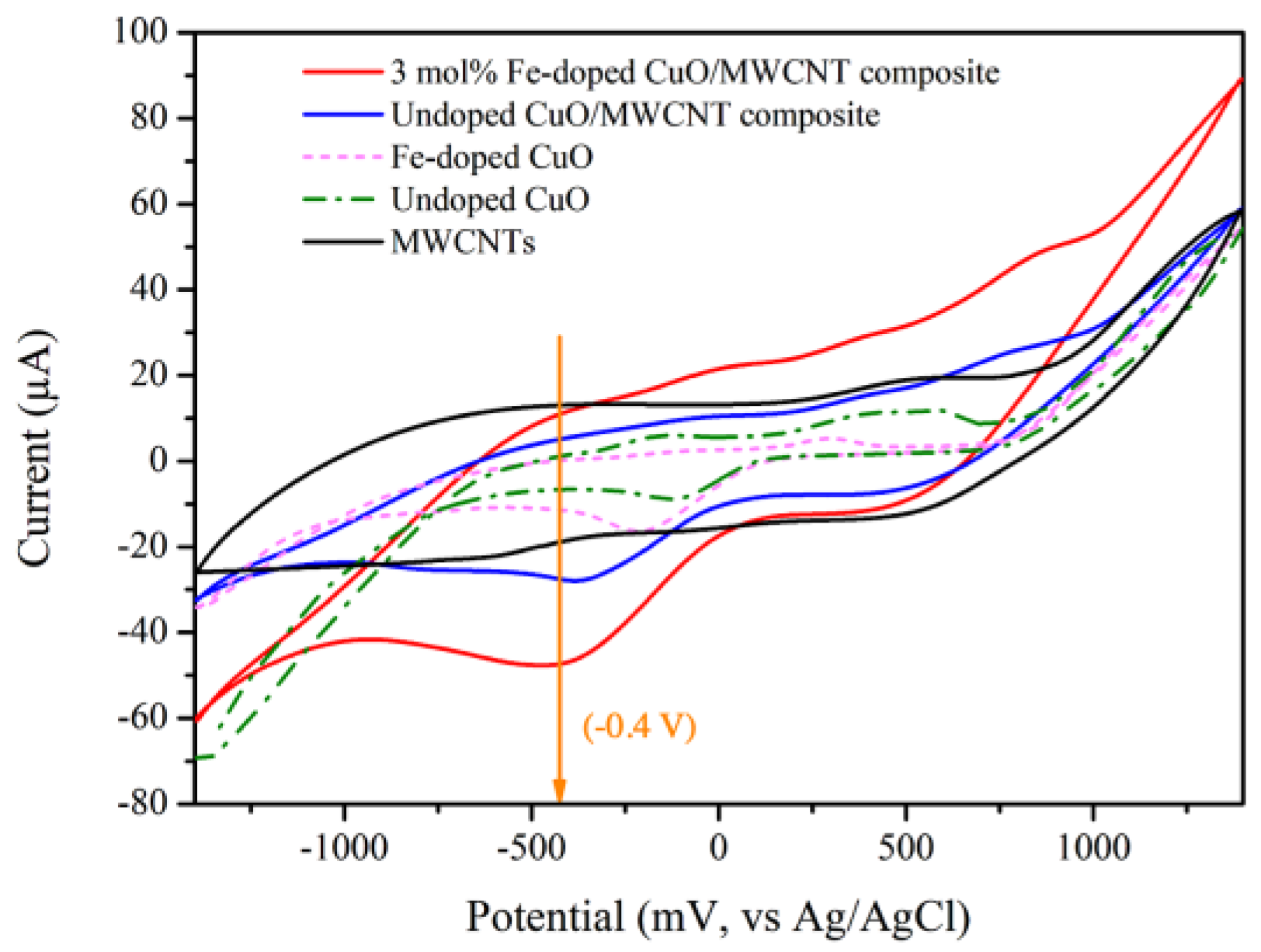
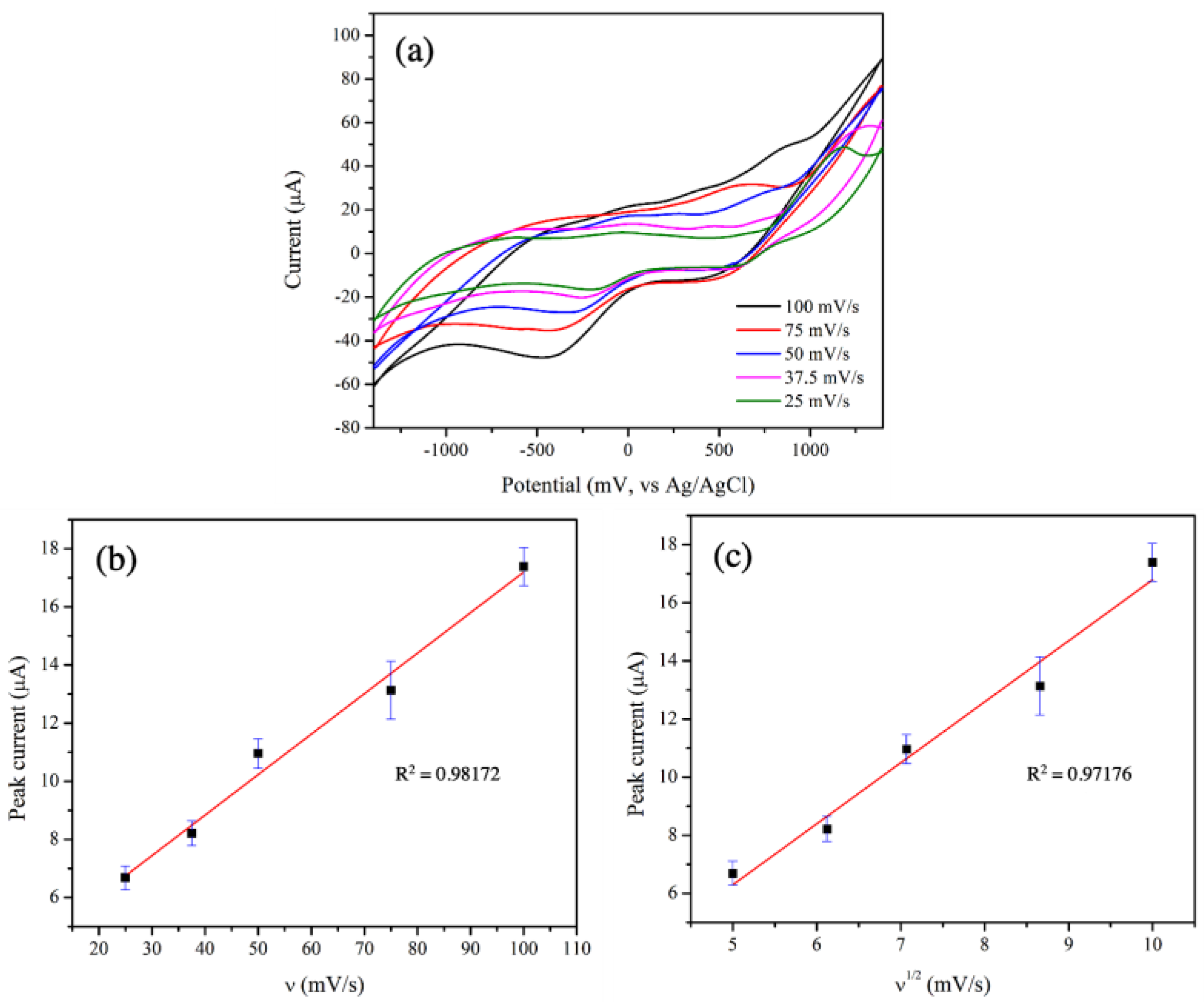
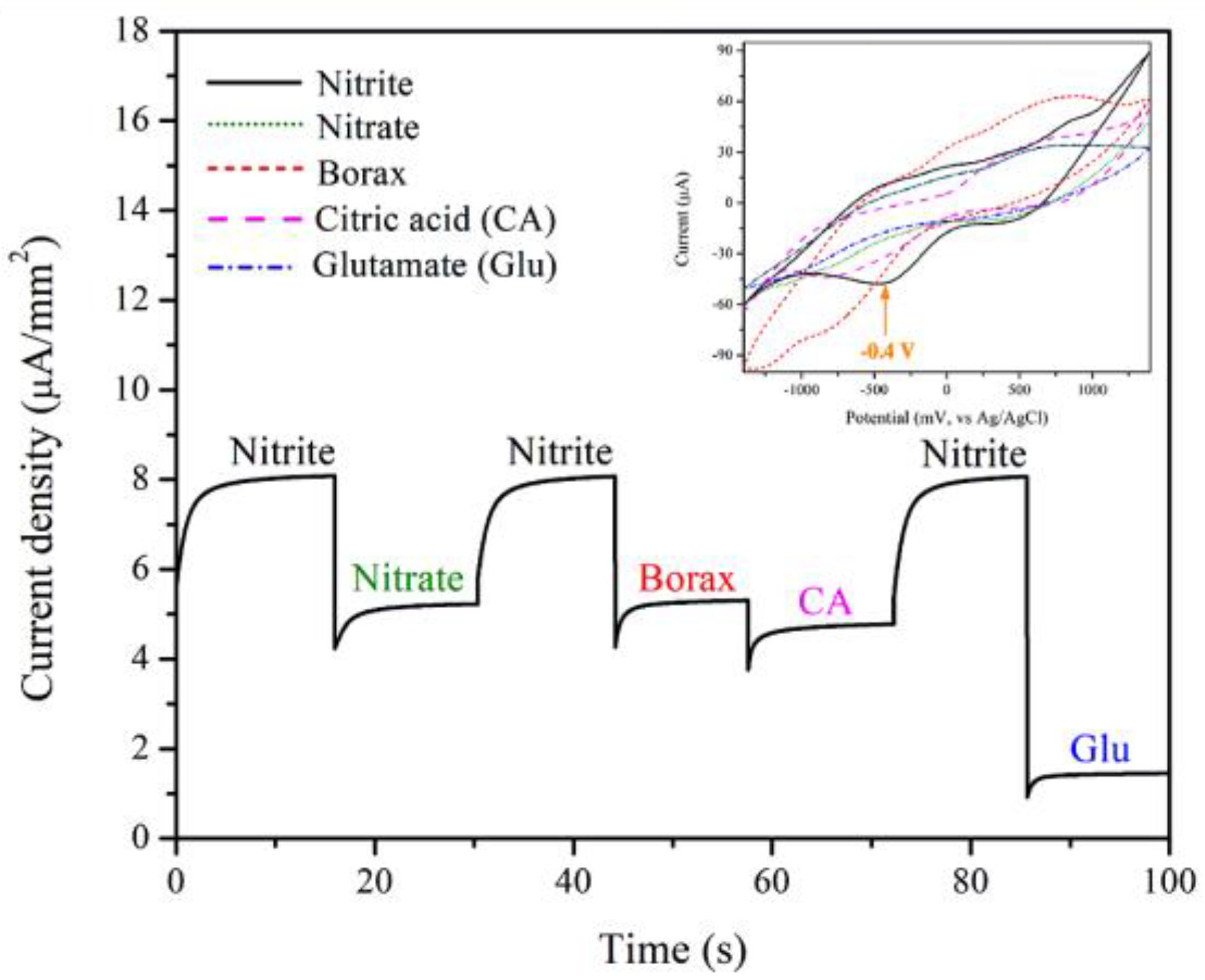
3.4. Electrocatalytic Activities
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mortensen, A.; Aguilar, F.; Crebelli, R.; Di Domenico, A.; Dusemund, B.; Frutos, M.J.; Galtier, P.; Gott, D.; Gundert-Remy, U.; Lambre, C.; et al. Re-evaluation of potassium nitrite (E 249) and sodium nitrite (E 250) as food additives. EFSA J. 2017, 15, e04786. [Google Scholar] [CrossRef] [PubMed]
- Griesenbeck, J.S.; Steck, M.D.; Huber, J.C.; Sharkey, J.R.; Rene, A.A.; Brender, J.D. Development of estimates of dietary nitrates, nitrites, and nitrosamines for use with the short willet food frequency questionnaire. Nutr. Metab. 2009, 6, 16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jakszyn, P.; Gonzalez, C.A. Nitrosamine and related food intake and gastric and oesophageal cancer risk: A systematic review of the epidemiological evidence. World J. Gastroenterol. 2006, 12, 4296–4303. [Google Scholar] [CrossRef] [PubMed]
- Sohn, C.H.; Seo, D.W.; Ryoo, S.M.; Lee, J.H.; Kim, W.Y.; Lim, K.S.; Oh, B.J. Life-threatening methemoglobinemia after unintentional ingestion of antifreeze admixtures containing sodium nitrite in the construction sites. Clin. Toxicol. 2014, 52, 44–47. [Google Scholar] [CrossRef] [PubMed]
- Schrenk, D.; Bignami, M.; Bodin, L.; Chipman, J.K.; Mazo, J.; Grasl-Kraupp, B.; Hoogenboom, L.R.; Leblanc, J.; Nebbia, C.S.; Nielsen, E.; et al. Risk assessment of nitrate and nitrite in feed. EFSA J. 2020, 18, e06290. [Google Scholar] [CrossRef]
- World Health Organization. Guidelines for Drinking-Water Quality, 4th ed.; WHO Publications: Geneva, Switzerland, 2022. [Google Scholar]
- Ensafi, A.A.; Rezaei, B.; Nouroozi, S. Simultaneous spectrophotometric determination of nitrite and nitrate by flow injection analysis. Anal. Sci. 2004, 20, 1749–1753. [Google Scholar] [CrossRef] [Green Version]
- Nagababu, E.; Rifkind, J.M. Measurement of plasma nitrite by chemiluminescence without interference of S-, N-nitroso and nitrated species. Free Radic. Biol. Med. 2007, 42, 1146–1154. [Google Scholar] [CrossRef] [Green Version]
- Ferreira, I.M.; Silva, S. Quantification of residual nitrite and nitrate in ham by reverse-phase high performance liquid chromatography/diode array detector. Talanta 2008, 74, 1598–1602. [Google Scholar] [CrossRef]
- Merusi, C.; Corradini, C.; Cavazza, A.; Borromei, C.; Salvadeo, P. Determination of nitrates, nitrites and oxalates in food products by capillary electrophoresis with pH-dependent electroosmotic flow reversal. Food Chem. 2010, 120, 615–620. [Google Scholar] [CrossRef]
- Moorcroft, M.J.; Davis, J.; Compton, R.G. Detection and determination of nitrate and nitrite: A review. Talanta 2001, 54, 785–803. [Google Scholar] [CrossRef]
- Wang, Q.-H.; Yu, L.-J.; Liu, Y.; Lin, L.; Lu, R.-g.; Zhu, J.-p.; He, L.; Lu, Z.-L. Methods for the detection and determination of nitrite and nitrate: A review. Talanta 2017, 165, 709–720. [Google Scholar] [CrossRef]
- Ramachandran, R.; Chen, T.-W.; Chen, S.-M.; Baskar, T.; Kannan, R.; Elumalai, P.; Raja, P.; Jeyapragasam, T.; Dinakaran, K.; Gnana Kumar, G.G. A review of the advanced developments of electrochemical sensors for the detection of toxic and bioactive molecules. Inorg. Chem. Front. 2019, 6, 3418–3439. [Google Scholar] [CrossRef]
- Simões, F.R.; Xavier, M.G. 6-Electrochemical Sensors. In Nanoscience and Its Applications, 1st ed.; de Oliveira, O.N., Jr., Ferreira, L., Marystela, G., de Lima Leite, F., Da Róz, A.L., Eds.; William Andrew Publishing: Boston, MA, USA, 2017; pp. 155–178. [Google Scholar]
- Fethi, A. Novel materials for electrochemical sensing platforms. Sens. Int. 2020, 1, 100035. [Google Scholar] [CrossRef]
- Tran, T.H.; Nguyen, V.T. Copper oxide nanomaterials prepared by solution methods, some properties, and potential applications: A brief review. Int. Sch. Res. Not. 2014, 2014, 856592. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Borgohain, K.; Singh, J.B.; Rama Rao, M.V.; Shripathi, T.; Mahamuni, S. Quantum size effects in CuO nanoparticles. Phys. Rev. B 2000, 61, 11093–11096. [Google Scholar] [CrossRef]
- Carnes, C.L.; Stipp, J.; Klabunde, K.J.; Bonevich, J. Synthesis, characterization, and adsorption studies of nanocrystalline copper oxide and nickel oxide. Langmuir 2002, 18, 1352–1359. [Google Scholar] [CrossRef]
- Sahooli, M.; Sabbaghi, S.; Saboori, R. Synthesis and characterization of mono sized CuO nanoparticles. Mater. Lett. 2012, 81, 169–172. [Google Scholar] [CrossRef]
- Wang, H.; Xu, J.-Z.; Zhu, J.-J.; Chen, H.-Y. Preparation of CuO nanoparticles by microwave irradiation. J. Cryst. Growth 2002, 244, 88–94. [Google Scholar] [CrossRef]
- Hossain, M.; Kecsenovity, E.; Varga, A.; Molnár, M.; Janáky, C.; Rajeshwar, K. Solution combustion synthesis of complex oxide semiconductors. Int. J. Self-Propagating High-Temp. Synth. 2018, 27, 129–140. [Google Scholar] [CrossRef]
- Kadhim, R.G.; Kzar, B.R.S. Effect of Cd doping on structural and some optical studies of nano CuO films prepared by sol-gel technique. World Sci. News 2017, 64, 69–83. [Google Scholar]
- Oh, J.; Ryu, H.; Lee, W.-J. Effects of Fe doping on the photoelectrochemical properties of CuO photoelectrodes. Compos. B Eng. 2019, 163, 59–66. [Google Scholar] [CrossRef]
- Chafi, F.Z.; Bahmad, L.; Hassanain, N.; Fares, B.; Laanab, L.; Mzerd, A. Characterization techniques of Fe-doped CuO thin films deposited by the Spray Pyrolysis method. arXiv 2018, arXiv:1807.09697. [Google Scholar]
- Sakthivel, B.; Gopalakrishnan, N. Effect of Fe doping on the NH3 sensing properties of CuO nanostructures. J. Mater. Sci. Mater. Electron. 2019, 30, 6920–6928. [Google Scholar] [CrossRef]
- Mishra, P.K.; Dobhal, R.; Rini, E.G.; Kumar, M.; Sen, S. Rapid organic dye degradation and wavelength dependent sensing study in Cu1-xFexO. Ceram. Int. 2022, 48, 5995–6006. [Google Scholar] [CrossRef]
- Fagan-Murphy, A.; Kataria, S.; Patel, B.A. Electrochemical performance of multi-walled carbon nanotube composite electrodes is enhanced with larger diameters and reduced specific surface area. J. Solid State Electrochem. 2016, 20, 785–792. [Google Scholar] [CrossRef]
- Yang, C.; Devasenathipathy, R.; Rani, K.K.; Wang, S. Synthesis of multi walled carbon nanotubes covered copper oxide nanoberries for the sensitive and selective electrochemical determination of hydrogen peroxide. Int. J. Electrochem. Sci. 2017, 12, 5910–5920. [Google Scholar] [CrossRef]
- Hussain, M.M.; Asiri, A.M.; Rahman, M.M. A non-enzymatic electrochemical approach for l-lactic acid sensor development based on CuO·MWCNT nanocomposites modified with a nafion matrix. New J. Chem. 2020, 44, 9775–9787. [Google Scholar] [CrossRef]
- Zi, Z.F.; Sun, Y.P.; Zhu, X.B.; Yang, Z.R.; Dai, J.M.; Song, W.H. Synthesis of magnetoresistive La0.7Sr0.3MnO3 nanoparticles by an improved chemical coprecipitation method. J. Magn. Magn. Mater. 2009, 321, 2378–2381. [Google Scholar] [CrossRef]
- Singh, J.; Sharma, S.; Soni, S.; Sharma, S.; Kohli, N. Influence of different milling media on structural, morphological and optical properties of the ZnO nanoparticles synthesized by ball milling process. Mater. Sci. Semicond. Process. 2019, 98, 29–38. [Google Scholar] [CrossRef]
- Chafi, F.Z.; Fares, B.; Hadri, A.; Nassiri, C.; Laaneb, L.; Hassanain, N.; Mzerd, A. Fe-doped CuO deposited by spray pyrolysis technique. In Proceedings of the 2015 3rd International Renewable and Sustainable Energy Conference (IRSEC), Marrakech, Morocco, 10–13 December 2015. [Google Scholar] [CrossRef]
- Layek, S.; Verma, H.C. Room temperature ferromagnetism in Fe-doped CuO nanoparticles. J. Nanosci. Nanotechnol. 2013, 13, 1848–1853. [Google Scholar] [CrossRef]
- Jongprateep, O.; Meesombad, K.; Techapiesancharoenkij, R.; Surawathanawises, K.; Siwayaprahm, P.; Watthanarat, P. Influences of chemical composition, microstructure and bandgap energy on photocatalytic and antimicrobial activities of ZnO and Ag-doped ZnO by solution combustion technique. J. Met. Mater. Miner. 2019, 29, 78–85. [Google Scholar]
- Ravichandran, K.; Sathish, P.; Snega, S.; Karthika, K.; Rajkumar, P.V.; Subha, K.; Sakthivel, B. Improving the antibacterial efficiency of ZnO nanopowders through simultaneous anionic (F) and cationic (Ag) doping. Powder Technol. 2015, 274, 250–257. [Google Scholar] [CrossRef]
- Bernard, P.; Stelmachowski, P.; Broś, P.; Makowski, W.; Kotarba, A. Demonstration of the influence of specific surface area on reaction rate in heterogeneous catalysis. J. Chem. Educ. 2021, 98, 935–940. [Google Scholar] [CrossRef] [PubMed]
- Dreyse, P.; Isaacs, M.; Calfumán, K.; Cáceres, C.; Aliaga, A.; Aguirre, M.J.; Villagra, D. Electrochemical reduction of nitrite at poly-[Ru(5-NO2-phen)2Cl] tetrapyridylporphyrin glassy carbon modified electrode. Electrochim. Acta 2011, 56, 5230–5237. [Google Scholar] [CrossRef]
- Fayemi, O.; Adekunle, A. Metal oxide nanoparticles/multi-walled carbon nanotube nanocomposite modified electrode for the detection of dopamine: Comparative electrochemical study. J. Biosens. Bioelectron. 2015, 6, 10–4172. [Google Scholar] [CrossRef]
- Wu, J.-J.; Wang, W.-T.; Wang, M.; Liu, H.; Pan, H.-C. Electrochemical behavior and direct quantitative determination of Tanshinone IIA in micro-emulsion. Int. J. Electrochem. Sci. 2016, 11, 5165–5179. [Google Scholar] [CrossRef]
- Li, Y.; Cheng, C.; Yang, Y.; Dun, X.; Gao, J.; Jin, X.-J. A novel electrochemical sensor based on CuO/H-C3N4/rGO nanocomposite for efficient electrochemical sensing nitrite. J. Alloys Compd. 2019, 798, 764–772. [Google Scholar] [CrossRef]
- Azad, U.P.; Ganesan, V. Efficient sensing of nitrite by Fe(bpy)32+ immobilized nafion modified electrodes. Chem. Commun. 2010, 46, 6156–6158. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Zhang, K.; Sun, Y.; Sun, C. Hemoglobin/colloidal silver nanoparticles immobilized in titania sol–gel film on glassy carbon electrode: Direct electrochemistry and electrocatalysis. Bioelectrochemistry 2006, 69, 10–15. [Google Scholar] [CrossRef]
- Salagare, S.; Shivappa Adarakatti, P.; Venkataramanappa, Y. Designing and construction of carboxyl functionalised MWCNTs/Co-MOFs-based electrochemical sensor for the sensitive detection of nitrite. Int. J. Environ. Anal. Chem. 2020, 2, 1–20. [Google Scholar] [CrossRef]
- Li, C.; Guo, B.; Guo, X.M.; Wang, F. The electrochemical sensor based on electrochemical oxidation of nitrite on metalloporphyrin–graphene modified glassy carbon electrode. RSC Adv. 2016, 6, 90480–90488. [Google Scholar] [CrossRef]
- Li, X.; Zou, N.; Wang, Z.; Sun, Y.; Li, H.; Gao, C.; Wang, T.; Wang, X. An electrochemical sensor for determination of nitrite based on Au nanoparticles decorated MoS2 nanosheets. Chem. Pap. 2020, 74, 441–449. [Google Scholar] [CrossRef]
- Radhakrishnan, S.; Krishnamoorthy, K.; Sekar, C.; Wilson, J.; Kim, S.J. A highly sensitive electrochemical sensor for nitrite detection based on Fe2O3 nanoparticles decorated reduced graphene oxide nanosheets. Appl. Catal. B Environ. 2014, 148–149, 22–28. [Google Scholar] [CrossRef]

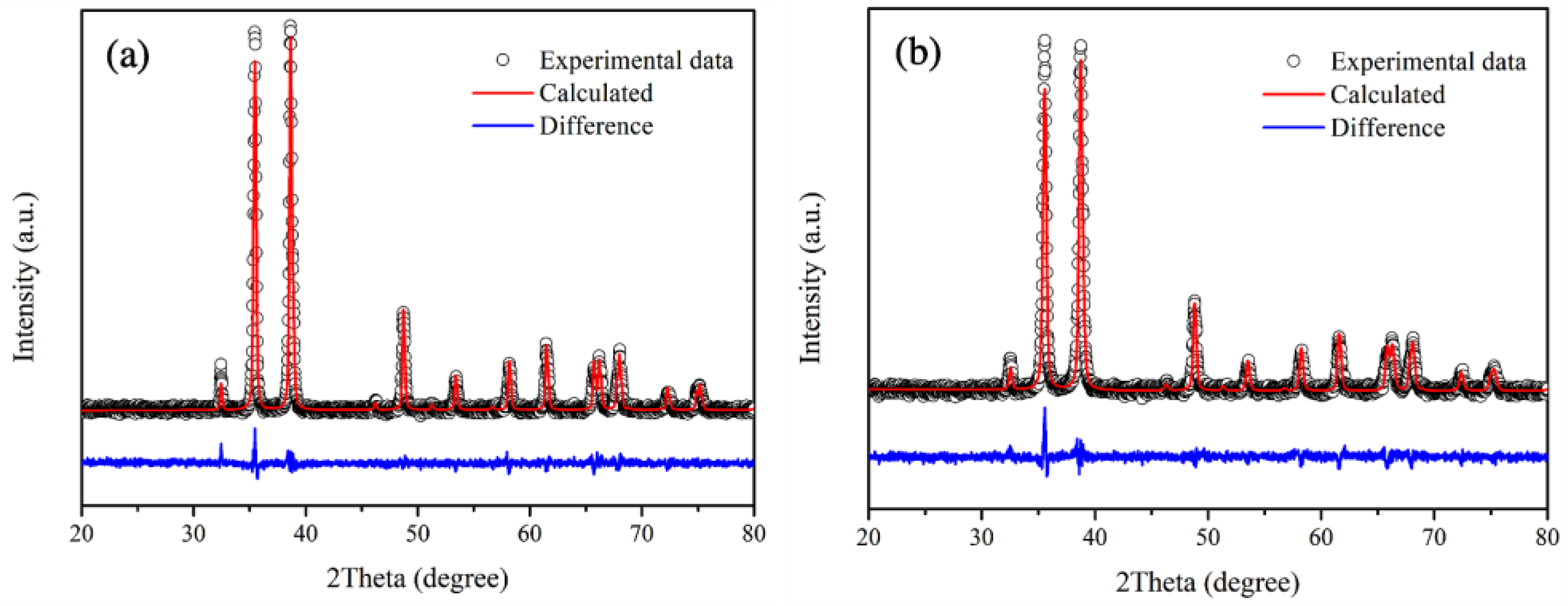
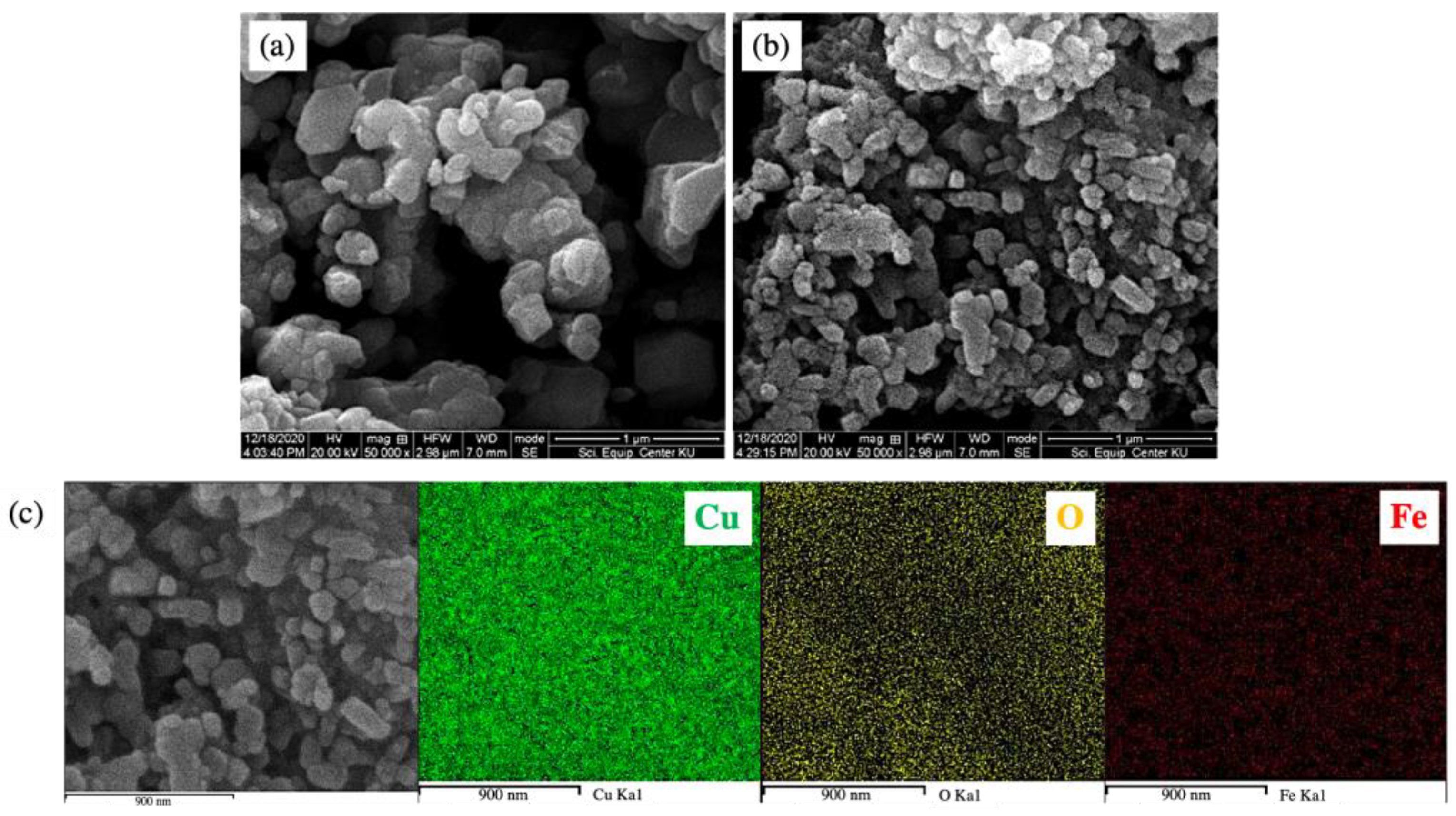
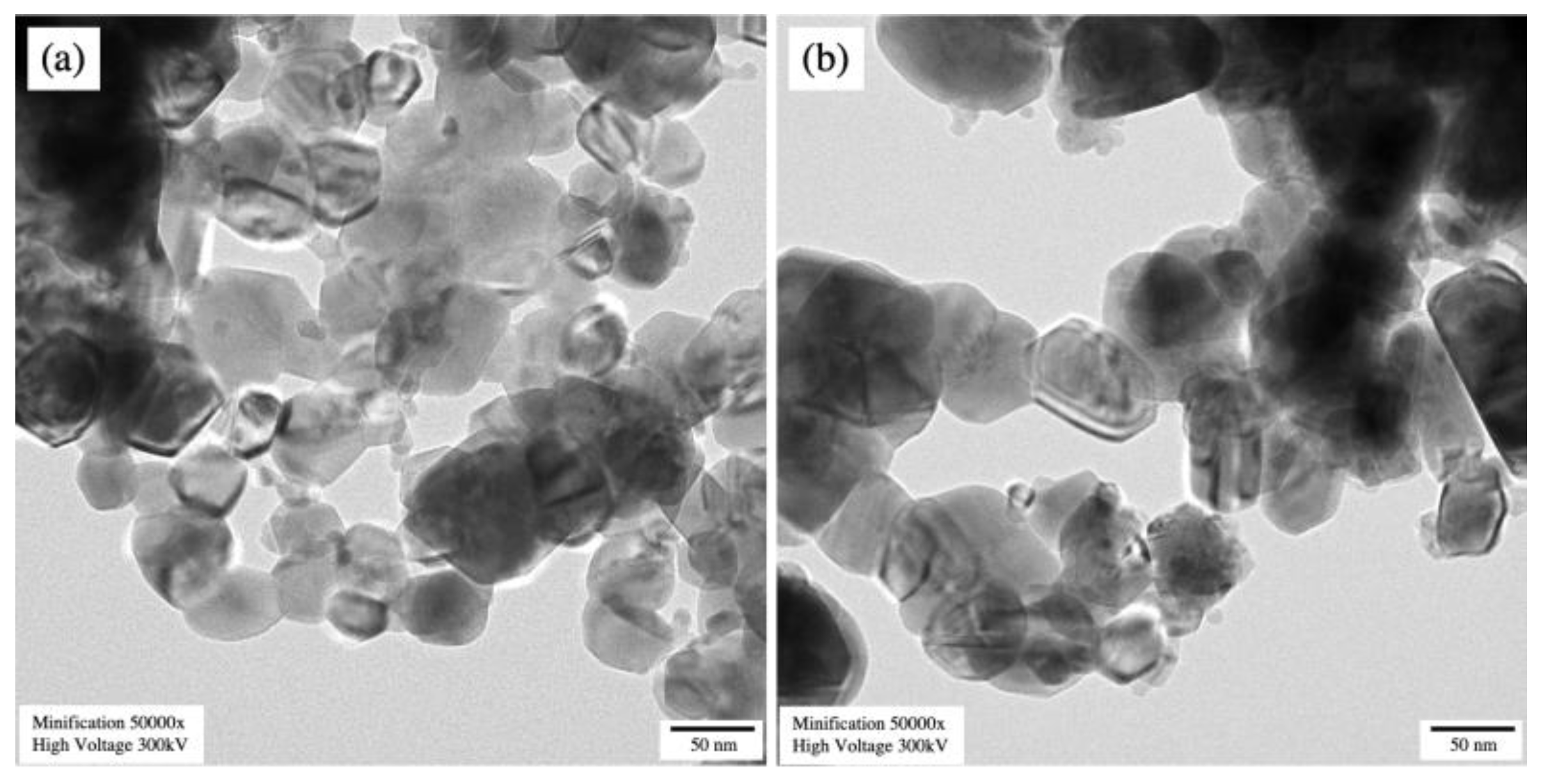

| Samples | Lattice Parameter (Å) | β (o) | Rwp | Rp | χ2 | ||
|---|---|---|---|---|---|---|---|
| a | b | c | |||||
| Undoped CuO | 4.6860 | 3.4251 | 5.1324 | 99.3957 | 7.4623 | 5.8034 | 1.1566 |
| 3 mol% Fe-doped CuO | 4.6866 | 3.4233 | 5.1321 | 99.3734 | 7.5311 | 5.9501 | 1.0150 |
| Sample | Average Crystallite Size (nm) | Particle Size (nm) | |
|---|---|---|---|
| SEM | TEM | ||
| Undoped CuO | 30.73 ± 2.83 | 112.61 ± 58.74 | 82.07 ± 30.94 |
| 3 mol% Fe-doped CuO | 24.57 ± 2.64 | 70.09 ± 29.95 | 52.58 ± 28.68 |
| Sample | Specific Surface Area (m2/g) |
|---|---|
| Undoped CuO | 2.46 |
| 3 mol% Fe-doped CuO | 4.67 |
| Undoped CuO/MWCNT composite | 129.25 |
| 3 mol% Fe-doped CuO/MWCNT composite | 141.25 |
| Sample | Peak Current (µA) |
|---|---|
| MWCNTs | – |
| Undoped CuO | 6.56 |
| 3 mol% Fe-doped CuO | 12.76 |
| Undoped CuO/MWCNT composite | 13.13 |
| 3 mol% Fe-doped CuO/MWCNT composite | 20.01 |
| pH | Peak Current (µA) |
|---|---|
| 4 | 16.9 |
| 5 | 17.1 |
| 6 | 17.1 |
| 7 | 18.0 |
| 8 | 19.5 |
| 9 | 16.5 |
| 10 | 13.2 |
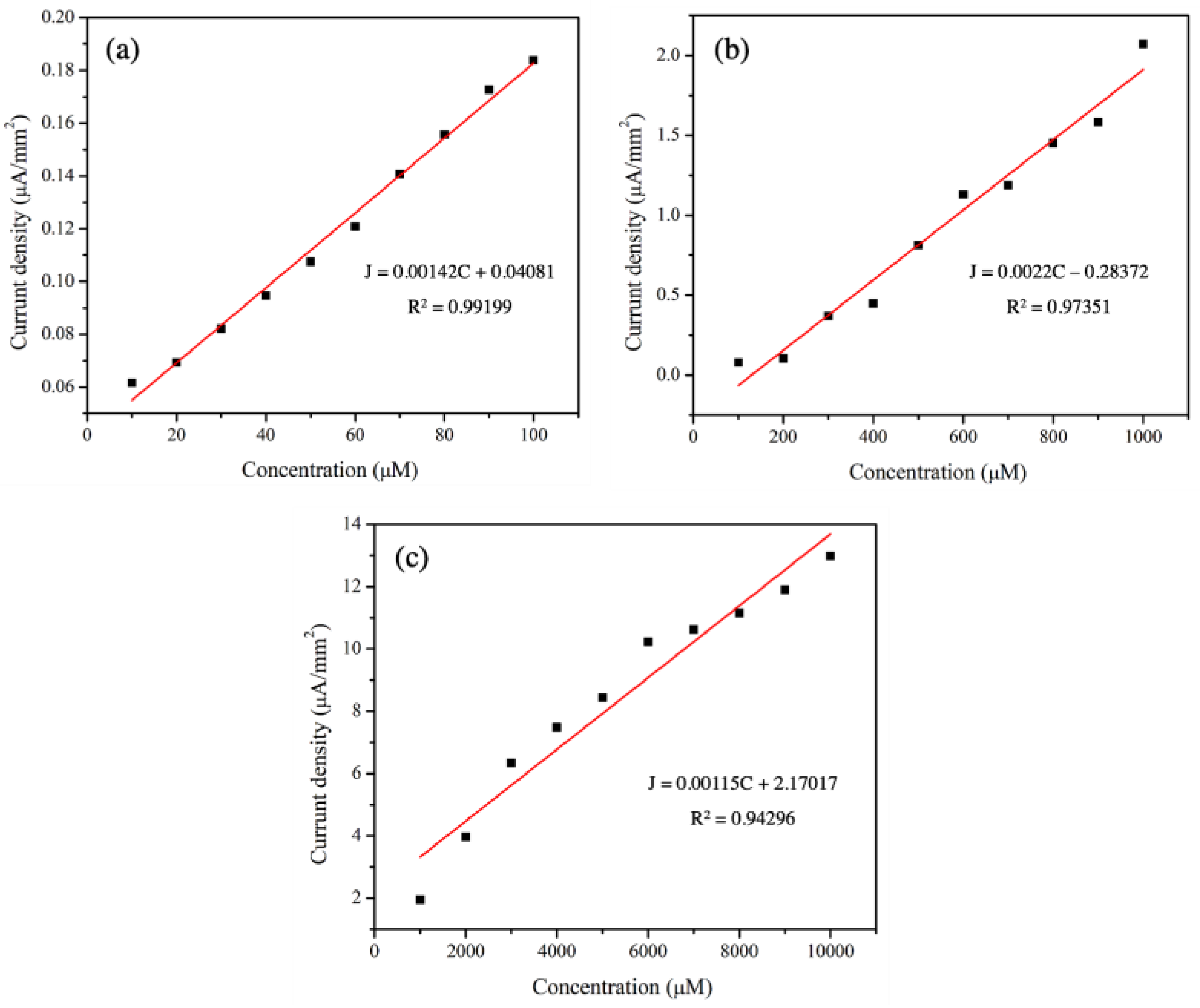
| Concentration | Sensitivity | R2 | ||
|---|---|---|---|---|
| µM | ppm | µA µM−1 mm−2 | µA ppm−1 mm−2 | |
| 10–100 | 0.46–4.6 | 1.42 × 10−3 | 3.09 × 10−2 | 0.992 |
| 100–1000 | 4.6–46 | 2.20 × 10−3 | 4.78 × 10−2 | 0.974 |
| 1000–10,000 | 46–460 | 1.15 × 10−3 | 2.50 × 10−2 | 0.943 |
| Electrodes | Linear Range (µM) | Sensitivity (µA µM−1 mm−2) | LOD (µM) | Ref. |
|---|---|---|---|---|
| Nf/Fe(bpy)32+/GCE a | 200–20,000 | 1.21 × 10−3 | 30 | Azada et al. [41] |
| Hb-CSNs/TiO2 Sol-Gel/GCE b | 200–6000 | 5.84 × 10−5 | 34 | Zhao et al. [42] |
| Carboxyl functionalised MWCNTs/Co-MOFs/Gr electrode c | 80–1160 | 1.00 × 10−4 | 18.8 | Salagare et al. [43] |
| 3 mol% Fe-doped CuO/MWCNT composite/GCE | 10–100 | 1.42 × 10−3 | 10.54 | This work |
| 100–1000 | 2.20 × 10−3 | |||
| 1000–10,000 | 1.15 × 10−3 |
| Samples | Added (µM) | Found (µM) | Recovery (%) |
|---|---|---|---|
| Drinking water | 1000 | 984.22 | 98.42% |
| 10 | 9.51 | 95.10% | |
| Tap water | 1000 | 1057.74 | 105.77% |
| 10 | 10.11 | 101.10% | |
| Rainwater | 1000 | 1064.17 | 106.42% |
| 10 | 9.21 | 92.10% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pitiphattharabun, S.; Auewattanapun, K.; Sato, N.; Janbooranapinij, K.; Techapiesancharoenkij, R.; Panomsuwan, G.; Ohta, J.; Jongprateep, O. Fe-Doped CuO/MWCNT as a Sensing Material for Electrochemical Detection of Nitrite. Crystals 2022, 12, 1536. https://doi.org/10.3390/cryst12111536
Pitiphattharabun S, Auewattanapun K, Sato N, Janbooranapinij K, Techapiesancharoenkij R, Panomsuwan G, Ohta J, Jongprateep O. Fe-Doped CuO/MWCNT as a Sensing Material for Electrochemical Detection of Nitrite. Crystals. 2022; 12(11):1536. https://doi.org/10.3390/cryst12111536
Chicago/Turabian StylePitiphattharabun, Siraprapa, Krittin Auewattanapun, Nicha Sato, Kasidit Janbooranapinij, Ratchatee Techapiesancharoenkij, Gasidit Panomsuwan, Jun Ohta, and Oratai Jongprateep. 2022. "Fe-Doped CuO/MWCNT as a Sensing Material for Electrochemical Detection of Nitrite" Crystals 12, no. 11: 1536. https://doi.org/10.3390/cryst12111536
APA StylePitiphattharabun, S., Auewattanapun, K., Sato, N., Janbooranapinij, K., Techapiesancharoenkij, R., Panomsuwan, G., Ohta, J., & Jongprateep, O. (2022). Fe-Doped CuO/MWCNT as a Sensing Material for Electrochemical Detection of Nitrite. Crystals, 12(11), 1536. https://doi.org/10.3390/cryst12111536







