Influence of the Arene/Perfluoroarene Ratio on the Structure and Non-Covalent Interactions in Crystals of Cd(II), Cd(II)-Tb(III) and Cu(II) Compounds
Abstract
:1. Introduction
2. Experimental
2.1. Materials and Methods
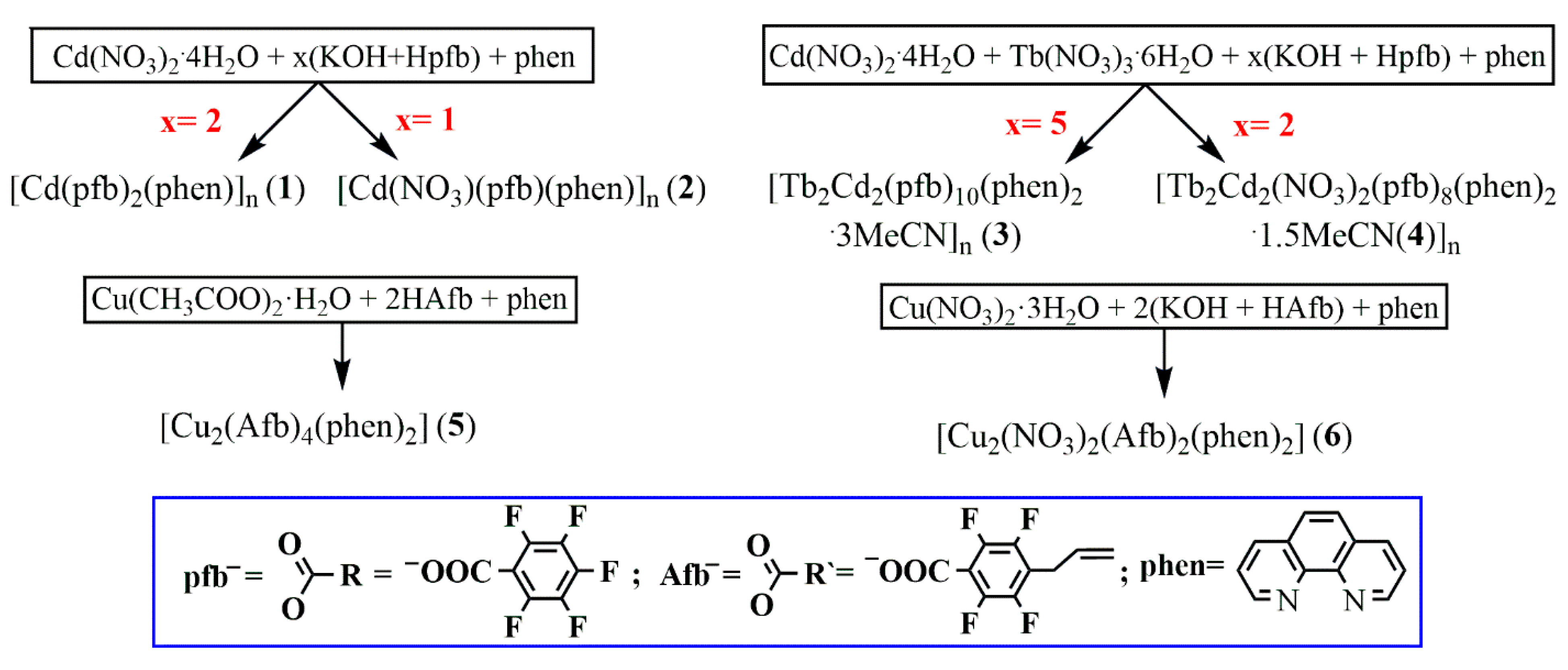
2.2. Synthesis of the Compounds
2.3. X-ray Diffraction Studies
3. Results
3.1. Synthesis of Complexes
3.2. The Structure of Complexes
3.2.1. The Structure of Complexes 1 and 2
3.2.2. The Structure of Complexes 3 and 4
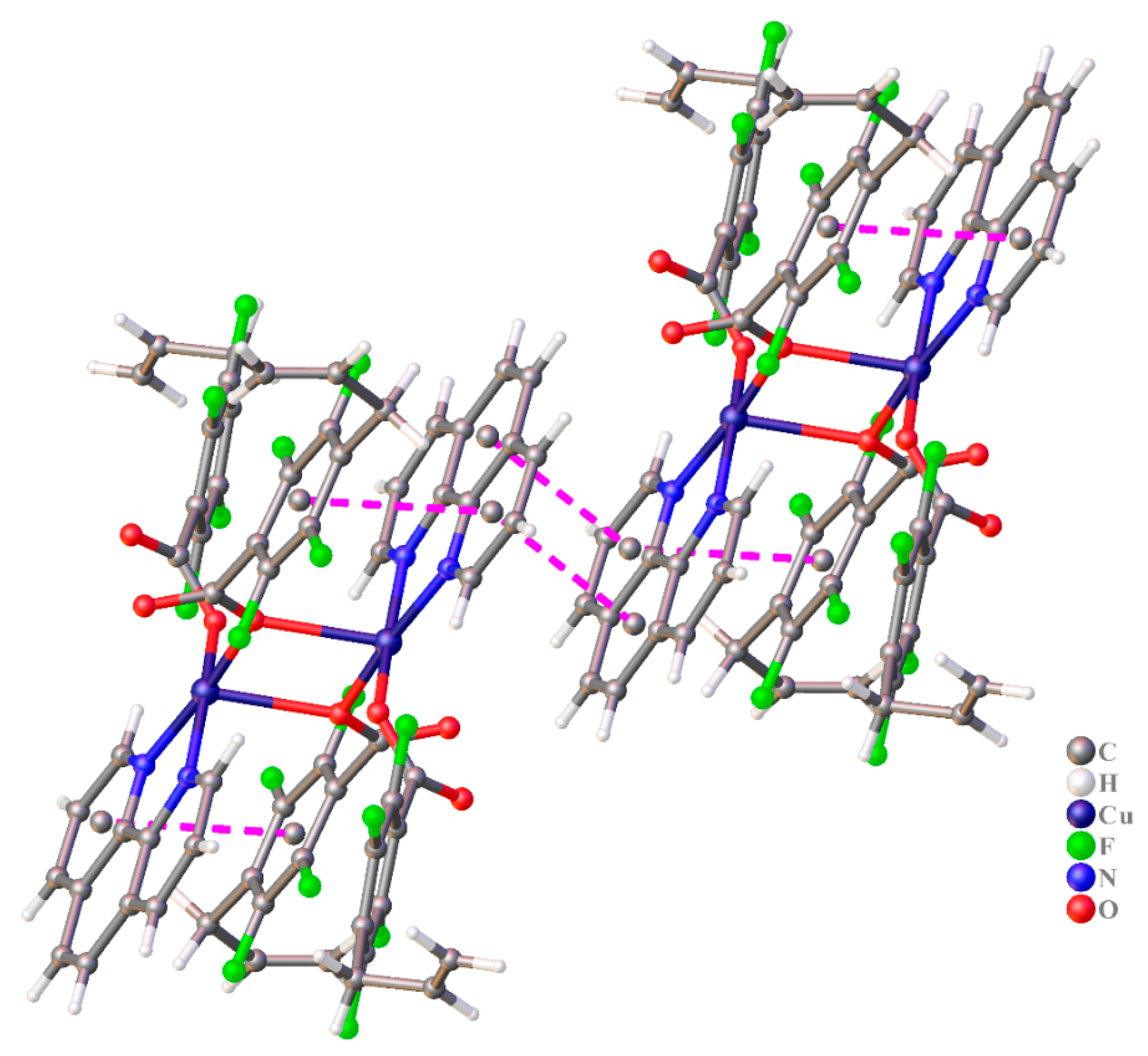
3.2.3. The Structure of Complexes 5 and 6
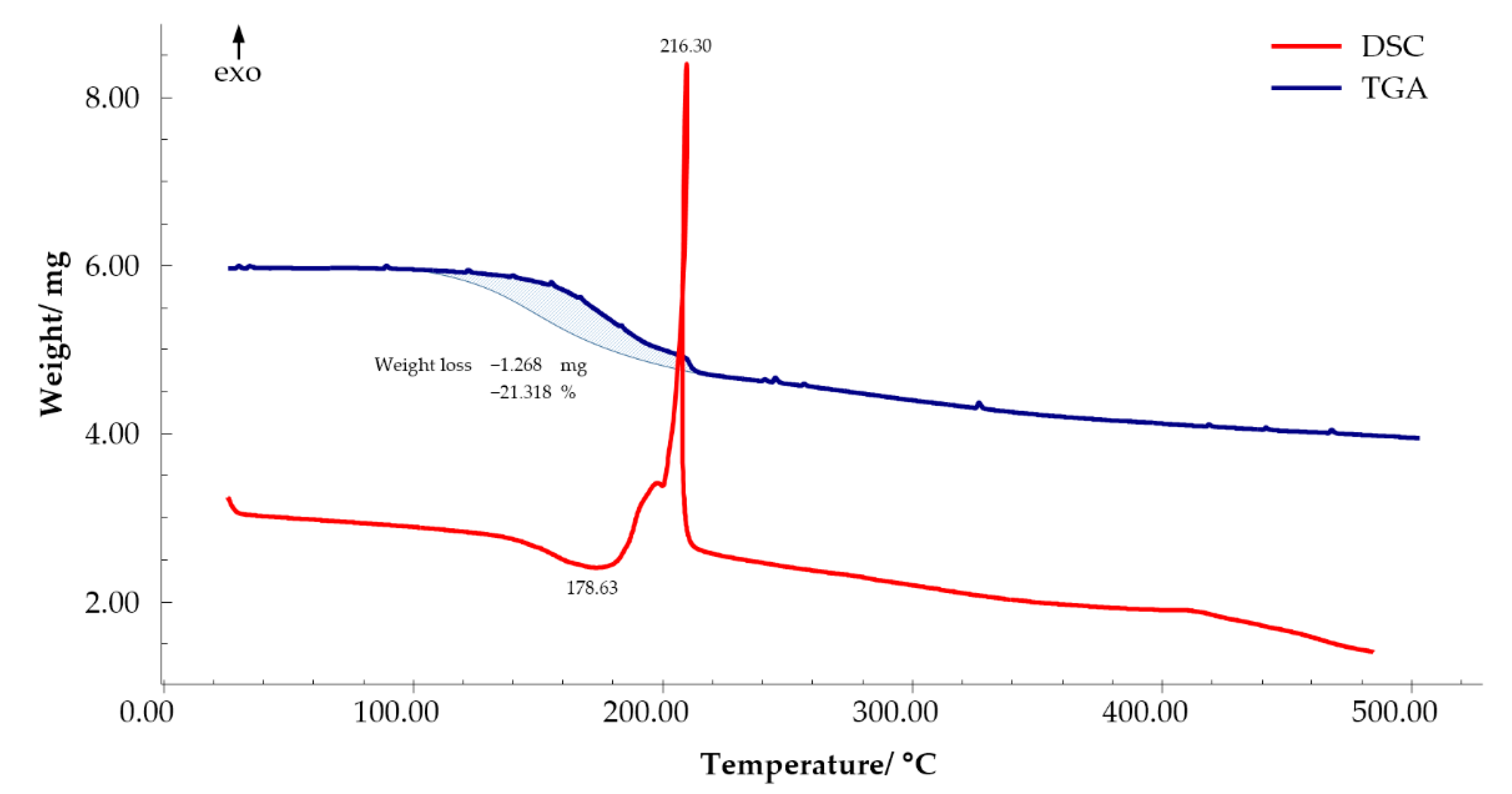
3.3. Thermal Decomposition
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Itoh, T.; Kondo, M.; Kanaike, M.; Masaoka, S. Arene–perfluoroarene interactions for crystal engineering of metal complexes: Controlled self-assembly of paddle-wheel dimers. CrystEngComm 2013, 15, 6122–6126. [Google Scholar] [CrossRef]
- Cockcroft, J.K.; Rosu-Finsen, A.; Fitch, A.N.; Williams, J.H. The temperature dependence of C–H⋯F–C interactions in benzene: hexafluorobenzene. CrystEngComm 2018, 20, 6677–6682. [Google Scholar] [CrossRef] [Green Version]
- Lee, G.Y.; Hu, E.; Rheingold, A.L.; Houk, K.N.; Sletten, E.M. Arene-perfluoroarene interactions in solution. J. Org. Chem. 2021, 86, 8425. [Google Scholar] [CrossRef]
- Hori, A.; Shinohe, A.; Yamasaki, M.; Nishibori, E.; Aoyagi, S.; Sakata, M. 1:1 Cross-assembly of two β-diketonate complexes through arene–perfluoroarene interactions. Angew. Chem. Int. Ed. 2007, 46, 7617–7620. [Google Scholar] [CrossRef] [PubMed]
- Götz, R.J.; Robertazzi, A.; Mutikainen, I.; Turpeinen, U.; Gamez, P.; Reedijk, J. Concurrent anion⋯π interactions between a perchlorate ion and two π-acidic aromatic rings, namely pentafluorophenol and 1,3,5-triazine. Chem. Commun. 2008, 29, 3384–3386. [Google Scholar] [CrossRef]
- Itoh, T.; Kondo, M.; Sakamoto, H.; Wakabayashi, K.; Kanaike, M.; Itami, K.; Masaoka, S. Porous frameworks constructed by non-covalent linking of substitution-inert metal complexes. J. Am. Chem. Soc. 2020, 142, 1731–1734. [Google Scholar] [CrossRef] [Green Version]
- Shmelev, M.A.; Gogoleva, N.V.; Ivanov, V.K.; Kovalev, V.V.; Razgonyaeva, G.A.; Kiskin, M.A.; Sidorov, A.A.; Eremenko, I.L. Heterometallic Ln(III)–Cd(II) complexes with anions of monocarboxylic acids: Synthetic approaches and analysis of structures and photoluminescence properties. Russ. J. Coord. Chem. 2022, 47, 539–556. [Google Scholar] [CrossRef]
- Bear, J.C.; Cockcroft, J.K.; Williams, J.H. Influence of Solvent in Crystal Engineering: A Significant change to the order–disorder transition in ferrocene. J. Am. Chem. Soc. 2020, 142, 1731–1734. [Google Scholar] [CrossRef]
- Sinnwell, M.A.; Baltrusaitis, J.; MacGillivray, L.R. Combination of argentophilic and perfluorophenyl-perfluorophenyl interactions supports a head-to-head [2 + 2] photodimerization in the solid state. Cryst. Growth Des. 2015, 15, 538–541. [Google Scholar] [CrossRef]
- Kong, Y.-J.; Li, P.; Han, L.-J.; Fan, L.-T.; Li, P.-P.; Yin, S. Two cadmium(II) fluorous coordination compounds tuned by different bi pyridines. Acta Cryst. 2017, C73, 424–429. [Google Scholar]
- Shmelev, M.A.; Gogoleva, N.V.; Kuznetsova, G.N.; Kiskin, M.A.; Voronina, Y.K.; Yakushev, I.A.; Ivanova, T.M.; Nelyubina, Y.V.; Sidorov, A.A.; Eremenko, I.L. Cd(II) and Cd(II)–Eu(III) complexes with pentafluorobenzoic acid anions and n-donor ligands: Synthesis and structures. Russ. J. Coord. Chem. 2020, 46, 557–572. [Google Scholar] [CrossRef]
- Shmelev, M.A.; Kuznetsova, G.N.; Dolgushin, F.M.; Voronina, Y.K.; Gogoleva, N.V.; Kiskin, M.A.; Ivanov, V.K.; Sidorov, A.A.; Eremenko, I.L. Influence of the fluorinated aromatic fragments on the structures of the cadmium and zinc carboxylate complexes using pentafluorobenzoates and 2,3,4,5-tetrafluorobenzoates as examples. Russ. J. Coord. Chem. 2021, 47, 127–143. [Google Scholar] [CrossRef]
- Shmelev, M.A.; Kiskin, M.A.; Voronina, J.K.; Babeshkin, K.A.; Efimov, N.N.; Varaksina, E.A.; Korshunov, V.M.; Taydakov, I.V.; Gogoleva, N.V.; Sidorov, A.A.; et al. Molecular and polymer Ln2M2 (Ln = Eu, Gd, Tb, Dy; M = Zn, Cd) complexes with pentafluorobenzoate anions: The role of temperature and stacking effects in the structure; magnetic and luminescent properties. Materials 2020, 13, 5689. [Google Scholar] [CrossRef]
- Shmelev, M.A.; Kuznetsova, G.N.; Gogoleva, N.V.; Dolgushin, F.M.; Nelyubina, Y.V.; Kiskin, M.A.; Sidorov, A.A.; Eremenko, I.L. Heteroleptic cadmium(II) and terbium(III) pentafluorobenzoate-benzoate and pentafluorobenzoate-2-furancarboxylate compounds. Russ. Chem. Bull. 2021, 70, 830–838. [Google Scholar] [CrossRef]
- Zorina-Tikhonova, E.N.; Chistyakov, A.S.; Kiskin, M.A.; Sidorov, A.A.; Dorovatovskii, P.V.; Zubavichus, Y.V.; Voronova, E.D.; Godovikov, I.A.; Korlyukov, A.A.; Eremenko, I.L.; et al. Exploitation of knowledge databases in the synthesis of zinc(II) malonates with photo-sensitive and photo-insensitive N, N’-containing linkers. IUCrJ 2018, 5, 293. [Google Scholar] [CrossRef] [Green Version]
- Gromov, S.P.; Vedernikov, A.I.; Lobova, N.A.; Kuz’mina, L.G.; Basok, S.S.; Strelenko, Y.A.; Alfimova, M.V.; Howard, J.A.K. Controlled self-assembly of bis(crown)stilbenes into unusual bis-sandwich complexes: Structure and stereoselective [2+2] photocycloaddition. New J. Chem. 2011, 35, 724. [Google Scholar] [CrossRef]
- Durand, P.-L.; Brège, A.; Chollet, G.; Grau, E.; Cramail, H. Simple and efficient approach toward photosensitive biobased aliphatic polycarbonate materials. ACS Macro Lett. 2018, 7, 250. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gong, L.L.; Feng, X.F.; Luo, F.; Yi, X.F.; Zheng, A.M. Removal and safe reuse of highly toxic allyl alcohol using a highly selective photo-sensitive metal–organic framework. Green Chem. 2016, 18, 2047. [Google Scholar] [CrossRef]
- Oguadinma, P.O.; Rodrigue-Witchel, A.; Rebera, C.; Schaper, F. Intramolecular π-stacking in copper(I) diketiminate phenanthroline complexes. Dalton Trans. 2010, 39, 8759. [Google Scholar] [CrossRef]
- Goldberg, A.; Kiskin, M.; Shalygina, O.; Kozyukhin, S.; Dobrokhotova, Z.; Nikolaevskii, S.; Sidorov, A.; Sokolov, S.; Timoshenko, V.; Goloveshkin, A.; et al. Tetranuclear heterometallic {Zn2Eu2} complexes with 1-Naphthoate anions: Synthesis, structure and photoluminescence properties. Chem. Asian J. 2016, 11, 604. [Google Scholar] [CrossRef]
- Koshevoy, I.O.; Krause, M.; Klein, A. Non-covalent intramolecular interactions through ligand-design promoting efficient photoluminescence from transition metal complexes. Coord. Chem. Rev. 2020, 405, 213094. [Google Scholar] [CrossRef]
- Shmelev, M.A.; Voronina, J.K.; Evtyukhin, M.A.; Dolgushin, F.M.; Varaksina, E.A.; Taydakov, I.V.; Sidorov, A.A.; Eremenko, I.L.; Kiskin, M.A. Synthesis, structure and photoluminescence properties of Cd and Cd-Ln pentafluorobenzoates with 2,2′:6′,2′-terpyridine derivatives. Inorganics 2022, 10, 194. [Google Scholar] [CrossRef]
- Krause, L.; Herbst-Irmer, R.; Sheldrick, G.M.; Stalke, D. Comparison of silver and molybdenum microfocus X-ray sources for single-crystal structure determination. J. Appl. Cryst. 2015, 48, 3–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sheldrick, G.M. SHELXT—Integrated space-group and crystal-structure determination. Acta Cryst. A 2015, 71, 3. [Google Scholar] [CrossRef] [Green Version]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Cryst. C 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Alvarez, S.; Avnir, D.; Llunell, M.; Pinsky, M. Continuous symmetry maps and shape classification. The case of six-coordinated metal compounds. New J. Chem. 2002, 26, 996–1009. [Google Scholar] [CrossRef]
- Spek, A.L. PLATON SQUEEZE: A tool for the calculation of the disordered solvent contribution to the calculated structure factors. Acta Cryst. C 2015, 71, 9–18. [Google Scholar] [CrossRef] [Green Version]
- Spackman, P.R.; Turner, M.J.; McKinnon, J.J.; Wolff, S.K.; Grimwood, D.J.; Jayatilaka, D.; Spackman, M.A. CrystalExplorer: A program for Hirshfeld surface analysis, visualization and quantitative analysis of molecular crystals. J. Appl. Cryst. 2021, 54, 1006–1011. [Google Scholar] [CrossRef]
- Edwards, A.J.; Mackenzie, C.F.; Spackman, P.R.; Jayatilaka, D.; Spackman, M.A. Intermolecular interactions in molecular crystals: What’s in a name? Faraday Discuss. 2017, 203, 93–112. [Google Scholar] [CrossRef] [Green Version]
- Ge, C.; Zhang, X.; Yin, J.; Zhang, R. Weak interactions involving fluorine in suppramolecular assemblies of Cu(II) complexes. Chin. J. Chem. 2010, 28, 2083–2088. [Google Scholar] [CrossRef]
- Bai, H.; Gao, H.; Hu, M. A Zigzag chain Cd (II) coordination polymer based on 2,4-Dinitro-benzoic acid ligand: Syntheses, structure and photoluminescence. Adv. Mater. Res. 2014, 997, 140–145. [Google Scholar] [CrossRef]
- Jassal, A.K.; Sran, B.S.; Suffren, Y.; Bernot, K.; Pointillart, F.; Cador, O.; Hundal, G. Structural diversity and photo-physical and magnetic properties of dimeric to 1D polymeric coordination polymers of lighter lanthanide(III) dinitrobenzoates. Dalton Trans. 2018, 47, 4722–4732. [Google Scholar] [CrossRef] [PubMed]





| Bond | d/Å | |||||
|---|---|---|---|---|---|---|
| 1 M = Cd | 2 M = Cd | 3 M = Cd | 4 M = Cd | 5 M = Cu | 6 M = Cu | |
| M-O (RCOO) | 2.425(1)–2.472(1) | 2.443(6)–2.510(6) | 2.215(4)–2.469(5) | 2.279(3)–2.535(4) | 1.930(4)–2.380(3) | 1.966(2), 2.366(2) |
| Tb-O (RCOO) | - | - | 2.350(3)–2.729(4) | 2.335(3)–2.576(3) | - | - |
| M-N (phen) | 2.366(1) | 2.313(7), 2.338(7) | 2.319(5), 2.324(6) | 2.274(4), 2.313(4) | 2.024(4), 2.032(4) | 1.997(3), 2.003 |
| M-O (NO3) | - | 2.324(7), 2.563(6) | - | - | - | 1.970(2) |
| Tb-O (NO3) | - | - | - | 2.418(3), 2.445(3) | - | - |
| Tb···Tb | - | - | 4.160(2) | 4.016(1) | - | - |
| M···M | 4.001(1) | 4.007(1), 4.099(1) | 3.926(1) | 3.730(1) | 3.435(1) | 3.364(1) |
| Interaction | Compound | |||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | |
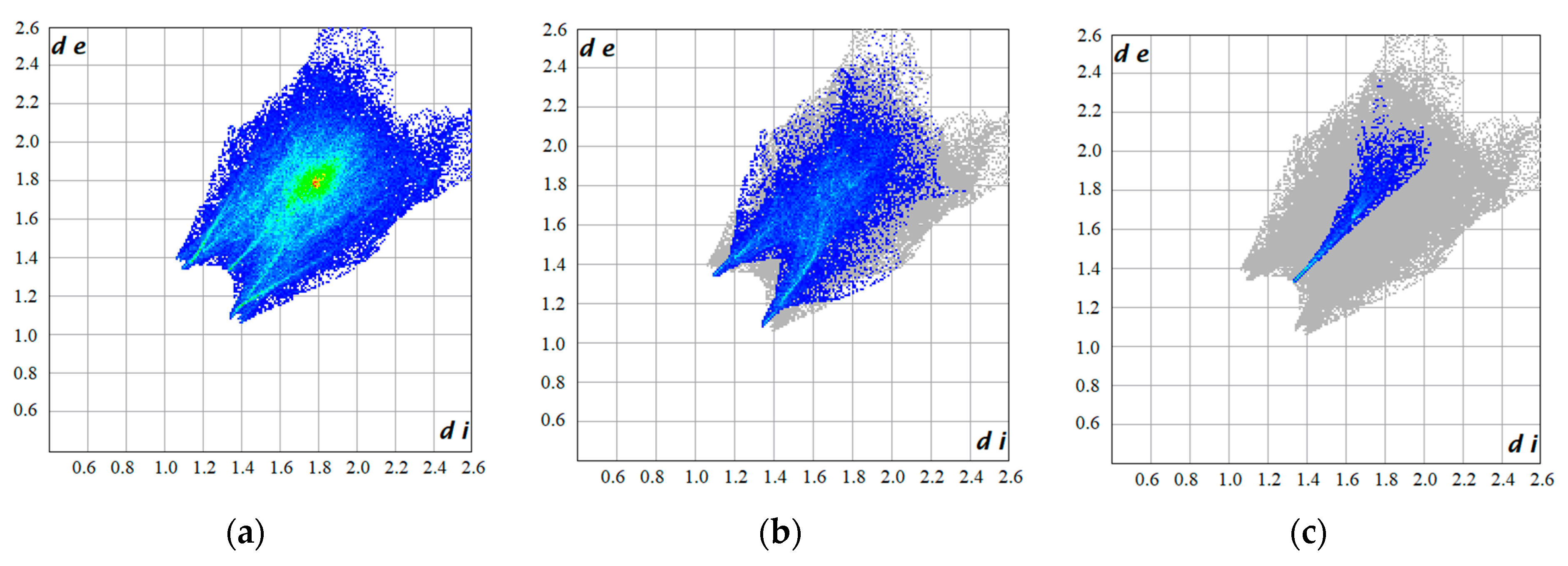
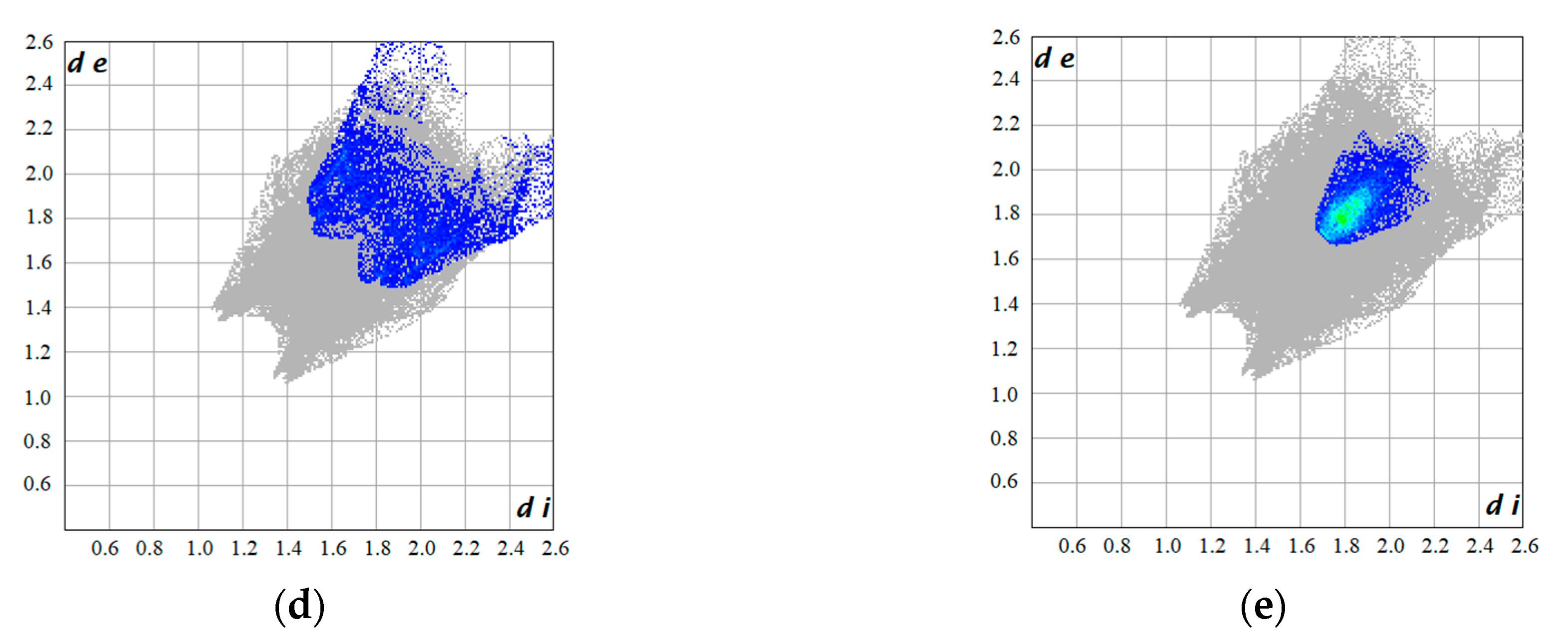
| C···C | 5.6% | 11.2% | 3.8% | 1.8% | 4.1% | 5.6% |
| C···F | 15.8% | 13.8% | 13.8% | 14.9% | 3.2% | 4.6% |
| H···F | 29.0% | 25.7% | 23.3% | 17.4% | 36.4% | 23.4% |
| F···F | 17.0% | 28.8% | 28.8% | 27.6% | 3.3% | 0.9% |
| C···H | 11.2% | 3.8% | 4.7% | 9.5% | 13.2% | 11.2% |
| O···H | 4.0% | 14.3% | 3.9% | 8.4% | 12.3% | 23.6% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Voronina, J.K.; Yambulatov, D.S.; Chistyakov, A.S.; Bolot’ko, A.E.; Efromeev, L.M.; Shmelev, M.A.; Sidorov, A.A.; Eremenko, I.L. Influence of the Arene/Perfluoroarene Ratio on the Structure and Non-Covalent Interactions in Crystals of Cd(II), Cd(II)-Tb(III) and Cu(II) Compounds. Crystals 2023, 13, 678. https://doi.org/10.3390/cryst13040678
Voronina JK, Yambulatov DS, Chistyakov AS, Bolot’ko AE, Efromeev LM, Shmelev MA, Sidorov AA, Eremenko IL. Influence of the Arene/Perfluoroarene Ratio on the Structure and Non-Covalent Interactions in Crystals of Cd(II), Cd(II)-Tb(III) and Cu(II) Compounds. Crystals. 2023; 13(4):678. https://doi.org/10.3390/cryst13040678
Chicago/Turabian StyleVoronina, Julia K., Dmitriy S. Yambulatov, Aleksander S. Chistyakov, Alena E. Bolot’ko, Leonid M. Efromeev, Maxim A. Shmelev, Alexey A. Sidorov, and Igor L. Eremenko. 2023. "Influence of the Arene/Perfluoroarene Ratio on the Structure and Non-Covalent Interactions in Crystals of Cd(II), Cd(II)-Tb(III) and Cu(II) Compounds" Crystals 13, no. 4: 678. https://doi.org/10.3390/cryst13040678





