Optimizing Struvite Crystallization at High Stirring Rates
Abstract
1. Introduction
2. Materials and Methods
3. Results
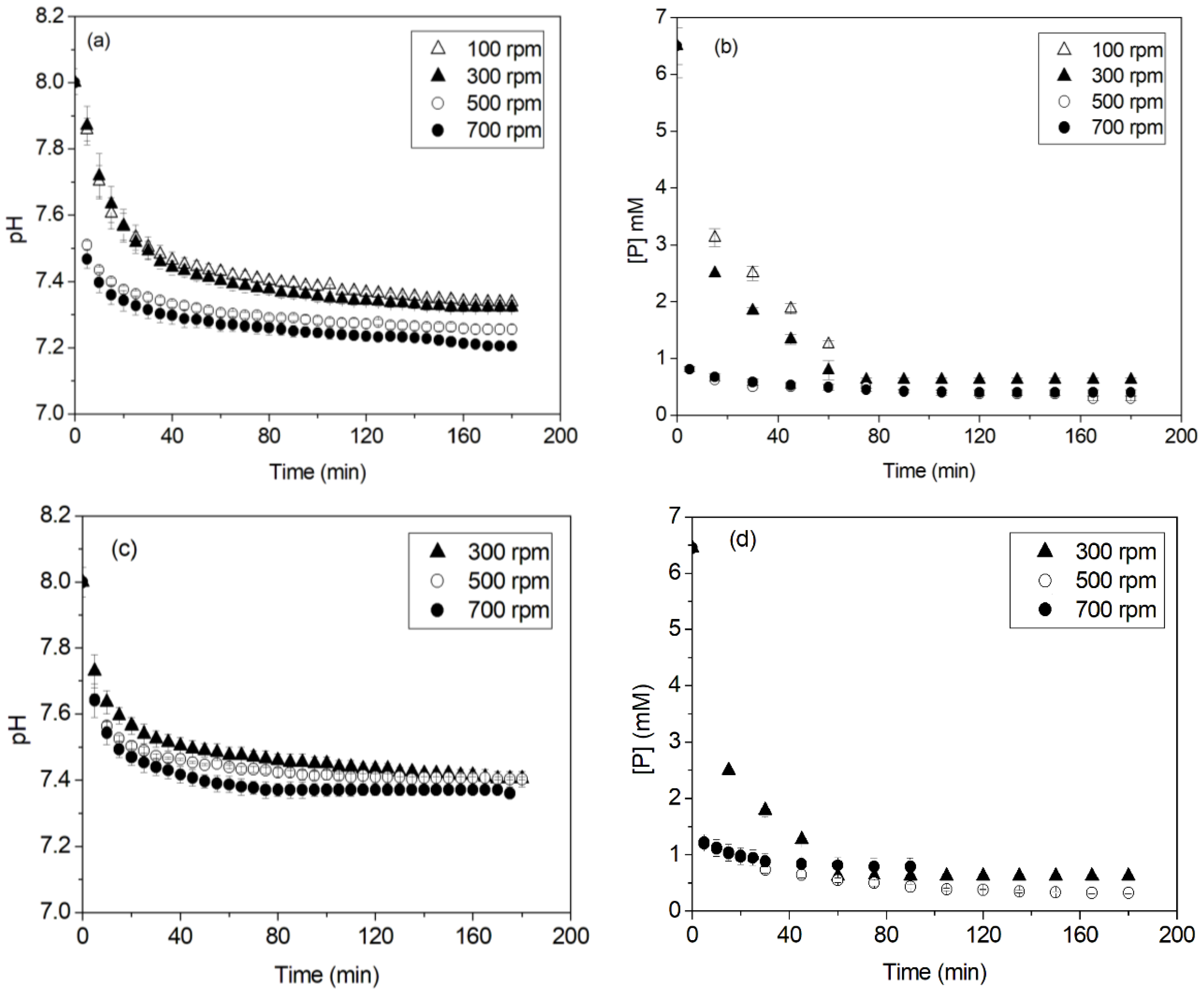
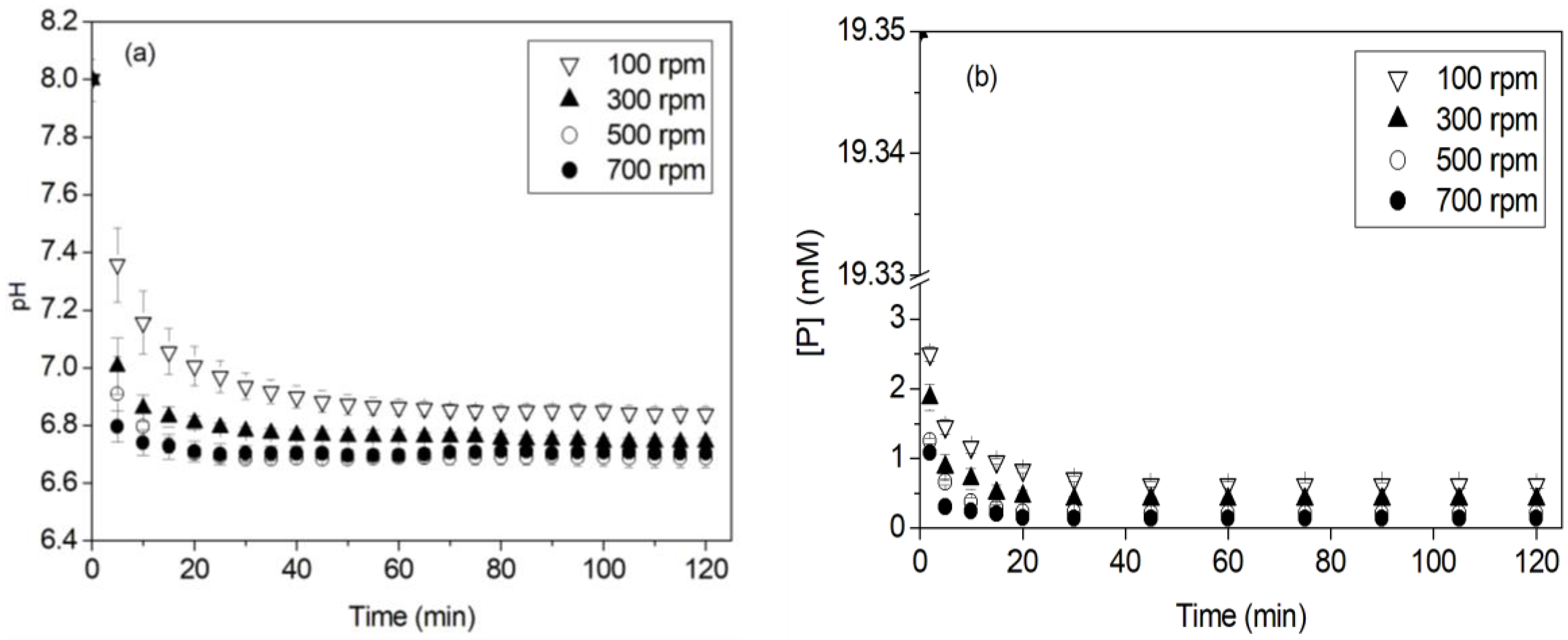
3.1. Effect of Stirring
3.2. Effect of Phosphorus Concentration
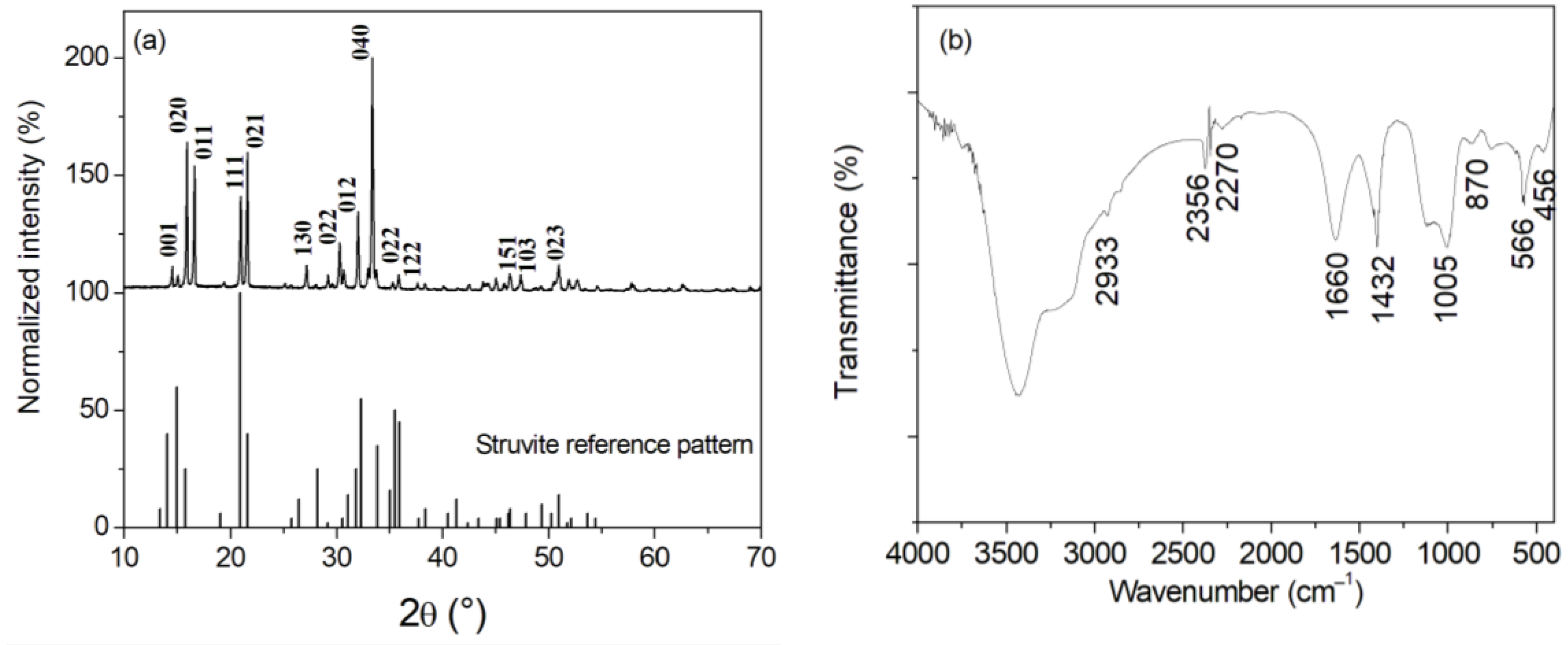
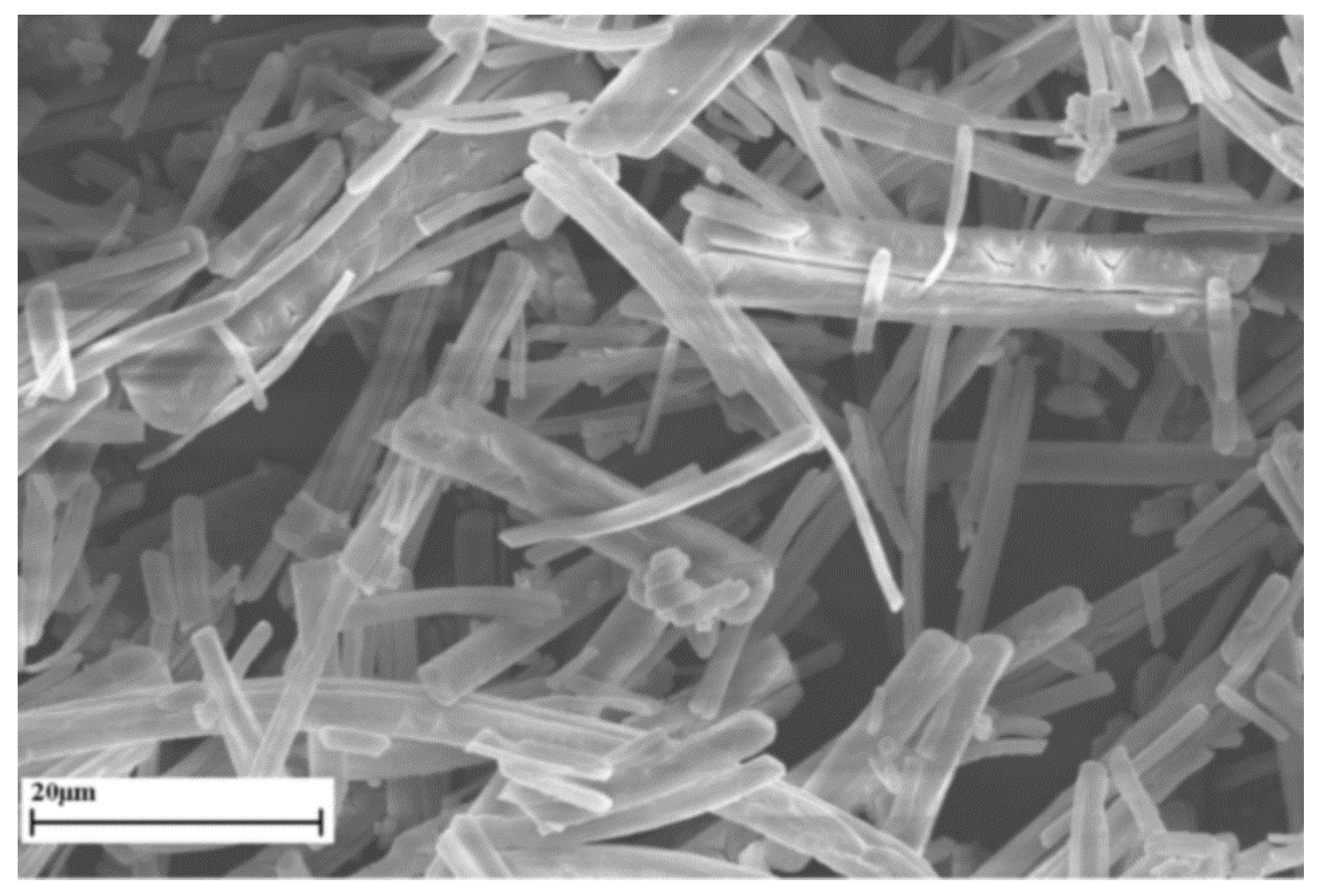
3.3. Particle Size
4. Conclusions
- For an initial phosphorus concentration of 200 mg·L−1, as the stirring rate increased from 100 to 500 rpm, the initial precipitation rate increased, the precipitation end time decreased, the phosphorus removal efficiency increased from 90% to 95%, respectively, and struvite particle size increased.
- A decrease in the removal efficiency and the struvite particle size was found at a higher stirring rate of 700 rpm. This was attributed to the solution turbulence, which caused the struvite precipitation reaction to be limited.
- At a fixed stirring rate and initial phosphorus concentration, the increase in the solution volume decreased the initial precipitation rate, increased the precipitation end time, and decreased the phosphorus removal efficiency.
- An increase in the phosphorus concentration overcame the limiting effect of turbulence. Indeed, it accelerated the precipitation of struvite and increased the phosphorus recovery (~99%) through large struvite crystals (~400 µm in size).
- Large struvite crystals formed in the solution facilitate the separation of struvite from the liquid phase and accelerate the release of ammonium and phosphate ions in soils when struvite is used as a fertilizer.
- Real wastewater contains ions of calcium, sulfate, and heavy metals, among others. The presence of these foreign ions influences the nature, shape, and size of the precipitated phase. Therefore, further study on the recycling of phosphorus and nitrogen through struvite crystallization from real wastewater is needed since it will allow us to identify these phases and to determine, in the case of insertion or adsorption, the rate of removal of these ions by the crystallization of struvite.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- US Bureau of Reclamation. Water Facts—Worldwide Water Supply. 2022. Available online: https://www.usbr.gov/mp/arwec/water-facts-ww-water-sup.html (accessed on 25 March 2023).
- EU. Drinking Water Directive; Council Directive 76/464/EEC of 4 May 1976 on Pollution Caused by Certain Dangerous Substances Discharged into the Aquatic Environment of the Community. 1976. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:31976L0464&from=EN (accessed on 25 March 2023).
- Wang, L.; Ye, C.; Gao, B.; Wang, X.; Li, Y.; Ding, K.; Li, H.; Ren, K.; Chen, S.; Wang, W.; et al. Applying struvite as a N-fertilizer to mitigate N2O emissions in agriculture: Feasibility and mechanism. J. Environ. Manag. 2023, 330, 117143. [Google Scholar] [CrossRef] [PubMed]
- Liang, Z.; Liu, J.; Wan, Y.; Feng, Z.; Zhang, P.; Wang, M.; Zhang, H. Preparation and fire extinguishing mechanism of novel fire extinguishing powder based on recyclable struvite. Mater. Today Comm. 2023, 34, 105410. [Google Scholar] [CrossRef]
- Giulio, G.; Ferretti, G.; Rosinger, C.; Huber, S.; Medoro, V.; Mentler, A.; Díaz-Pinés, E.; Gorfer, M.; Faccini, B.; Keiblinger, K.M. Recycling nitrogen from liquid digestate via novel reactive struvite and zeolite minerals to mitigate agricultural pollution. Chemosphere 2023, 317, 137881. [Google Scholar] [CrossRef]
- Ha, T.-H.; Mahasti, N.N.; Lu, M.C.; Huang, Y.H. Ammonium-nitrogen recovery as struvite from swine wastewater using various magnesium sources. Sep. Purif. Technol. 2023, 308, 122870. [Google Scholar] [CrossRef]
- Natsi, P.D.; Goudas, K.-A.; Koutsoukos, P.G. Phosphorus recovery from municipal wastewater: Brucite from MgO hydrothermal treatment as magnesium source. Crystals 2023, 13, 208. [Google Scholar] [CrossRef]
- Sultana, R.; Kékedy-Nagy, L.; Daneshpour, R.; Greenlee, L. Electrochemical recovery of phosphate from synthetic wastewater with enhanced salinity. Electrochim. Acta 2022, 426, 140848. [Google Scholar] [CrossRef]
- Song, L.; Li, Z.; Wang, G.; Tian, Y.; Yang, C. Supersaturation control of struvite growth by operating pH. J. Mol. Liq. 2021, 336, 116293. [Google Scholar] [CrossRef]
- Abbona, F.; Madsen, H.L.; Boistelle, R. Crystallization of two magnesium phosphates, struvite and newberyite: Effect of pH and concentration. J. Cryst. Growth 1982, 57, 6–14. [Google Scholar] [CrossRef]
- Aage, H.; Andersen, B.; Blom, A.; Jensen, I. The solubility of struvite. J. Radioanal. Nucl. Chem. 1997, 223, 213–215. [Google Scholar] [CrossRef]
- Li, M.; Zhang, H.; Sun, H.; Mohammed, A.; Liu, Y.; Lu, Q. Effect of phosphate and ammonium concentrations, total suspended solids and alkalinity on lignin-induced struvite precipitation. Sci. Rep. 2022, 12, 2901. [Google Scholar] [CrossRef]
- Korchef, A.; Saidou, H.; Ben Amor, M. Phosphate recovery through struvite precipitation by CO2 removal: Effect of magnesium, phosphate and ammonium concentrations. J. Hazard. Mater. 2011, 186, 602–613. [Google Scholar] [CrossRef]
- Jaffer, Y.; Clark, T.A.; Pearce, P.; Parsons, S.A. Potential phosphorus recovery by struvite formation. Water Res. 2002, 36, 1834–1842. [Google Scholar] [CrossRef]
- Kruk, D.J.; Elektorowicz, M.; Oleszkiewicz, J.A. Struvite precipitation and phosphorus removal using magnesium sacrificial anode. Chemosphere 2014, 101, 28–33. [Google Scholar] [CrossRef]
- Wang, Y.; Da, J.; Deng, Y.; Wang, R.; Liu, X.; Chang, J. Competitive adsorption of heavy metals between Ca-P and Mg-P products from wastewater during struvite crystallization. J. Environ. Manag. 2023, 335, 117552. [Google Scholar] [CrossRef]
- Wang, Y.; Deng, Y.; Liu, X.; Chang, J. Adsorption behaviors and reduction strategies of heavy metals in struvite recovered from swine wastewater. J. Chem. Eng. 2022, 437, 135288. [Google Scholar] [CrossRef]
- Hutnik, N.; Stanclik, A.; Piotrowski, K.; Matynia, A. Kinetic conditions of struvite continuous reaction crystallisation from wastewater in presence of aluminium(III) and iron(III) ions. Int. J. Environ. Pollut. 2019, 64, 358–374. [Google Scholar] [CrossRef]
- Saidou, H.; Korchef, A.; Moussa, S.B.; Amor, M.B. Study of Cd2+, Al3+, and SO42− ions influence on struvite precipitation from synthetic water by dissolved CO2 degasification technique. Open J. Inorg. Chem. 2015, 5, 41. [Google Scholar] [CrossRef]
- Lu, X.; Xu, W.; Zeng, Q.; Liu, W.; Wang, F. Quantitative, morphological, and structural analysis of Ni incorporated with struvite during precipitation. Sci. Total Environ. 2022, 817, 152976. [Google Scholar] [CrossRef]
- Rabinovich, A.; Rouff, A.A. Effect of phenolic organics on the precipitation of struvite from simulated dairy wastewater. ACS EST Water 2021, 1, 910–918. [Google Scholar] [CrossRef]
- Capdevielle, A.; Sýkorová, E.; Biscans, B.; Béline, F.; Daumer, M.L. Optimization of struvite precipitation in synthetic biologically treated swine wastewater-Determination of the optimal process parameters. J. Hazard. Mater. 2013, 244, 357–369. [Google Scholar] [CrossRef]
- Bouropoulos, N.C.; Koutsoukos, P.G. Spontaneous precipitation of struvite from aqueous solutions. J. Cryst. Growth 2000, 213, 381–388. [Google Scholar] [CrossRef]
- Fattah, K.P.; Zhang, Y.; Mavinic, D.S.; Koch, F.A. Application of carbon dioxide stripping for struvite crystallization-I: Development of a carbon dioxide stripper model to predict CO2 removal and pH changes. J. Environ. Eng. Sci. 2008, 7, 345–356. [Google Scholar] [CrossRef]
- Saidou, H.; Korchef, A.; Moussa, S.B.; Amor, M.B. Struvite precipitation by the dissolved CO2 degasification technique: Impact of the airflow rate and pH. Chemosphere 2009, 74, 338–343. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Gu, K.; Zhang, Y.; Sun, J.; Gu, Z.; Zhao, B.; Hu, C. Enhanced struvite generation and separation by magnesium anode electrolysis coupled with cathode electrodeposition. Sci. Total Environ. 2022, 804, 150101. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Shashvatt, U.; Amurrio, F.; Stewart, K.; Blaney, L. Sustainable nutrient recovery from synthetic urine by Donnan dialysis with tubular ion-exchange membranes. J. Chem. Eng. 2023, 460, 141625. [Google Scholar] [CrossRef]
- Perwitasari, D.S.; Santi, S.S.; Yahya, A. The effect of stirrer rotation on crystallization of struvite that can be used as fertilizer. Int. J. Mech. Eng. 2022, 7, 2969–2972. [Google Scholar]
- Perera, P.W.A.; Wu, W.-X.; Chen, Y.-X.; Han, Z.-Y. Struvite Recovery from Swine Waste Biogas Digester Effluent through a Stainless Steel Device under Constant pH Conditions. Biomed. Environ. Sci. 2009, 22, 201–209. [Google Scholar] [CrossRef]
- Xu, K.; Wang, C.; Wang, X.; Qian, Y. Laboratory experiments on simultaneous removal of K and P from synthetic and real urine for nutrient recycle by crystallization of magnesium–potassium–phosphate–hexahydrate in a draft tube and baffle reactor. Chemosphere 2012, 88, 219–223. [Google Scholar] [CrossRef]
- Zhang, D.; Chen, Y.; Jilani, G.; Wu, W.; Liu, W.; Han, Z. Optimization of struvite crystallization protocol for pretreating the swine wastewater and its impact on subsequent anaerobic biodegradation of pollutants. Bioresour. Technol. 2012, 116, 386–395. [Google Scholar] [CrossRef]
- International Centre for Diffraction Data, Ammonium Magnesium Phosphate Hydrate (Standard #15-0762), A Computer Database. 1996. Available online: https://www.icdd.com (accessed on 25 March 2023).
- Zhang, T.; Ding, L.; Ren, H. Pretreatment of ammonium removal from landfill leachate by chemical precipitation. J. Hazard. Mater. 2009, 166, 911–915. [Google Scholar] [CrossRef]
- Korchef, A.; Naffouti, S.; Souid, I. Recovery of high concentrations of phosphorus and ammonium through struvite crystallization by CO2 repelling. Cryst. Res. Technol. 2022, 57, 2200123. [Google Scholar] [CrossRef]
- Nelson, N.O.; Mikkelsen, R.L.; Hesterberg, D.L. Struvite precipitation in anaerobic swine lagoon liquid: Effect of pH and Mg:P ratio and determination of rate constant. Bioresour. Technol. 2003, 89, 229–236. [Google Scholar] [CrossRef]
- Stratful, I.; Scrimshaw, M.D.; Lester, J.N. Conditions influencing the precipitation of magnesium ammonium phosphate. Water Res. 2001, 35, 4191–4199. [Google Scholar] [CrossRef]
- Muys, M.; Cámara, S.J.G.; Derese, S.; Spiller, M.; Verliefde, A.; Vlaeminck, S.E. Dissolution rate and growth performance reveal struvite as a sustainable nutrient source to produce a diverse set of microbial protein. Sci. Total Environ. 2023, 866, 161172. [Google Scholar] [CrossRef]
- González-Morales, C.; Fernández, B.; Molina, F.J.; Naranjo-Fernández, D.; Matamoros-Veloza, A.; Camargo-Valero, M.A. Influence of pH and temperature on struvite purity and recovery from anaerobic digestate. Sustainability 2021, 13, 10730. [Google Scholar] [CrossRef]
- Buchanan, J.R.; Mote, C.R.; Robinson, R.B. Thermodynamics of struvite formation. Trans. ASAE. 1994, 37, 617–621. [Google Scholar] [CrossRef]
- Mancho, C.; Diez-Pascual, S.; Alonso, J.; Gil-Díaz, M.; Lobo, M.C. Assessment of recovered struvite as a safe and sustainable phosphorous fertilizer. Environments 2023, 10, 22. [Google Scholar] [CrossRef]
- Li, B.; Boiarkina, I.; Young, B.; Yu, W. Quantification and mitigation of the negative impact of calcium on struvite purity. Adv. Powder Technol. 2016, 27, 2354–2362. [Google Scholar] [CrossRef]
- Yang, Y.; Peng, Y.; Cheng, J.; Zhang, S.; Liu, C.; Zhang, L. A novel two-stage aerobic granular sludge system for simultaneous nutrient removal from municipal wastewater with low C/N ratios. Chem. Eng. J. 2023, 462, 142318. [Google Scholar] [CrossRef]
- Guan, Q.; Zeng, G.; Gong, B.; Li, Y.; Ji, H.; Zhang, J.; Song, J.; Liu, C.; Wang, Z.; Deng, C. Phosphorus recovery and iron, copper precipitation from swine wastewater via struvite crystallization using various magnesium compounds. J. Clean. Prod. 2021, 328, 129588. [Google Scholar] [CrossRef]
- Pastor, L.; Mangin, D.; Barat, R.; Seco, A. A pilot-scale study of struvite precipitation in a stirred tank reactor: Conditions influencing the process. Bioressour. Technol. 2008, 99, 6285–6291. [Google Scholar] [CrossRef] [PubMed]
- Le Corre, K.S.; Valsami-Jones, E.; Hobbs, P.; Parsons, S.A. Impact of calcium on struvite crystal size, shape and purity. J. Cryst. Growth. 2005, 283, 514–522. [Google Scholar] [CrossRef]
- Rouff, A.A.; Juarez, K.M. Zinc interaction with struvite during and after mineral formation. Environ. Sci. Technol. 2014, 48, 6342. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Zhong, R.; Liu, Y.; Li, Z.; Yang, J.; Wang, F. The incorporation of Pb2+ during struvite precipitation: Quantitative, morphological and structural analysis. J. Environ. Manag. 2020, 276, 111359. [Google Scholar] [CrossRef]
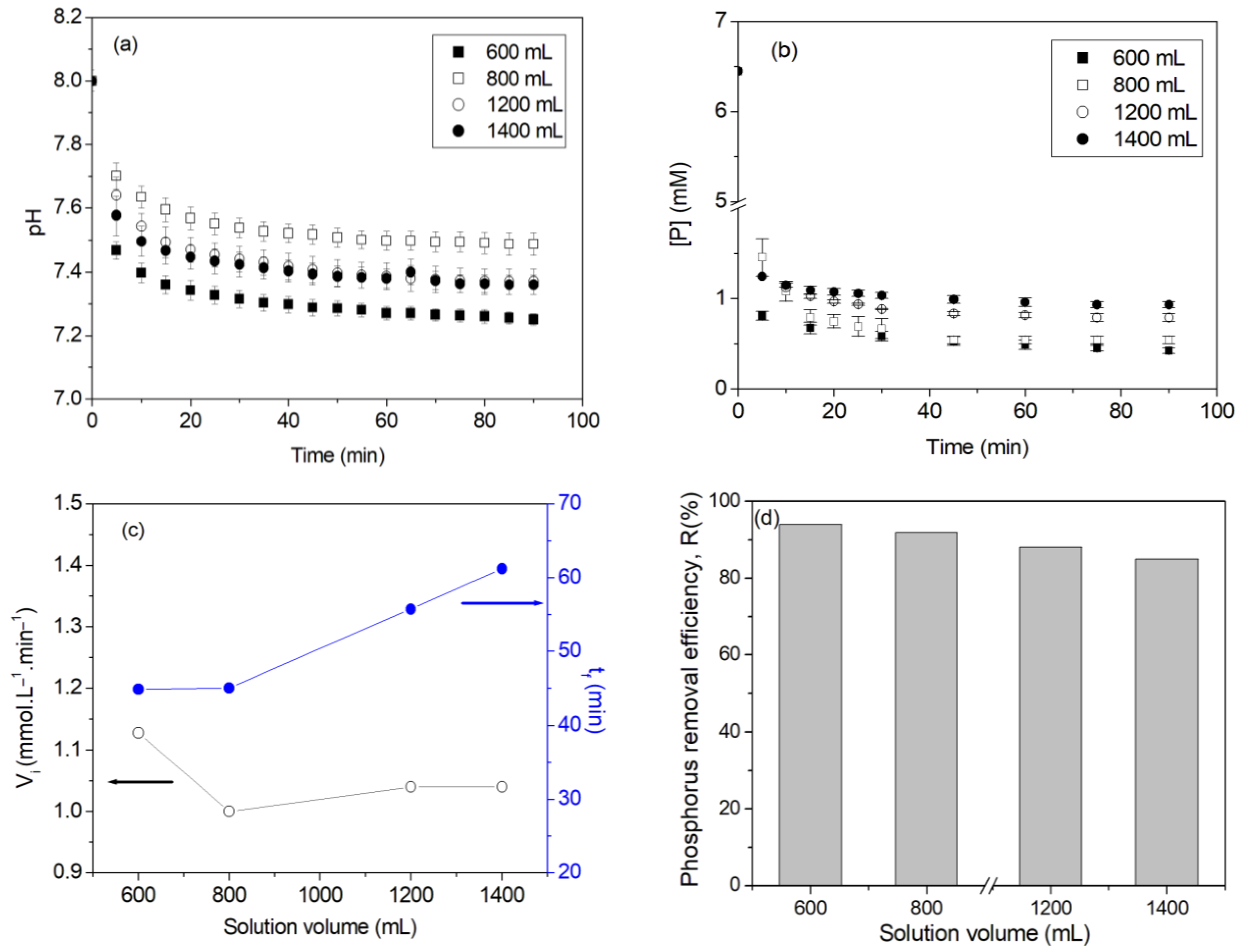
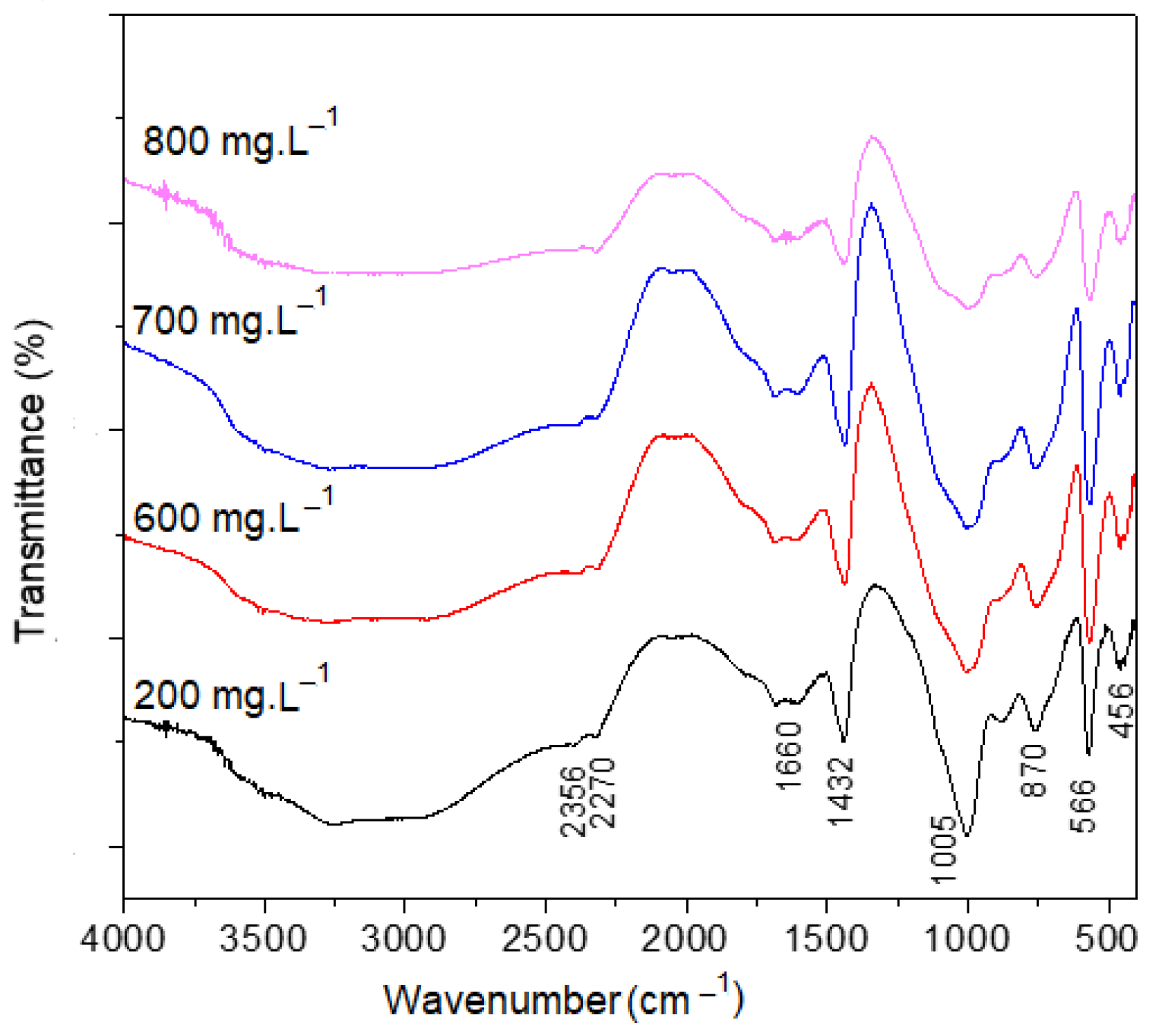
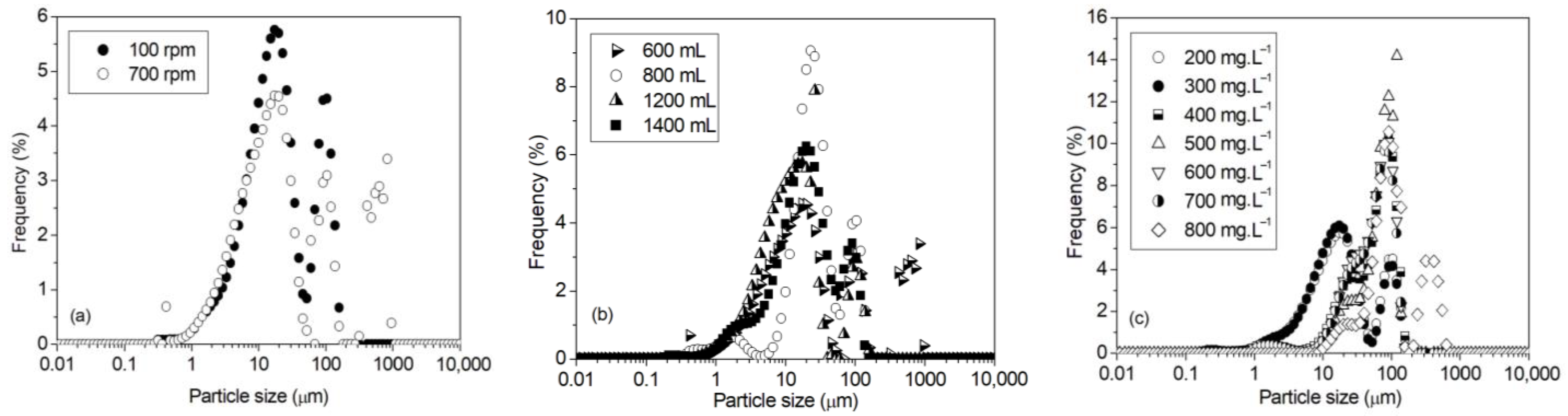
| Solution Volume | Stirring Rate (rpm) | Vi (mmol·L−1·min−1) | tf (min) | R (%) |
|---|---|---|---|---|
| 600 mL | 100 | 0.22 | 75 | 90.31 |
| 300 | 0.26 | 75 | 90.31 | |
| 500 | 0.39 | 45 | 95.48 | |
| 700 | 1.13 | 45 | 94.01 | |
| 1200 mL | 100 | 0.22 | 75 | 90.31 |
| 300 | 0.26 | 60 | 90.31 | |
| 500 | 0.36 | 90 | 95.04 | |
| 700 | 1.04 | 45 | 88.71 |
| Stirring Rate (rpm) | Vi (mmol·L−1·min−1) | tf (min) | R (%) |
|---|---|---|---|
| 100 | 8.42 | 45 | 97.51 |
| 300 | 8.74 | 30 | 97.92 |
| 500 | 9.05 | 20 | 98.73 |
| 700 | 9.13 | 30 | 99.42 |
| [P]0 (mg·L−1) | Vi (mmol·L−1·min−1) | tf (min) | R (%) |
|---|---|---|---|
| 300 | 2.33 | 50 | 87.32 |
| 400 | 4.43 | 40 | 91.74 |
| 500 | 7.03 | 25 | 95.77 |
| 600 | 8.42 | 22 | 96.15 |
| 700 | 10.79 | 20 | 98.21 |
| 800 | 12.55 | 20 | 98.94 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Korchef, A.; Abouda, S.; Souid, I. Optimizing Struvite Crystallization at High Stirring Rates. Crystals 2023, 13, 711. https://doi.org/10.3390/cryst13040711
Korchef A, Abouda S, Souid I. Optimizing Struvite Crystallization at High Stirring Rates. Crystals. 2023; 13(4):711. https://doi.org/10.3390/cryst13040711
Chicago/Turabian StyleKorchef, Atef, Salwa Abouda, and Imen Souid. 2023. "Optimizing Struvite Crystallization at High Stirring Rates" Crystals 13, no. 4: 711. https://doi.org/10.3390/cryst13040711
APA StyleKorchef, A., Abouda, S., & Souid, I. (2023). Optimizing Struvite Crystallization at High Stirring Rates. Crystals, 13(4), 711. https://doi.org/10.3390/cryst13040711







