FeCo2S4/Ni foam: A Bimetallic Sulfide Electrocatalyst with Efficient and Robust Behavior
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials Preparation
2.2. Synthesis Process
2.3. Characterization and Electrochemical Measurements
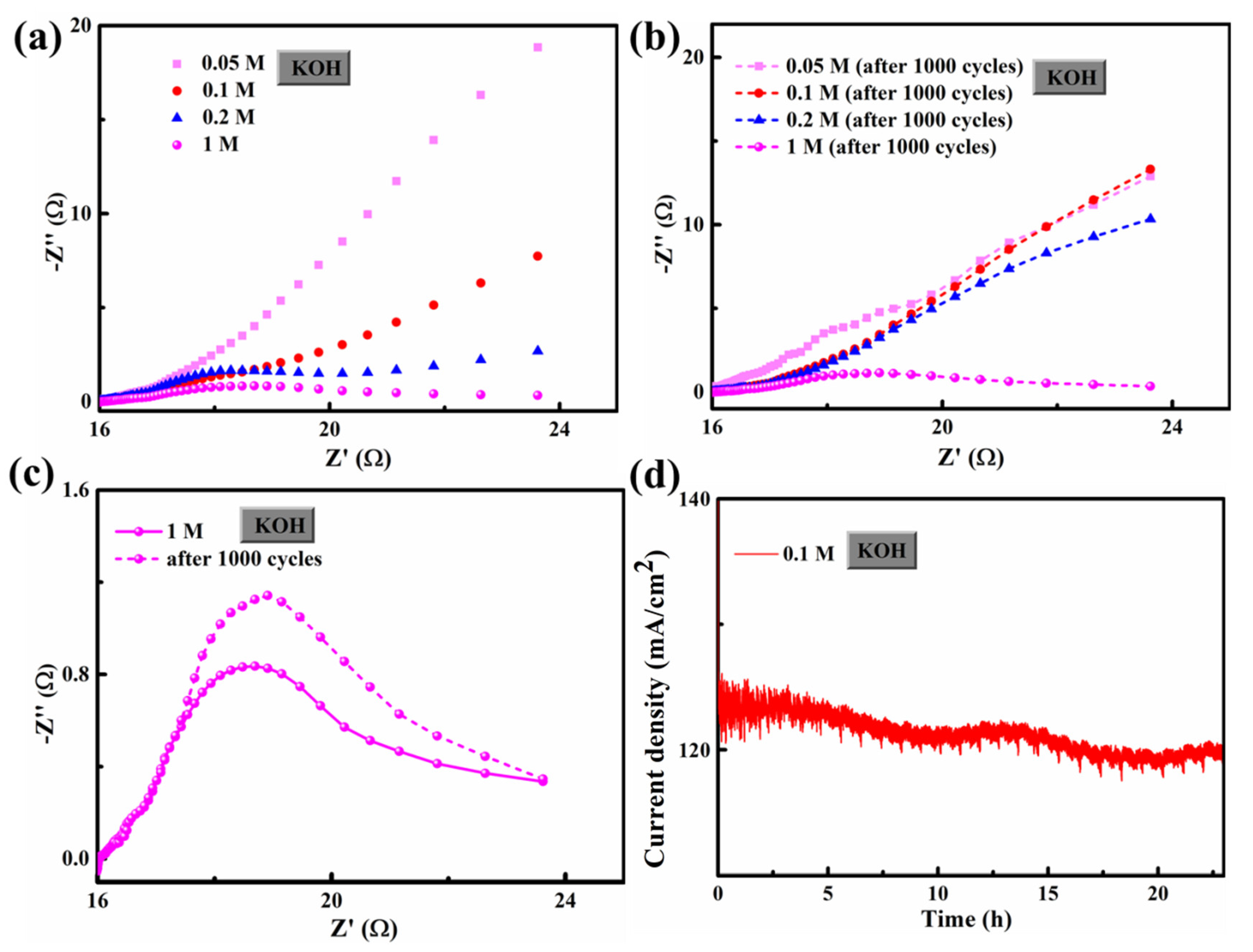
3. Results and Discussion
3.1. Morphology and Microstructure Characterization
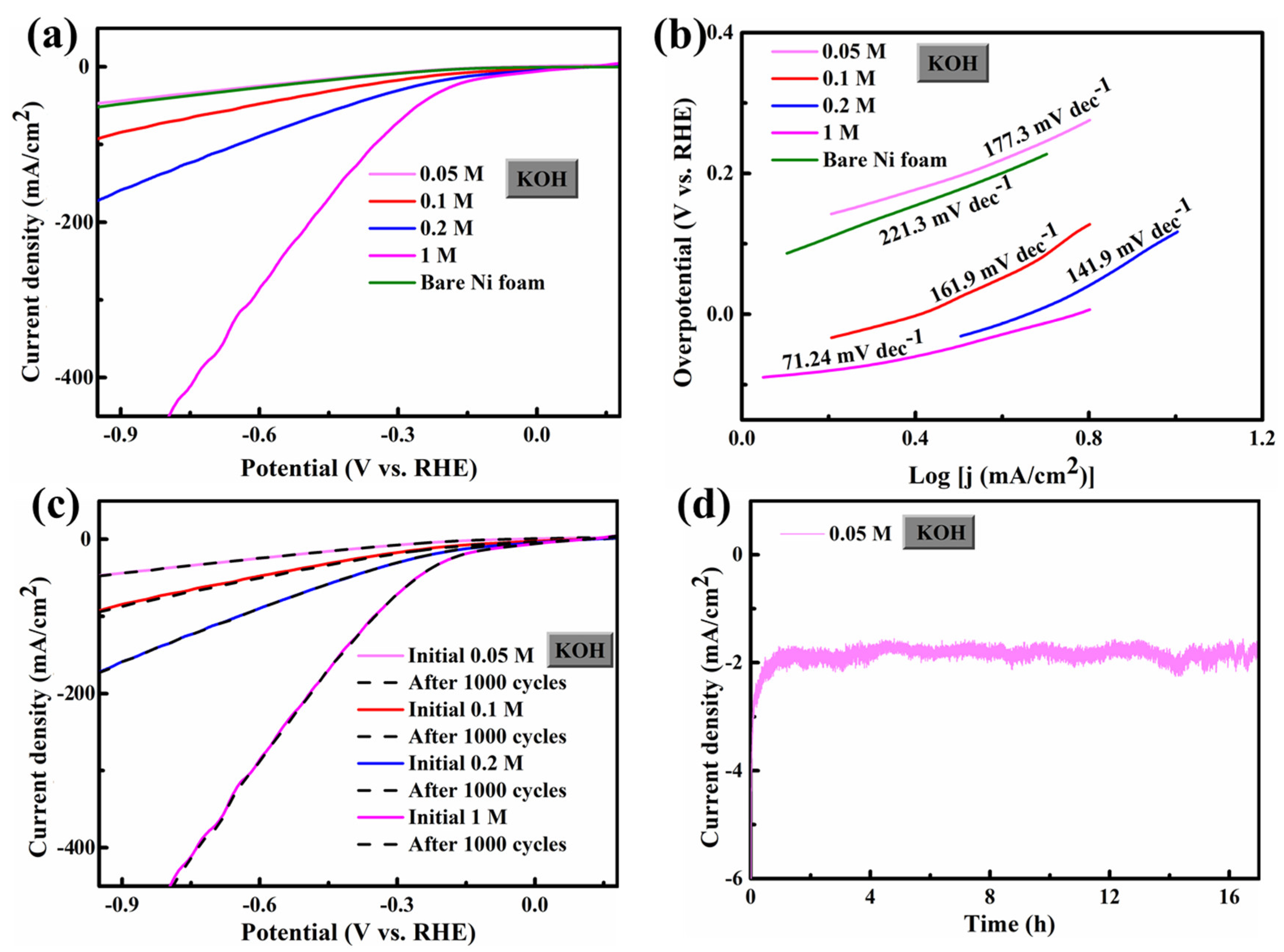
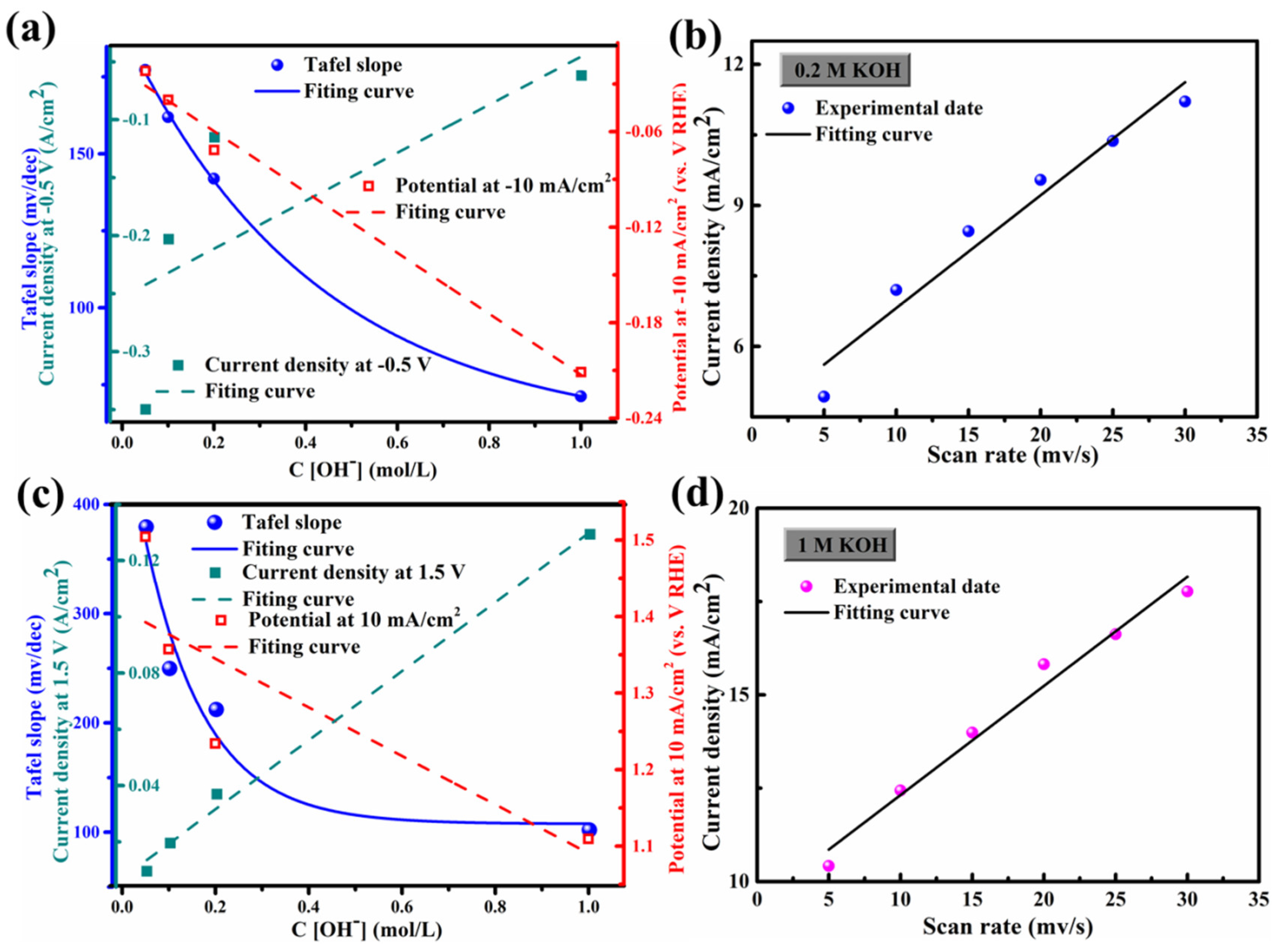
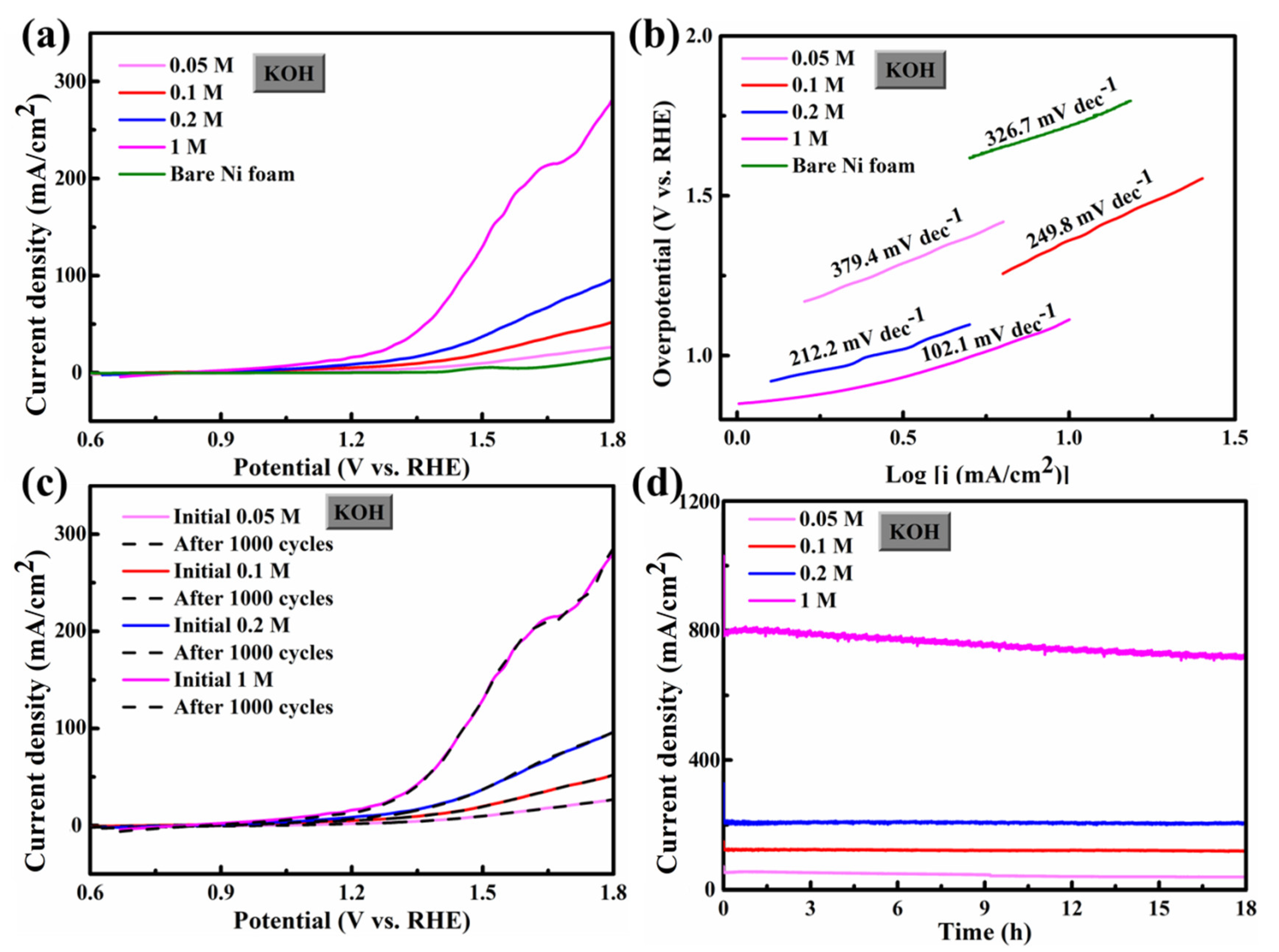
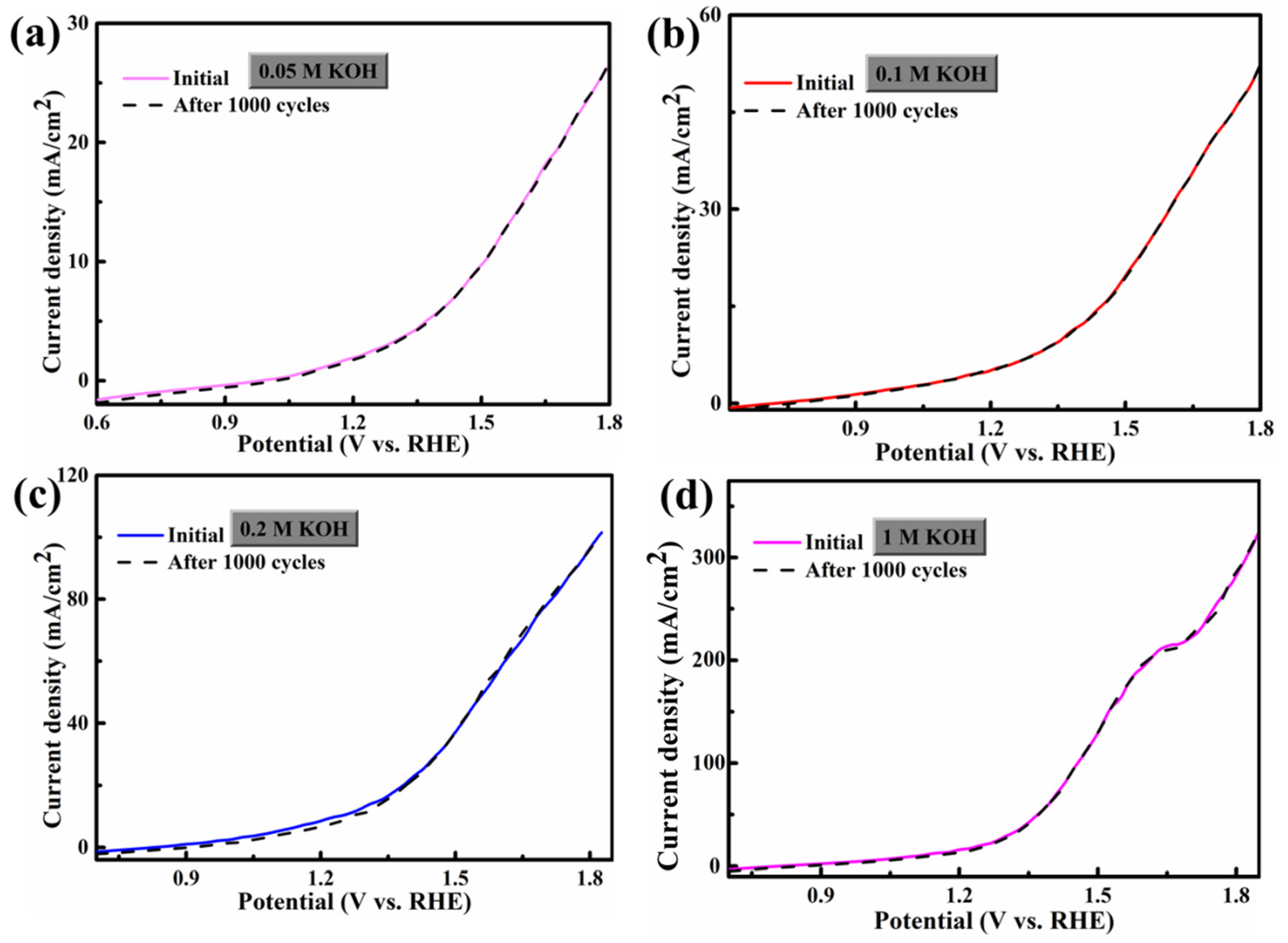
3.2. HER/OER Performance
| Materials | Electrolyte | J (mA/cm2) | Overpotential (V) | Tafel Slope (mV dec−1) | References | |
|---|---|---|---|---|---|---|
| HER | FeCo2S4/Ni foam | 1 M KOH 0.2 M KOH 0.1 M KOH 0.05 M KOH | 10 10 10 10 | 0.0626 0.11597 0.2037 0.35052 | 71.24 141.9 161.9 177.3 | This work |
| OER | FeCo2S4/Ni foam | 1 M KOH 0.2 M KOH 0.1 M KOH 0.05 M KOH | 10 10 10 10 | 1.1124 1.237 1.3603 1.50748 | 102.1 212.2 249.8 379.4 | This work |
| HER/ OER | Ni3Se2/Ni foam | 1 M KOH | 10 100 | 0.097 0.353 | 79 144 | [32] |
| HER/ OER | NiSe2 NSs-120 | 1 M KOH 1 M KOH | 10 40 | 0.207 1.562 | 186.5 109.4 | [33] |
| HER/ OER | NiCo2S4/CC | 1 M KOH | 50 50 | 0.263 0.31 | 141 89 | [34] |
| OER | CuCo2O4 | 1 M KOH | 20 | 0.29 | 117 | [17] |
| HER | CoOx@CN | 1 M KOH | 10 | 0.232 | … | [35] |
| OER | FeCoW oxyhydroxides | 1 M KOH | 10 | 0.191 | … | [36] |
| HER | β-InSe | 1 M KOH | 10 | 0.483 | 135 | [37] |
| OER | CuCo2S4/CF | 1 M KOH | 60 | 0.259 | 110 | [38] |
| HER/ OER | NiSe2 NCs | 1 M KOH | 10 10 | 0.54 0.25 | 139 38 | [39] |
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Guo, Y.; Park, T.; Yi, J.W.; Henzie, J.; Kim, J.; Wang, Z.; Jiang, B.; Bando, Y.; Sugahara, Y.; Tang, J. Nanoarchitectonics for Transition-Metal-Sulfide-Based Electrocatalysts for Water Splitting. Adv. Mater. 2019, 31, 1807134. [Google Scholar] [CrossRef] [PubMed]
- Xi, X.; Wang, X.W.; Fan, Y.; Wang, Q.; Lu, Y.; Li, J.; Shao, L.; Luo, J.L.; Fu, X.Z. Efficient bifunctional electrocatalysts for solid oxide cells based on the structural evolution of perovskites with abundant defects and exsolved CoFe nanoparticles. J. Power Sources 2021, 482, 228981. [Google Scholar] [CrossRef]
- Lei, Z.; Wang, T.; Zhao, B.; Cai, W.; Liu, Y.; Jiao, S.; Li, Q.; Cao, R.; Liu, M. Recent Progress in Electrocatalysts for Acidic Water Oxidation. Adv. Energy Mater. 2020, 10, 2000478. [Google Scholar] [CrossRef]
- Luo, Y.; Zhou, H.; Sun, J.; Fan, Q.; Dan, L.; Xie, L.; Fang, Y.; Bao, J.; Yong, L.; Ying, Y. Hierarchical Cu@CoFe layered double hydroxide core-shell nanoarchitectures as bifunctional electrocatalysts for efficient overall water splitting. Nano Energy 2017, 41, 327–336. [Google Scholar]
- Ledendecker, M.; Krick Calderón, S.; Papp, C.; Steinrück, H.P.; Antonietti, M.; Shalom, M. The Synthesis of Nanostructured Ni5P4 Films and their Use as a Non-Noble Bifunctional Electrocatalyst for Full Water Splitting. Angew. Chem. Int. Ed. 2015, 54, 12361–12365. [Google Scholar] [CrossRef]
- Wang, B.; Ye, Y.; Xu, L.; Quan, Y.; Wei, W.; Zhu, W.; Li, H.; Xia, J. Space-Confined Yolk-Shell Construction of Fe3O4 Nanoparticles Inside N-Doped Hollow Mesoporous Carbon Spheres as Bifunctional Electrocatalysts for Long-Term Rechargeable Zinc-Air Batteries. Adv. Funct. Mater. 2020, 30, 2005834. [Google Scholar] [CrossRef]
- Zhang, C.; Liu, J.; Ye, Y.; Aslam, Z.; Brydson, R.; Liang, C. Fe-N-Doped Mesoporous Carbon with Dual Active Sites Loaded on Reduced Graphene Oxides for Efficient Oxygen Reduction Catalysts. ACS Appl. Mater. Interfaces 2018, 10, 2423–2429. [Google Scholar] [CrossRef]
- Luque-Centeno, J.M.; Martínez-Huerta, M.V.; Sebastián, D.; Lemes, G.; Pastor, E.; Lázaro, M.J. Bifunctional N-doped graphene Ti and Co nanocomposites for the oxygen reduction and evolution reactions. Renew. Energy 2018, 125, 182–192. [Google Scholar] [CrossRef]
- Devaguptapu, S.V.; Hwang, S.; Karakalos, S.; Zhao, S.; Gupta, S.; Su, D.; Xu, H.; Wu, G. Morphology Control of Carbon-Free Spinel NiCo2O4 Catalysts for Enhanced Bifunctional Oxygen Reduction and Evolution in Alkaline Media. ACS Appl. Mater. Interfaces 2018, 9, 44567–44578. [Google Scholar] [CrossRef]
- Liu, K.; Li, J.; Li, J.; Wang, J.; Zhao, D.; Jiang, J.; Qian, D. A facile and low-cost synthesis of MnOx/Mn2N-N-C nanohybrid as an advanced oxygen reduction electrocatalyst. Mater. Res. Bull. 2018, 104, 60–64. [Google Scholar] [CrossRef]
- Wang, D.; Luo, D.; Zhang, Y.; Zhao, Y.; Zhou, G.; Shui, L.; Chen, Z.; Wang, X. Deciphering interpenetrated interface of transition metal oxides/phosphates from atomic level for reliable Li/S electrocatalytic behavior. Nano Energy 2021, 81, 105602. [Google Scholar] [CrossRef]
- Zhou, Y.; Xi, S.; Wang, J.; Sun, S.; Wei, C.; Feng, Z.; Du, Y.; Xu, Z.J. Revealing the Dominant Chemistry for Oxygen Reduction Reaction on Small Oxide Nanoparticles. ACS Catal. 2017, 8, 673–677. [Google Scholar] [CrossRef]
- Ting, B.; Hui, Z.; Jiang, Y.; Jin, C.; Wu, J.; Hong, Y.; Deren, Y. Epitaxial Growth of Twinned Au-Pt Core-Shell Star-Shaped Decahedra as Highly Durable Electrocatalysts. Nano Lett. 2015, 15, 7808–7815. [Google Scholar]
- Wu, X.; Niu, Y.; Feng, B.; Yu, Y.; Huang, X.; Zhong, C.; Hu, W.; Li, C.M. Mesoporous Hollow Nitrogen-Doped Carbon Nanospheres with Embedded MnFe2O4/Fe Hybrid Nanoparticles as Efficient Bifunctional Oxygen Electrocatalysts in Alkaline Media. ACS Appl. Mater. Interfaces 2018, 10, 20440–20447. [Google Scholar] [CrossRef] [PubMed]
- Suntivich, J.; May, K.J.; Gasteiger, H.A.; Goodenough, J.B.; Shaohorn, Y. A Perovskite Oxide Optimized for Oxygen Evolution Catalysis from Molecular Orbital Principles. Chem. Inform. 2012, 43, 1383–1385. [Google Scholar] [CrossRef]
- Hogg, S.A. Oxidation states of Co and Fe in Ba1−xSrxCo1−yFeyO3−δ (x,y = 0.2–0.8) and oxygen desorption in the temperature range 300–1273 K. Phys. Chem. Chem. Phys. 2009, 11, 3090–3098. [Google Scholar]
- Aqueel Ahmed, A.T.; Hou, B.; Chavan, H.S.; Jo, Y.; Cho, S.; Kim, J.; Pawar, S.M.; Cha, S.; Inamdar, A.I.; Kim, H. Self-Assembled Nanostructured CuCo2O4 for Electrochemical Energy Storage and the Oxygen Evolution Reaction via Morphology Engineering. Small 2018, 14, 1800742. [Google Scholar] [CrossRef]
- Shi, J.; Li, X.; He, G.; Zhang, L.; Li, M. Electrodeposition of high-capacitance 3D CoS/graphene nanosheets on nickel foam for high-performance aqueous asymmetric supercapacitors. J. Mater. Chem. A 2015, 3, 20619–20626. [Google Scholar] [CrossRef]
- Sivanantham, A.; Ganesan, P.; Shanmugam, S. Bifunctional Electrocatalysts: Hierarchical NiCo2S4 Nanowire Arrays Supported on Ni Foam: An Efficient and Durable Bifunctional Electrocatalyst for Oxygen and Hydrogen Evolution Reactions. Adv. Funct. Mater. 2016, 26, 4660. [Google Scholar] [CrossRef]
- Zhu, J.; Tang, S.; Wu, J.; Shi, X.; Zhu, B.; Meng, X. Wearable High-Performance Supercapacitors Based on Silver-Sputtered Textiles with FeCo2S4@NiCo2S4 Composite Nanotube-Built Multitripod Architectures as Advanced Flexible Electrodes. Adv. Energy Mater. 2017, 7, 1601234. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, X.; Cui, G.; Zhang, A.; Zhou, X.; Xu, H.; Gu, L. NiCo2S4 sub-micron spheres: An efficient non-precious metal bifunctional electrocatalyst. Nanoscale 2014, 6, 3540–3544. [Google Scholar] [CrossRef]
- Yang, L.; Xie, L.; Ren, X.; Wang, Z.; Liu, Z.; Du, G.; Asiri, A.M.; Yao, Y.; Sun, X. Hierarchical CuCo2S4 nanoarrays for high-efficient and durable water oxidation electrocatalysis. Chem. Commun. 2017, 52, 78–81. [Google Scholar] [CrossRef]
- Hao, Z.; He, X.; Li, H.; Trefilov, D.; Song, Y.; Li, Y.; Fu, X.; Cui, Y.; Tang, S.; Ge, H. Vertically Aligned and Ordered Arrays of 2D MCo2S4@Metal with Ultrafast Ion/Electron Transport for Thickness-Independent Pseudocapacitive Energy Storage. ACS Nano 2020, 14, 12719–12731. [Google Scholar] [CrossRef] [PubMed]
- Xu, G.; Zhang, Z.; Qi, X.; Ren, X.; Liu, S.; Chen, Q.; Huang, Z.; Zhong, J. Hydrothermally synthesized FeCo2O4 nanostructures: Structural manipulation for high-performance all solid-state supercapacitors. Ceram. Int. 2018, 4, 120–127. [Google Scholar] [CrossRef]
- Tang, S.; Zhu, B.; Shi, X.; Wu, J.; Meng, X. General Controlled Sulfidation toward Achieving Novel Nanosheet-Built Porous Square-FeCo2S4-Tube Arrays for High-Performance Asymmetric All-Solid-State Pseudocapacitors. Adv. Energy Mater. 2017, 7, 1601985. [Google Scholar] [CrossRef]
- Huang, Y.; Chen, X.; Ge, S.; Zhang, Q.; Zhang, X.; Li, W.; Cui, Y. Hierarchical FeCo2S4@CoFe layered double hydroxide on Ni foam as bifunctional electrocatalyst for overall water splitting. Catal. Sci. Technol. 2020, 10, 1292–1298. [Google Scholar] [CrossRef]
- Qiao, H.; Liu, H.; Huang, Z.; Ma, Q.; Luo, S.; Li, J.; Liu, Y.; Zhong, J.; Qi, X. Black Phosphorus Nanosheets Modified with Au Nanoparticles as High Conductivity and High Activity Electrocatalyst for Oxygen Evolution Reaction. Adv. Energy Mater. 2020, 10, 2002424. [Google Scholar] [CrossRef]
- Jaramillo, T.F.; Jørgensen, K.P.; Bonde, J.; Nielsen, J.H.; Horch, S.; Chorkendorff, I. Identification of Active Edge Sites for Electrochemical H2 Evolution from MoS2 Nanocatalysts. Science 2007, 317, 100–102. [Google Scholar] [CrossRef]
- Menezes, P.; Panda, C.; Loos, S.; Bunscheibruns, F.; Walter, C.; Schwarze, M.; Deng, X.; Dau, H.; Driess, M. A structurally versatile nickel phosphite acting as a robust bifunctional electrocatalyst for overall water splitting. Energy Environ. Sci. 2018, 5, 1287–1298. [Google Scholar] [CrossRef]
- Zhang, H.; Li, X.; Hähnel, A.; Naumann, V.; Lin, C.; Azimi, S.; Schweizer, S.L.; Maijenburg, A.W.; Wehrspohn, R.B. Bifunctional Heterostructure Assembly of NiFe LDH Nanosheets on NiCoP Nanowires for Highly Efficient and Stable Overall Water Splitting. Adv. Funct. Mater. 2018, 28, 1706847. [Google Scholar] [CrossRef]
- Liu, B.; Yang, M.; Yang, D.; Chen, H.; Li, H. Graphene-like porous carbon nanosheets for ultra-high rate performance supercapacitors and efficient oxygen reduction electrocatalysts. J. Power Sources 2020, 456, 227999. [Google Scholar] [CrossRef]
- Xu, R.; Wu, R.; Shi, Y.; Zhang, J.; Zhang, B. Ni3Se2 nanoforest/Ni foam as a hydrophilic, metallic, and self-supported bifunctional electrocatalyst for both H2 and O2 generations. Nano Energy 2016, 24, 103–110. [Google Scholar] [CrossRef]
- Jie, Z.; Liu, Y.; Zhen, Z.; Huang, Z.; Chen, X.; Ren, X.; Long, R.; Xiang, Q.; Zhong, J. Hierarchical NiSe2 sheet-like nano-architectures as an efficient and stable bifunctional electrocatalyst for overall water splitting: Phase and morphology engineering. Electrochim. Acta 2018, 279, 195–203. [Google Scholar]
- Kang, J.; Wood, J.D.; Wells, S.A.; Lee, J.H.; Liu, X.; Chen, K.S.; Hersam, M.C. Solvent Exfoliation of Electronic-Grade, Two-Dimensional Black Phosphorus. ACS Nano 2015, 9, 3596–3604. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.; Wang, J.; Su, D.; Wei, Z.; Pang, Z.; Wang, Y.; Am, J. In situ Cobalt-Cobalt Oxide/N-Doped Carbon Hybrids as Superior Bifunctional Electrocatalysts for Hydrogen and Oxygen Evolution. Chem. Soc. 2015, 137, 2688–2694. [Google Scholar] [CrossRef]
- Zhang, B.; Zheng, X.; Voznyy, O.; Comin, R.; Bajdich, M.; Garcíamelchor, M.; Han, L.; Xu, J.; Liu, M.; Zheng, L. Homogeneously dispersed multimetal oxygen-evolving catalysts. Sci. Found. China 2016, 352, 51. [Google Scholar] [CrossRef]
- Elisa, P.; Emanuele, L.; Sebastiano, B.; Danil, W.B.; Antonio, P.; Bekir, G.; Songül, D.; Mirko, P.; Silvia, G.; Reinier, O.N.; et al. Liquid-Phase Exfoliated Indium-Selenide Flakes and Their Application in Hydrogen Evolution Reaction. Small 2018, 26, 1800749. [Google Scholar]
- Hu, Z.; Li, Q.; Lei, B.; Zhou, Q.; Xiang, D.; Lyu, Z.; Hu, F.; Wang, J.; Ren, Y.; Guo, R. Water-Catalyzed Oxidation of Few-Layer Black Phosphorous in a Dark Environment. Angew. Chem. Int. Ed. 2017, 56, 9131–9135. [Google Scholar] [CrossRef]
- Kwak, I.H.; Im, H.S.; Dong, M.J.; Kim, Y.W.; Park, K.; Lim, Y.R.; Cha, E.H.; Park, J. CoSe2 and NiSe2 Nanocrystals as Superior Bifunctional Catalysts for Electrochemical and Photoelectrochemical Water Splitting. ACS Appl. Mater. Interfaces 2016, 8, 5327–5334. [Google Scholar] [CrossRef]



| Agents | Company |
|---|---|
| Co(NO3)2•6H2O | Xilong Chemical Co., Ltd. |
| NH4F | Xilong Chemical Co., Ltd. |
| Urea | Xilong Chemical Co., Ltd. |
| Fe(NO3)3•9H2O | Chengdu Kelong Chemical Co., Ltd. |
| Na2S | Chengdu Kelong Chemical Co., Ltd. |
| HCl (34 wt%–37 wt%) | Alfa Aesar (Tianjin) Reagent Co., Ltd. |
| Acetone | Alfa Aesar (Tianjin) Reagent Co., Ltd. |
| KOH (AR) | Alfa Aesar (Tianjin) Reagent Co., Ltd. |
| The Nernst equation: E (vs. RHE) = E (vs. SCE) + ESCE + 0.0591 pH = E (SCE) + 0.241 + 0.0591 pH | ||
| Electrolyte (M/KOH) | pH | E (RHE) |
| 0.05 | 12.8 | E (SCE) + 0.99748 V |
| 0.1 | 13 | E (SCE) + 1.0093 V |
| 0.2 | 13.3 | E (SCE) + 1.02703 V |
| 1 | 14 | E (SCE) + 1.0684 V |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tao, J.; Liu, S.; Liao, Y.; Qiao, H.; Liu, Y.; Chen, H.; Teng, M.; Wei, Y.; Liu, S.; Qiu, Z.; et al. FeCo2S4/Ni foam: A Bimetallic Sulfide Electrocatalyst with Efficient and Robust Behavior. Crystals 2023, 13, 717. https://doi.org/10.3390/cryst13050717
Tao J, Liu S, Liao Y, Qiao H, Liu Y, Chen H, Teng M, Wei Y, Liu S, Qiu Z, et al. FeCo2S4/Ni foam: A Bimetallic Sulfide Electrocatalyst with Efficient and Robust Behavior. Crystals. 2023; 13(5):717. https://doi.org/10.3390/cryst13050717
Chicago/Turabian StyleTao, Jiayou, Shuhua Liu, Yanmo Liao, Hui Qiao, Yu Liu, Hui Chen, Min Teng, Yong Wei, Sanjie Liu, Zongyi Qiu, and et al. 2023. "FeCo2S4/Ni foam: A Bimetallic Sulfide Electrocatalyst with Efficient and Robust Behavior" Crystals 13, no. 5: 717. https://doi.org/10.3390/cryst13050717
APA StyleTao, J., Liu, S., Liao, Y., Qiao, H., Liu, Y., Chen, H., Teng, M., Wei, Y., Liu, S., Qiu, Z., Li, C., & Qi, X. (2023). FeCo2S4/Ni foam: A Bimetallic Sulfide Electrocatalyst with Efficient and Robust Behavior. Crystals, 13(5), 717. https://doi.org/10.3390/cryst13050717





