First-Principles Modeling of Bottom-Up Synthesis of Carbon Quantum Dots
Abstract
:1. Introduction
2. Computational Method
3. Formation of Carbon Nanostructures from Citric Acid (CA)
3.1. Review of Experimental Results
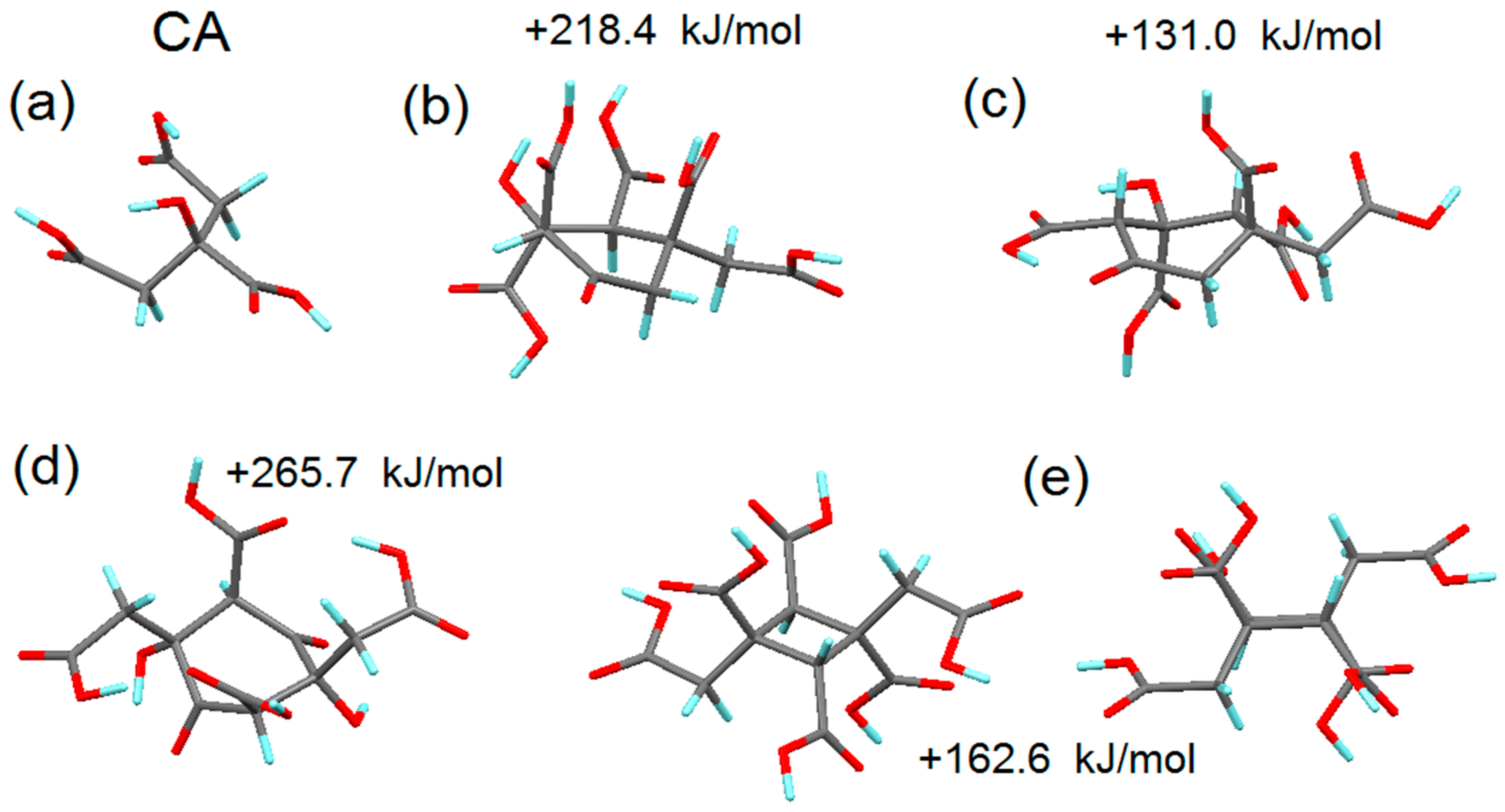
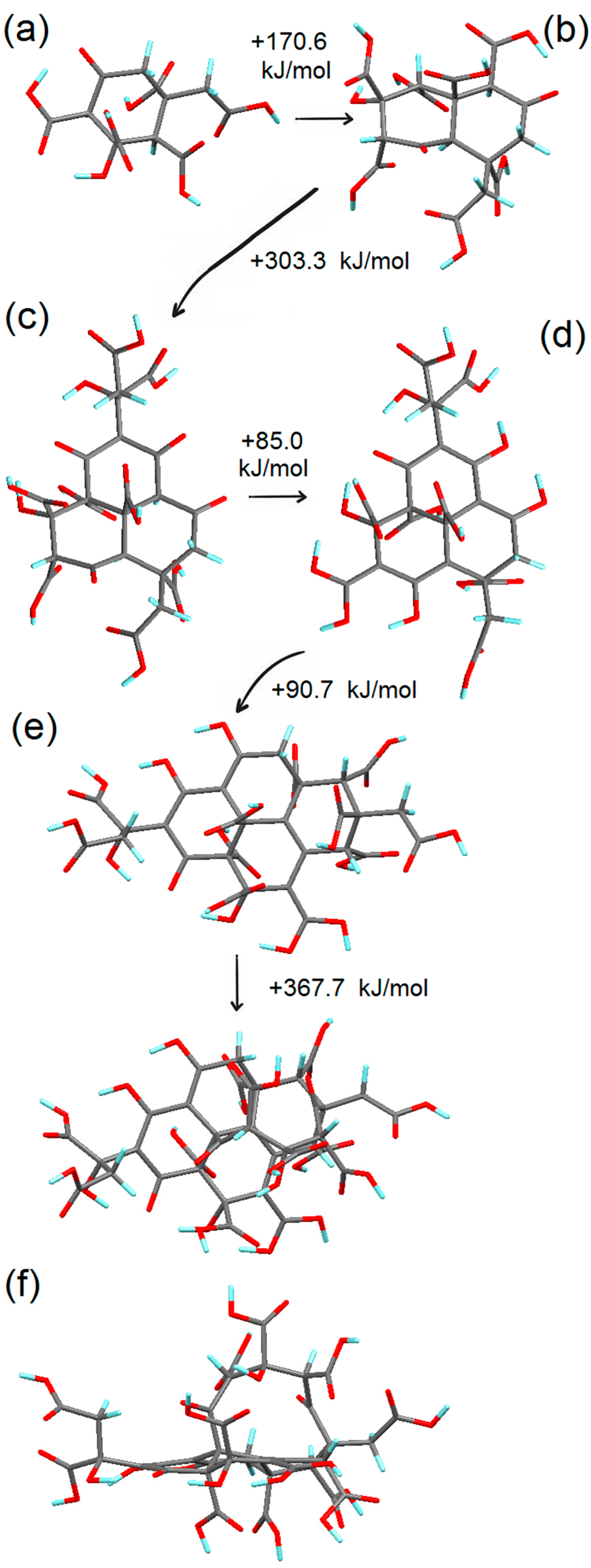
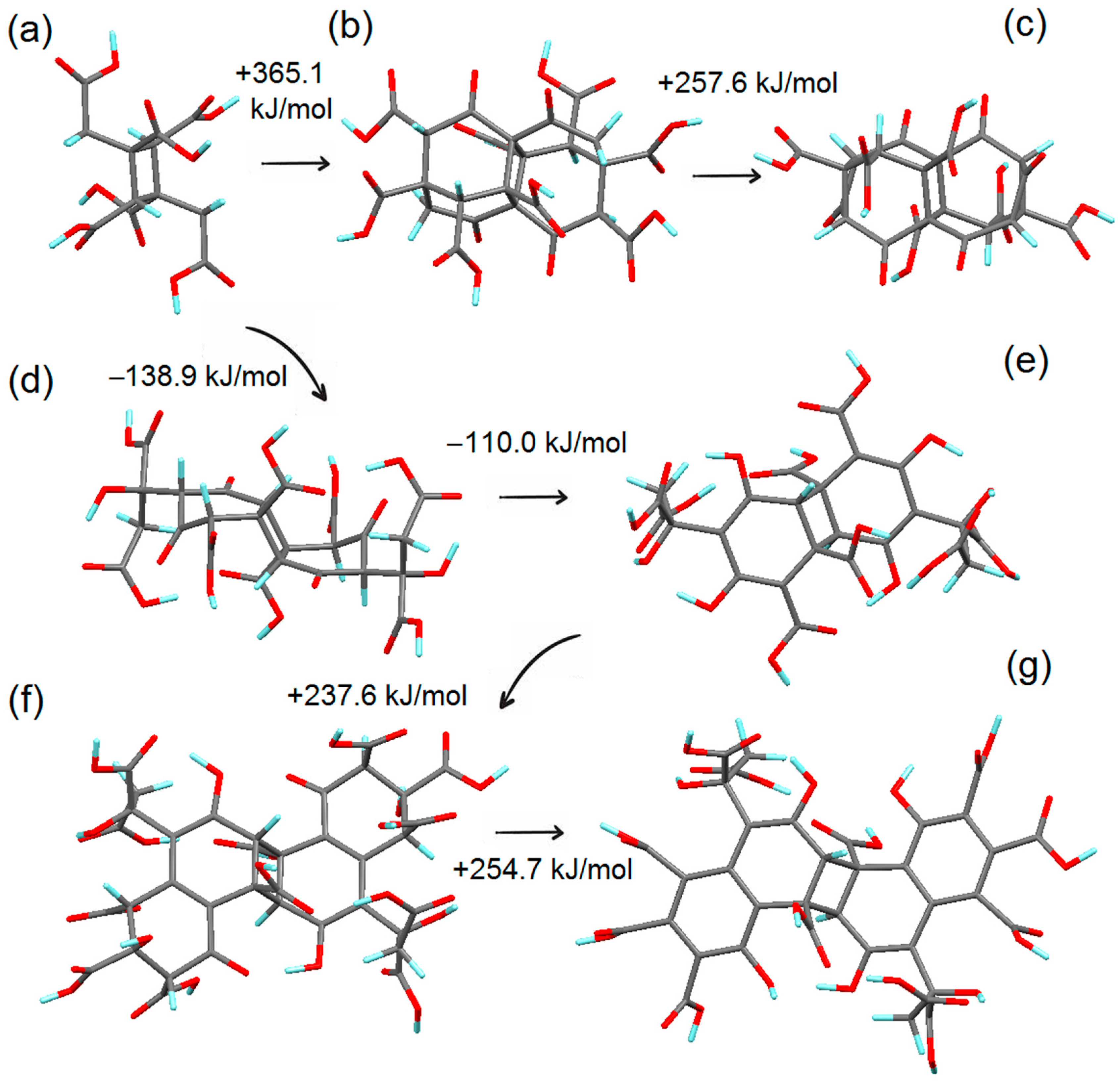
3.2. Simulation of the First Steps of Carbon Dot Assembly from CA
4. Formation of Carbon Nanostructures from o-Phenylenediamine (OPD)
4.1. Review of Experimental Results
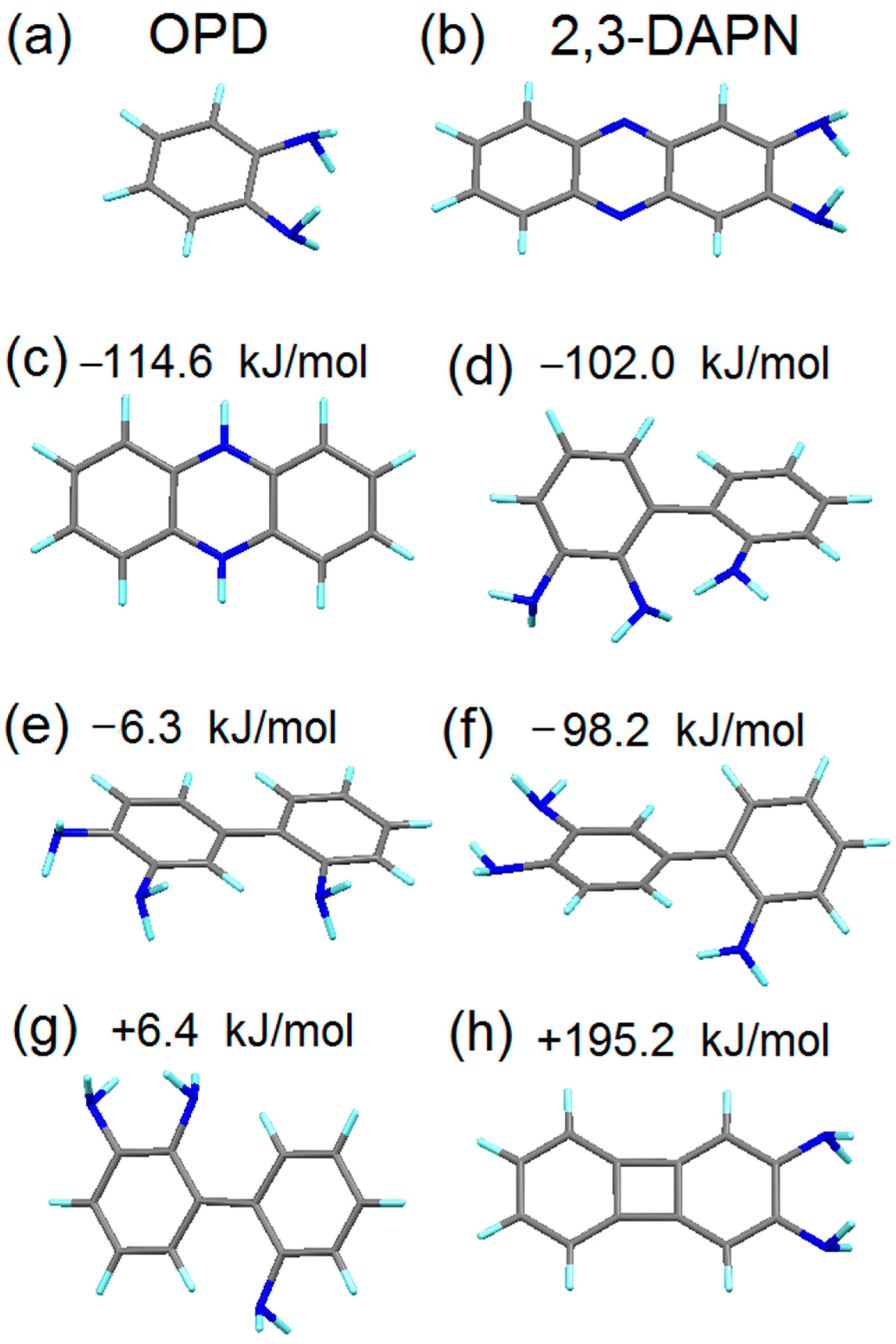
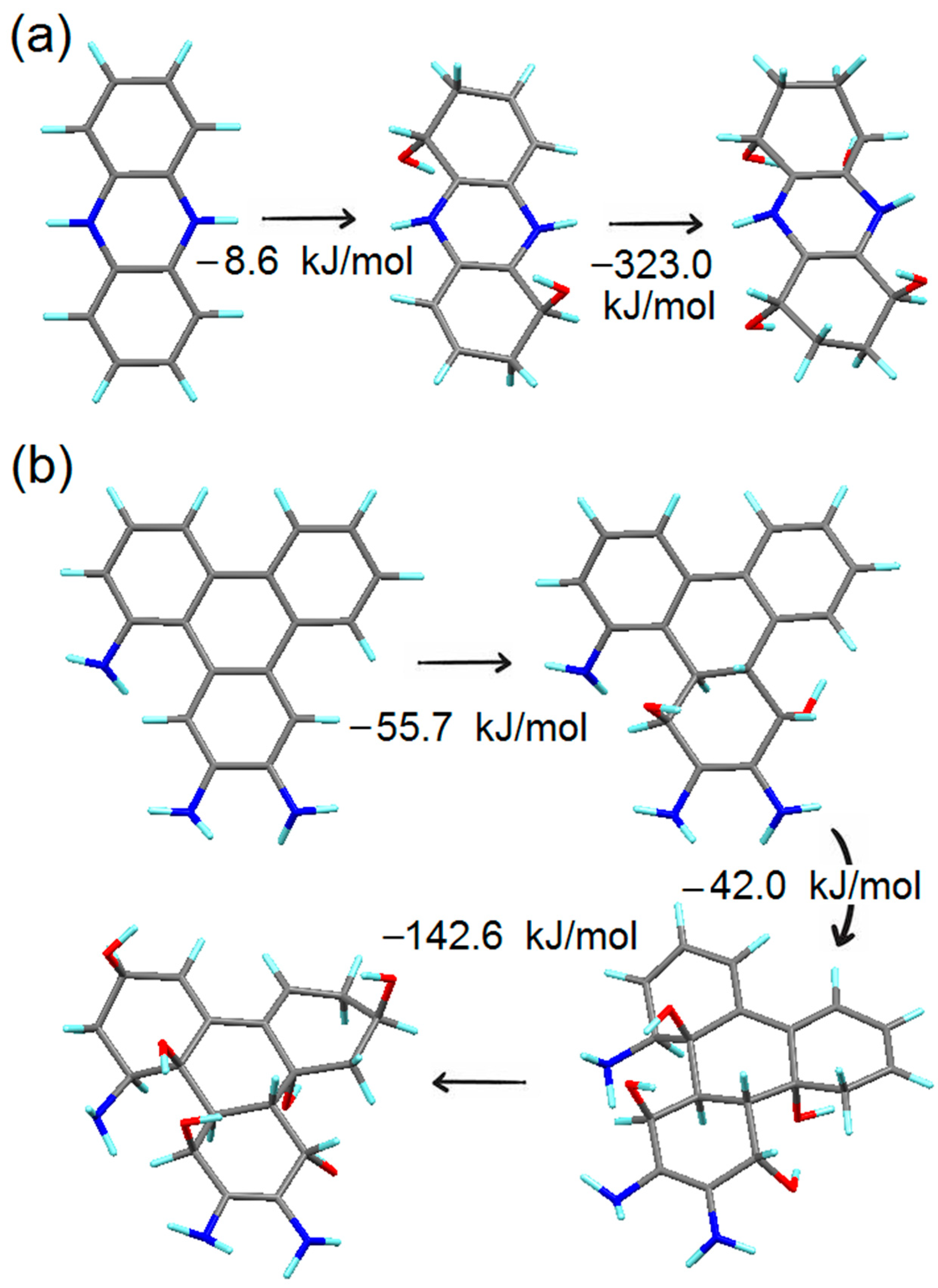
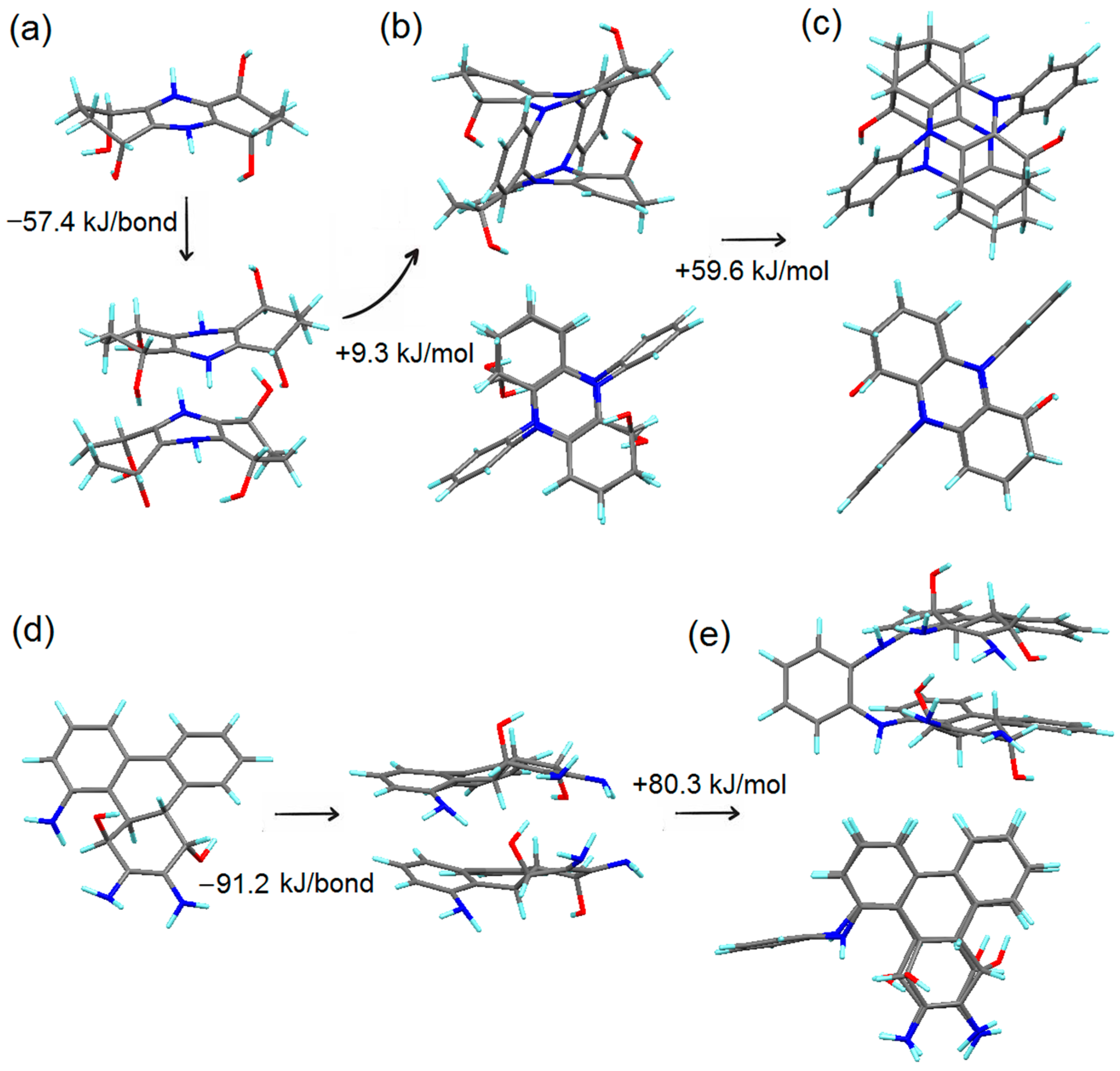
4.2. Simulation of the First Steps of Carbon Dot Assembly from OPD
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Enoki, T.; Takai, K.; Osipov, V.; Baidakova, M.; Vul’, A. Nanographene and Nanodiamond; New Members in the Nanocarbon Family. Chem. Asian J. 2009, 4, 796–804. [Google Scholar] [CrossRef] [PubMed]
- Xia, C.; Zhu, S.; Feng, T.; Yang, M.; Yang, B. Evolution and Synthesis of Carbon Dots: From Carbon Dots to Carbonized Polymer Dots. Adv. Sci. 2019, 6, 1901316. [Google Scholar] [CrossRef]
- Wang, X.; Feng, Y.; Dong, P.; Huang, J. A Mini Review on Carbon Quantum Dots: Preparation, Properties, and Electrocatalytic Application. Front. Chem. 2019, 7, 671. [Google Scholar] [CrossRef] [PubMed]
- Das, R.; Bandyopadhyay, R.; Pramanik, P. Carbon quantum dots from natural resource: A review. Mater. Today Chem. 2018, 8, 96. [Google Scholar] [CrossRef]
- Fan, H.; Zhang, M.; Bhandari, B.; Yang, C. Food waste as a carbon source in carbon quantum dots technology and their applications in food safety detection. Trends Food Sci. Technol. 2020, 95, 86. [Google Scholar] [CrossRef]
- Ren, J.; Malfatti, L.; Innocenzi, P. Citric Acid Derived Carbon Dots, the Challenge of Understanding the Synthesis-Structure Relationship. C J. Carbon Res. 2021, 7, 2. [Google Scholar] [CrossRef]
- Yang, S.; Li, Y.; Chen, L.; Wang, H.; Shang, L.; He, P.; Dong, H.; Wang, G.; Ding, G. Fabrication of Carbon-Based Quantum Dots via a “Bottom-Up” Approach: Topology, Chirality, and Free Radical Processes in “Building Blocks”. Small 2023, 2205957. [Google Scholar] [CrossRef]
- Molaei, M.J. The optical properties and solar energy conversion applications of carbon quantum dots: A review. Solar Ener. 2020, 196, 549. [Google Scholar] [CrossRef]
- Rasal, A.S.; Yadav, S.; Yadav, A.; Kashale, A.A.; Manjunatha, S.T.; Altaee, A.; Chang, J.-Y. Carbon Quantum Dots for Energy Applications: A Review. ACS Appl. Nano Mater. 2021, 4, 6515. [Google Scholar] [CrossRef]
- Sharma, S.; Dutta, V.; Singh, P.; Raizada, P.; Rahmani-Sani, A.; Hosseini-Bandegharaei, A.; Thakur, V.K. Carbon quantum dot supported semiconductor photocatalysts for efficient degradation of organic pollutants in water: A review. J. Clean. Product. 2019, 228, 755. [Google Scholar] [CrossRef]
- Singh, A.; Qu, Z.; Sharma, A.; Singh, M.; Tse, B.; Ostrikov, K.; Popat, A.; Sonar, P.; Kumeria, T. Ultra-bright green carbon dots with excitation-independent fluorescence for bioimaging. J. Nanostruct. Chem. 2022, 13, 377–387. [Google Scholar] [CrossRef]
- Molaei, M.J. Carbon quantum dots and their biomedical and therapeutic applications: A review. RSC Adv. 2019, 9, 6460. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Ding, H.; Li, Z.; Tedesco, A.C.; Bi, H. Carbon Dots Derived from Tea Polyphenols as Photosensitizers for Photodynamic Therapy. Molecules 2022, 27, 8627. [Google Scholar] [CrossRef]
- Šafranko, S.; Goman, D.; Stanković, A.; Medvidović-Kosanović, M.; Moslavac, T.; Jerković, I.; Jokić, S. An Overview of the Recent Developments in Carbon Quantum Dots—Promising Nanomaterials for Metal Ion Detection and (Bio)Molecule Sensing. Chemosensors 2021, 9, 138. [Google Scholar] [CrossRef]
- Mansuriya, B.D.; Altintas, Z. Carbon Dots: Classification, Properties, Synthesis, Characterization, and Applications in Health Care—An Updated Review (2018–2021). Nanomaterials 2021, 11, 2525. [Google Scholar] [CrossRef] [PubMed]
- Dugam, S.; Nangare, S.; Patil, P.; Jadhav, N. Carbon dots: A novel trend in pharmaceutical applications. Ann. Pharm. Fran. 2021, 79, 335–345. [Google Scholar] [CrossRef]
- Santonocito, R.; Intravaia, M.; Caruso, I.M.; Pappalardo, A.; Sfrazzetto, G.T.; Tuccitto, N. Fluorescence sensing by carbon nanoparticles. Nanoscale Adv. 2022, 4, 1926–1948. [Google Scholar] [CrossRef]
- Shi, W.; Han, Q.; Wu, J.; Ji, C.; Zhou, Y.; Li, S.; Gao, L.; Leblanc, R.M.; Peng, Z. Synthesis Mechanisms, Structural Models, and Photothermal Therapy Applications of Top-Down Carbon Dots from Carbon Powder, Graphite, Graphene, and Carbon Nanotubes. Int. J. Mol. Sci. 2022, 23, 1456. [Google Scholar] [CrossRef]
- Jones, S.S.; Sahatiya, P.; Badhulik, S. One step, high yield synthesis of amphiphilic carbon quantum dots derived from chia seeds: A solvatochromic study. New J. Chem. 2017, 41, 13130. [Google Scholar] [CrossRef]
- Atchudan, R.; Edison, T.N.J.I.; Shanmugam, M.; Perumal, S.; Somanathan, T.; Lee, Y.R. Sustainable synthesis of carbon quantum dots from banana peel waste using hydrothermal process for in vivo bioimaging. Phys. E Low-Dimens. Syst. Nanostructures 2021, 126, 114417. [Google Scholar] [CrossRef]
- Dong, Y.; Shao, J.; Chen, C.; Hao Li, H.; Wang, R.; Chi, Y.; Lin, X.; Chen, G. Blue luminescent graphene quantum dots and graphene oxide prepared by tuning the carbonization degree of citric acid. Carbon 2012, 50, 4738–4743. [Google Scholar] [CrossRef]
- Wang, F.; Pang, S.; Wang, L.; Li, Q.; Kreiter, M.; Liu, C.-Y. One-Step Synthesis of Highly Luminescent Carbon Dots in Noncoordinating Solvents. Chem. Mater. 2010, 22, 4528–4530. [Google Scholar] [CrossRef]
- Merlen, A.; Buijnsters, J.G.; Pardanaud, C. A Guide to and Review of the Use of Multiwavelength Raman Spectroscopy for Characterizing Defective Aromatic Carbon Solids: From Graphene to Amorphous Carbons. Coatings 2017, 7, 153. [Google Scholar] [CrossRef]
- Bokobza, L.; Bruneel, J.-L.; Couzi, M. Raman Spectra of Carbon-Based Materials (from Graphite to Carbon Black) and of Some Silicone Composites. C J. Carbon Res. 2015, 1, 77–94. [Google Scholar] [CrossRef]
- Wang, S.; Chen, Z.-G.; Cole, I.; Li, Q. Structural evolution of graphene quantum dots during thermal decomposition of citric acid and the corresponding photoluminescence. Carbon 2015, 82, 304–313. [Google Scholar] [CrossRef]
- Ludmerczki, R.; Mura, S.; Carbonaro, C.M.; Mandity, I.M.; Carraro, M.; Senes, N.; Garroni, S.; Granozzi, G.; Calvillo, L.; Marras, S.; et al. Carbon Dots from Citric Acid and its Intermediates Formed by Thermal Decomposition. Chem. Eur. J. 2019, 25, 11963–11974. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.; Ma, S.; Li, J.; Dai, K.; Xiao, X.; Zhao, D.; Gong, W. Rational design of high quality citric acid-derived carbon dots by selecting efficient chemical structure motifs. Carbon 2017, 112, 131–141. [Google Scholar] [CrossRef]
- Kundu, A.; Jungpyo Lee, J.; Park, B.; Ray, C.; Sankar, K.V.; Kim, W.S.; Lee, S.H.; Cho, I.-J.; Jun, S.C. Facile approach to synthesize highly fluorescent multicolor emissive carbon dots via surface functionalization for cellular imaging. J. Coll. Interf. Sci. 2018, 513, 505–514. [Google Scholar] [CrossRef]
- Dhenadhayalan, N.; Lin, K.-C.; Raghupathy Suresh, R.; Ramamurthy, P. Unravelling the Multiple Emissive States in Citric-Acid-Derived Carbon Dots. J. Phys. Chem. C 2016, 120, 1252–1261. [Google Scholar] [CrossRef]
- Zhang, Q.; Wang, R.; Feng, B.; Zhong, X.; Ostrikov, K. Photoluminescence mechanism of carbon dots: Triggering high-color-purity red fluorescence emission through edge amino protonation. Nat. Commun. 2021, 12, 6856. [Google Scholar] [CrossRef]
- Dong, Y.; Li, T.; Bateer, B.; Wang, H.; Fu, Q.; Zhang, F. Preparation of yellow emissive nitrogen-doped carbon dots from o-phenylenediamine and their application in curcumin sensing. New J. Chem. 2022, 46, 9543. [Google Scholar] [CrossRef]
- Li, P.; Xue, S.; Sun, L.; Zong, X.; An, L.; Qu, D.; Wang, X.; Sun, Z. Formation and fluorescent mechanism of red emissive carbon dots from o-phenylenediamine and catechol system. Light Sci. Appl. 2022, 11, 298. [Google Scholar] [CrossRef]
- Wang, B.; Wei, Z.; Sui, L.; Yu, J.; Zhang, B.; Wang, X.; Feng, S.; Song, H.; Yong, X.; Tian, Y.; et al. Electron–phonon coupling-assisted universal red luminescence of o-phenylenediamine-based carbon dots. Light Sci. Appl. 2022, 11, 172. [Google Scholar] [CrossRef] [PubMed]
- Vedernikova, A.A.; Miruschenko, M.D.; Arefina, I.A.; Stepanidenko, E.A.; Cherevkov, S.A.; Spiridonov, I.G.; Danilov, D.V.; Koroleva, A.V.; Zhizhin, E.V.; Ushakova, E.V. Dual-Purpose Sensing Nanoprobe Based on Carbon Dots from o-Phenylenediamine: pH and Solvent Polarity Measurement. Nanomaterials 2022, 12, 3314. [Google Scholar] [CrossRef] [PubMed]
- An, Y.; Lin, X.; Zhou, Y.; Li, Y.; Zheng, Y.; Wu, C.; Xu, K.; Chaib, X.; Liu, C. Red, green, and blue light-emitting carbon dots prepared from o-phenylenediamine. RSC Adv. 2021, 11, 26915. [Google Scholar] [CrossRef]
- Song, L.; Cui, Y.; Zhang, C.; Hu, Z.; Liu, X. Microwave-assisted facile synthesis of yellow fluorescent carbon dots from o-phenylenediamine for cell imaging and sensitive detection of Fe3+ and H2O2. RSC Adv. 2016, 6, 17704–17712. [Google Scholar] [CrossRef]
- Ji, C.; Han, Q.; Zhou, Y.; Wu, J.; Shi, W.; Gao, L.; Leblanc, R.M.; Peng, Z. Phenylenediamine-derived near infrared carbon dots: The kilogram-scale preparation, formation process, photoluminescence tuning mechanism and application as red phosphors. Carbon 2022, 192, 198–208. [Google Scholar] [CrossRef]
- Wu, Z.; Chen, R.; Pan, S.; Liu, H.; Hu, X. A ratiometric fluorescence strategy based on dual-signal response of carbon dots and o-phenylenediamine for ATP detection. Microchem. J. 2021, 164, 105976. [Google Scholar] [CrossRef]
- Zammataro, A.; Gangemi CM, A.; Pappalardo, A.; Toscano, R.M.; Puglisi, R.; Nicotra, G.; Sfrazzetto, G.T. Covalently functionalized carbon nanoparticles with a chiral Mn-Salen: A new nanocatalyst for enantioselective epoxidation of alkenes. Chem. Commun. 2019, 55, 5255–5258. [Google Scholar] [CrossRef]
- Salman, B.I.; Hassan, A.I.; Hassan, Y.F.; Saraya, R.E.; Batakoushy, H.A. Rapid One-Pot Microwave Assisted Green Synthesis Nitrogen Doped Carbon Quantum Dots as Fluorescent Precursor for Estimation of Modafinil as Post-Covid Neurological Drug in Human Plasma with Greenness Assessments. J. Fluoresc. 2022, 1–10. [Google Scholar] [CrossRef]
- Abbasi, A.; Abushad, M.; Khan, A.; Bhat, Z.U.H.; Hanif, S.; Shakir, M. Bare undoped nontoxic carbon dots as a visible light photocatalyst for the degradation of methylene blue and congo red. Carbon Trends 2023, 10, 100238. [Google Scholar] [CrossRef]
- Esmail, L.A.; Jabbar, H.S. Violuric acid carbon dots as a highly fluorescence probe for ultrasensitive determination of Zn (II) in tomato paste. Food Chem. 2023, 413, 135638. [Google Scholar] [CrossRef]
- Carbonaro, C.M.; Corpino, R.; Salis, M.; Mocci, F.; Thakkar, S.V.; Olla, C.; Ricci, P.C. On the Emission Properties of Carbon Dots: Reviewing Data and Discussing Models. C J. Carbon Res. 2019, 5, 60. [Google Scholar] [CrossRef]
- Mocci, F.; de Villiers Engelbrecht, L.; Olla, C.; Cappai, A.; Casula, M.F.; Melis, C.; Stagi, L.; Laaksonen, A.; Carbonaro, C.M. Carbon Nanodots from an In Silico Perspective. Chem. Rev. 2022, 122, 13709–13799. [Google Scholar] [CrossRef] [PubMed]
- Boukhvalov, D.W.; Osipov, V.Y.; Hogan, B.T.; Baldycheva, A. A Comprehensive Model of Nitrogen-Free Ordered Carbon Quantum Dots. Nanoscale Res. Lett. 2023, 18, 1. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.; Cong, R.; Zhu, S.; Zhao, X.; Liu, J.; STse, J.; Yang, B. pH-Dependent Synthesis of Novel Structure-Controllable Polymer-Carbon NanoDots with High Acidophilic Luminescence and Super Carbon Dots Assembly for White Light-Emitting Diodes. ACS Appl. Mater. Interf. 2016, 8, 4062–4068. [Google Scholar] [CrossRef]
- Wongso, V.; Sambudi, N.S.; Sufian, S.; Isnaeni; Abdullah, B. The effect of pH in the synthesis of carbon quantum dots from rice husk on their photoluminescence properties. IOP Conf. Ser. Earth Environ. Sci. 2019, 268, 012087. [Google Scholar] [CrossRef]
- Soler, J.M.; Artacho, E.; Gale, J.D.; Garsia, A.; Junquera, J.; Orejon, P.; Sanchez-Portal, D. The SIESTA Method for Ab-Initio Order-N Materials Simulation. J. Phys. Condens. Matter. 2002, 14, 2745. [Google Scholar] [CrossRef]
- Boukhvalov, D.W.; Dreyer, D.R.; Bielawski, C.W.; Son, Y.W. A Computational Investigation of the Catalytic Properties of Graphene Oxide: Exploring Mechanisms by using DFT Methods. ChemCatChem 2012, 4, 1844–1849. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1996, 77, 3865. [Google Scholar] [CrossRef]
- Dion, M.; Rydberg, H.; Schröder, E.; Langreth, D.C.; Lundqvist, B.I. Van der Waals density functional for general geometries. Phys. Rev. Lett. 2004, 92, 246401. [Google Scholar] [CrossRef] [PubMed]
- Troullier, O.N.; Martins, J.L. Efficient Pseudopotentials for Plane-Wave Calculations. Phys. Rev. B 1991, 43, 1993. [Google Scholar] [CrossRef] [PubMed]
- Miao, X.; Qu, D.; Yang, D.; Nie, B.; Zhao, Y.; Fan, H.; Sun, Z. Synthesis of carbon dots with multiple color emission by controlled graphitization and surface functionalization. Adv. Mater. 2018, 30, 1704740. [Google Scholar] [CrossRef] [PubMed]
- Boukhvalov, D.W.; Feng, X.; Müllen, K. First-principles modeling of the polycyclic aromatic hydrocarbons reduction. J. Phys. Chem. C 2011, 115, 16001–16005. [Google Scholar] [CrossRef]
- Zhu, Y.; Wang, J. Helical Synthetic Nanographenes with Atomic Precision. Acc. Chem. Res. 2023, 56, 363–373. [Google Scholar] [CrossRef]
- Haynes, W.M. CRC Handbook of Chemistry and Physics; CRC Press: Boca Raton, FL, USA, 2014. [Google Scholar]
- Lee, J.-K.; Kim, J.-G.; Hembram, K.P.S.S.; Kim, Y.-I.; Min, B.-K.; Park, Y.; Lee, J.-K.; Moon, D.J.; Lee, W.; Lee, S.-G.; et al. The Nature of Metastable AA’ Graphite: Low Dimensional Nano- and Single-Crystalline Forms. Sci. Rep. 2016, 6, 39624. [Google Scholar] [CrossRef]

| Number | Reference | Co-Precursor | Approximate Ratio of XPS Peak Intensities for O1s and N1s Signals | FTIR, Registered Vibrations from Surface Functional Groups | HRTEM, Period of Lattice Fringes (nm) | |
|---|---|---|---|---|---|---|
| O1s C=O/C–O | N1s G:P:A | |||||
| 1 | [30] | water | 1:4 1:3 | 1:4:0 1:3:0 | –OH, CN, –NH2, –CH | 0.21 |
| 2 | [31] | ethanol | 6:4 | 1:2:7 | –OH | 0.21 |
| 3 | [32] | catechol | only C=O | 3:1:6 | –OH, NH, C=N (C=O), CN, CO, CH | 0.24 |
| 4 | [33] | acids | from 4:1 to 1:1 | from 9:0:1 to 4:0:6 8:0:1 to 4:0:6 | –OH, C-N=, C=O, –NH, C–O, | 0.206–0.208 |
| 5 | [34] | benzoic acid, urea | none | 1:2:1 | –OH, –NH2, C–N, C–H, C=C, imidazole and protonated amines, C=O/N–C | --- |
| 6 | [35] | ethanol, potassium bisulfate | none | none | –OH, N–H, C–H, C=O, N–H, C–NH–C, aromatic –CH, C–C, C=C | 0.19 |
| 7 | [36] | water | 1:5 | 5:1:0 | --- | --- |
| 8 | [37] | acids | 3:2 2:3 | 1: 2:0 | –OH, C=C, C–N=C, C2–N–H | --- |
| 9 | [38] | acids | --- | --- | –OH, C–H, O–H, N–H, C=O, C–N, C–O | --- |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boukhvalov, D.W.; Osipov, V.Y. First-Principles Modeling of Bottom-Up Synthesis of Carbon Quantum Dots. Crystals 2023, 13, 716. https://doi.org/10.3390/cryst13050716
Boukhvalov DW, Osipov VY. First-Principles Modeling of Bottom-Up Synthesis of Carbon Quantum Dots. Crystals. 2023; 13(5):716. https://doi.org/10.3390/cryst13050716
Chicago/Turabian StyleBoukhvalov, Danil W., and Vladimir Yu. Osipov. 2023. "First-Principles Modeling of Bottom-Up Synthesis of Carbon Quantum Dots" Crystals 13, no. 5: 716. https://doi.org/10.3390/cryst13050716






