Ionic Conductivity of Lithium Phosphides
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Marom, R.; Amalraj, S.F.; Leifer, N.; Jacob, D.; Aurbach, D. A review of advanced and practical lithium battery materials. J. Mater. Chem. 2011, 21, 9938–9954. [Google Scholar] [CrossRef]
- Nazri, G.A.; Pistoia, G. Lithium Batteries: Science and Technology; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2008. [Google Scholar]
- Girishkumar, G.; McCloskey, B.; Luntz, A.C.; Swanson, S.; Wilcke, W. Lithium- air battery: Promise and challenges. J. Phys. Chem. Lett. 2010, 1, 2193–2203. [Google Scholar] [CrossRef]
- Scrosati, B.; Garche, J. Lithium batteries: Status, prospects and future. J. Power Sources 2010, 195, 2419–2430. [Google Scholar] [CrossRef]
- Armand, M.; Tarascon, J.M. Building better batteries. Nature 2008, 451, 652–657. [Google Scholar] [CrossRef]
- Kim, T.H.; Park, J.S.; Chang, S.K.; Choi, S.; Ryu, J.H.; Song, H.K. The current move of lithium ion batteries towards the next phase. Adv. Energy Mater. 2012, 2, 860–872. [Google Scholar] [CrossRef]
- Goodenough, J.B.; Park, K.S. The Li-ion rechargeable battery: A perspective. J. Am. Chem. Soc. 2013, 135, 1167–1176. [Google Scholar] [CrossRef]
- Etacheri, V.; Marom, R.; Elazari, R.; Salitra, G.; Aurbach, D. Challenges in the development of advanced Li-ion batteries: A review. Energy Environ. Sci. 2011, 4, 3243–3262. [Google Scholar] [CrossRef]
- Goriparti, S.; Miele, E.; De Angelis, F.; Di Fabrizio, E.; Zaccaria, R.P.; Capiglia, C. Review on recent progress of nanostructured anode materials for Li-ion batteries. J. Power Sources 2014, 257, 421–443. [Google Scholar] [CrossRef]
- Winter, M.; Brodd, R.J. What are batteries, fuel cells, and supercapacitors? Chem. Rev. 2004, 104, 4245–4270. [Google Scholar] [CrossRef]
- Cheng, F.; Liang, J.; Tao, Z.; Chen, J. Functional materials for rechargeable batteries. Adv. Mater. 2011, 23, 1695–1715. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Wang, Z.; Chen, L.; Huang, X. Research on advanced materials for Li-ion batteries. Adv. Mater. 2009, 21, 4593–4607. [Google Scholar] [CrossRef]
- Goodenough, J.B.; Kim, Y. Challenges for rechargeable Li batteries. Chem. Mater. 2010, 22, 587–603. [Google Scholar] [CrossRef]
- Tarascon, J.M.; Armand, M. Issues and challenges facing rechargeable lithium batteries. Nature 2001, 414, 359–367. [Google Scholar] [CrossRef]
- Xu, B.; Qian, D.; Wang, Z.; Meng, Y.S. Recent progress in cathode materials research for advanced lithium ion batteries. Mater. Sci. Eng. R Rep. 2012, 73, 51–65. [Google Scholar] [CrossRef]
- Li, J.; Barillas, J.K.; Guenther, C.; Danzer, M.A. A comparative study of state of charge estimation algorithms for LiFePO4 batteries used in electric vehicles. J. Power Sources 2013, 230, 244–250. [Google Scholar] [CrossRef]
- Chacko, S.; Chung, Y.M. Thermal modelling of Li-ion polymer battery for electric vehicle drive cycles. J. Power Sources 2012, 213, 296–303. [Google Scholar] [CrossRef]
- Dunn, J.; Gaines, L.; Kelly, J.; James, C.; Gallagher, K. The significance of Li-ion batteries in electric vehicle life-cycle energy and emissions and recycling’s role in its reduction. Energy Environ. Sci. 2015, 8, 158–168. [Google Scholar] [CrossRef]
- Fathabadi, H. Combining a proton exchange membrane fuel cell (PEMFC) stack with a Li-ion battery to supply the power needs of a hybrid electric vehicle. Renew. Energy 2019, 130, 714–724. [Google Scholar] [CrossRef]
- Hannan, M.A.; Hoque, M.M.; Hussain, A.; Yusof, Y.; Ker, P.J. State-of-the-art and energy management system of lithium-ion batteries in electric vehicle applications: Issues and recommendations. IEEE Access 2018, 6, 19362–19378. [Google Scholar] [CrossRef]
- Lin, D.; Liu, Y.; Cui, Y. Reviving the lithium metal anode for high-energy batteries. Nat. Nanotechnol. 2017, 12, 194–206. [Google Scholar] [CrossRef]
- Leisegang, T.; Meutzner, F.; Zschornak, M.; Münchgesang, W.; Schmid, R.; Nestler, T.; Eremin, R.A.; Kabanov, A.A.; Blatov, V.A.; Meyer, D.C. The aluminum-ion battery: A sustainable and seminal concept? Front. Chem. 2019, 7, 268. [Google Scholar] [CrossRef] [PubMed]
- Duehnen, S.; Betz, J.; Kolek, M.; Schmuch, R.; Winter, M.; Placke, T. Toward green battery cells: Perspective on materials and technologies. Small Methods 2020, 4, 2000039. [Google Scholar] [CrossRef]
- Nitta, N.; Yushin, G. High-capacity anode materials for lithium-ion batteries: Choice of elements and structures for active particles. Part. Part. Syst. Charact. 2014, 31, 317–336. [Google Scholar] [CrossRef]
- Obrovac, M.; Christensen, L. Structural changes in silicon anodes during lithium insertion/extraction. Electrochem. Solid-State Lett. 2004, 7, A93. [Google Scholar] [CrossRef]
- Xu, W.; Wang, J.; Ding, F.; Chen, X.; Nasybulin, E.; Zhang, Y.; Zhang, J.G. Lithium metal anodes for rechargeable batteries. Energy Environ. Sci. 2014, 7, 513–537. [Google Scholar] [CrossRef]
- Famprikis, T.; Canepa, P.; Dawson, J.A.; Islam, M.S.; Masquelier, C. Fundamentals of inorganic solid-state electrolytes for batteries. Nat. Mater. 2019, 18, 1278–1291. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Ma, C.; Chi, M.; Liang, C.; Dudney, N.J. Solid electrolyte: The key for high-voltage lithium batteries. Adv. Energy Mater. 2015, 5, 1401408. [Google Scholar] [CrossRef]
- Sakuda, A.; Kitaura, H.; Hayashi, A.; Tadanaga, K.; Tatsumisago, M. All-solid-state lithium secondary batteries with oxide-coated LiCoO2 electrode and Li2S-P2S5 electrolyte. J. Power Sources 2009, 189, 527–530. [Google Scholar] [CrossRef]
- Sakuda, A.; Hayashi, A.; Tatsumisago, M. Interfacial observation between LiCoO2 electrode and Li2S-P2S5 solid electrolytes of all-solid-state lithium secondary batteries using transmission electron microscopy. Chem. Mater. 2010, 22, 949–956. [Google Scholar] [CrossRef]
- Nagao, M.; Hayashi, A.; Tatsumisago, M.; Kanetsuku, T.; Tsuda, T.; Kuwabata, S. In situ SEM study of a lithium deposition and dissolution mechanism in a bulk-type solid-state cell with a Li2S-P2S5 solid electrolyte. Phys. Chem. Chem. Phys. 2013, 15, 18600–18606. [Google Scholar] [CrossRef]
- Liu, Z.; Fu, W.; Payzant, E.A.; Yu, X.; Wu, Z.; Dudney, N.J.; Kiggans, J.; Hong, K.; Rondinone, A.J.; Liang, C. Anomalous high ionic conductivity of nanoporous β-Li3PS4. J. Am. Chem. Soc. 2013, 135, 975–978. [Google Scholar] [CrossRef]
- Lepley, N.; Holzwarth, N. Modeling interfaces between solids: Application to Li battery materials. Phys. Rev. B 2015, 92, 214201. [Google Scholar] [CrossRef]
- Wenzel, S.; Weber, D.A.; Leichtweiss, T.; Busche, M.R.; Sann, J.; Janek, J. Interphase formation and degradation of charge transfer kinetics between a lithium metal anode and highly crystalline Li7P3S11 solid electrolyte. Solid State Ionics 2016, 286, 24–33. [Google Scholar] [CrossRef]
- Rangasamy, E.; Liu, Z.; Gobet, M.; Pilar, K.; Sahu, G.; Zhou, W.; Wu, H.; Greenbaum, S.; Liang, C. An iodide-based Li7P2S8I superionic conductor. J. Am. Chem. Soc. 2015, 137, 1384–1387. [Google Scholar] [CrossRef] [PubMed]
- Wenzel, S.; Sedlmaier, S.J.; Dietrich, C.; Zeier, W.G.; Janek, J. Interfacial reactivity and interphase growth of argyrodite solid electrolytes at lithium metal electrodes. Solid State Ionics 2018, 318, 102–112. [Google Scholar] [CrossRef]
- Mayo, M.; Griffith, K.J.; Pickard, C.J.; Morris, A.J. Ab initio study of phosphorus anodes for lithium-and sodium-ion batteries. Chem. Mater. 2016, 28, 2011–2021. [Google Scholar] [CrossRef]
- Li, L.; Yu, Y.; Ye, G.J.; Ge, Q.; Ou, X.; Wu, H.; Feng, D.; Chen, X.H.; Zhang, Y. Black phosphorus field-effect transistors. Nat. Nanotechnol. 2014, 9, 372–377. [Google Scholar] [CrossRef]
- Kulish, V.V.; Malyi, O.I.; Persson, C.; Wu, P. Phosphorene as an anode material for Na-ion batteries: A first-principles study. Phys. Chem. Chem. Phys. 2015, 17, 13921–13928. [Google Scholar] [CrossRef]
- Zhao, S.; Kang, W.; Xue, J. The potential application of phosphorene as an anode material in Li-ion batteries. J. Mater. Chem. A 2014, 2, 19046–19052. [Google Scholar] [CrossRef]
- Sun, J.; Lee, H.W.; Pasta, M.; Yuan, H.; Zheng, G.; Sun, Y.; Li, Y.; Cui, Y. A phosphorene–graphene hybrid material as a high-capacity anode for sodium-ion batteries. Nat. Nanotechnol. 2015, 10, 980–985. [Google Scholar] [CrossRef]
- Fu, Y.; Wei, Q.; Zhang, G.; Sun, S. Advanced phosphorus-based materials for lithium/sodium-ion batteries: Recent developments and future perspectives. Adv. Energy Mater. 2018, 8, 1703058. [Google Scholar] [CrossRef]
- Amine, R.; Daali, A.; Zhou, X.; Liu, X.; Liu, Y.; Ren, Y.; Zhang, X.; Zhu, L.; Al-Hallaj, S.; Chen, Z.; et al. A practical phosphorus-based anode material for high-energy lithium-ion batteries. Nano Energy 2020, 74, 104849. [Google Scholar] [CrossRef]
- Fang, K.; Liu, D.; Xiang, X.; Zhu, X.; Tang, H.; Qu, D.; Xie, Z.; Li, J.; Qu, D. Air-stable red phosphorus anode for potassium/sodium-ion batteries enabled through dual-protection design. Nano Energy 2020, 69, 104451. [Google Scholar] [CrossRef]
- Feng, W.; Wang, H.; Jiang, Y.; Zhang, H.; Luo, W.; Chen, W.; Shen, C.; Wang, C.; Wu, J.; Mai, L. A Strain-Relaxation Red Phosphorus Freestanding Anode for Non-Aqueous Potassium Ion Batteries. Adv. Energy Mater. 2022, 12, 2103343. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmüller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 1996, 54, 11169. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmüller, J. Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set. Comput. Mater. Sci. 1996, 6, 15–50. [Google Scholar] [CrossRef]
- Blöchl, P.E. Projector augmented-wave method. Phys. Rev. B 1994, 50, 17953. [Google Scholar] [CrossRef]
- Kresse, G.; Joubert, D. From ultrasoft pseudopotentials to the projector augmented-wave method. Phys. Rev. B 1999, 59, 1758. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 1996, 77, 3865. [Google Scholar] [CrossRef]
- Parlinski, K.; Li, Z.Q.; Kawazoe, Y. First-Principles Determination of the Soft Mode in Cubic ZrO2. Phys. Rev. Lett. 1997, 78, 4063–4066. [Google Scholar] [CrossRef]
- Togo, A.; Tanaka, I. First principles phonon calculations in materials science. Scr. Mater. 2015, 108, 1–5. [Google Scholar] [CrossRef]
- Kresse, G.; Hafner, J. Ab initio molecular dynamics for liquid metals. Phys. Rev. B 1993, 47, 558. [Google Scholar] [CrossRef] [PubMed]
- Evans, D.J. Computer “experiment” for nonlinear thermodynamics of Couette flow. J. Chem. Phys. 1983, 78, 3297–3302. [Google Scholar] [CrossRef]
- Hoover, W.G.; Ladd, A.J.; Moran, B. High-strain-rate plastic flow studied via nonequilibrium molecular dynamics. Phys. Rev. Lett. 1982, 48, 1818. [Google Scholar] [CrossRef]
- Shapeev, A.V. Moment tensor potentials: A class of systematically improvable interatomic potentials. Multiscale Model. Simul. 2016, 14, 1153–1173. [Google Scholar] [CrossRef]
- Zuo, Y.; Chen, C.; Li, X.; Deng, Z.; Chen, Y.; Behler, J.; Csányi, G.; Shapeev, A.V.; Thompson, A.P.; Wood, M.A.; et al. Performance and cost assessment of machine learning interatomic potentials. J. Phys. Chem. A 2020, 124, 731–745. [Google Scholar] [CrossRef] [PubMed]
- Podryabinkin, E.V.; Tikhonov, E.V.; Shapeev, A.V.; Oganov, A.R. Accelerating crystal structure prediction by machine-learning interatomic potentials with active learning. Phys. Rev. B 2019, 99, 064114. [Google Scholar] [CrossRef]
- Körmann, F.; Kostiuchenko, T.; Shapeev, A.; Neugebauer, J. B2 ordering in body-centered-cubic AlNbTiV refractory high-entropy alloys. Phys. Rev. Mater. 2021, 5, 053803. [Google Scholar] [CrossRef]
- Shapeev, A.V.; Podryabinkin, E.V.; Gubaev, K.; Tasnádi, F.; Abrikosov, I.A. Elinvar effect in β-Ti simulated by on-the-fly trained moment tensor potential. New J. Phys. 2020, 22, 113005. [Google Scholar] [CrossRef]
- Novoselov, I.; Yanilkin, A.; Shapeev, A.; Podryabinkin, E. Moment tensor potentials as a promising tool to study diffusion processes. Comput. Mater. Sci. 2019, 164, 46–56. [Google Scholar] [CrossRef]
- Novikov, I.S.; Suleimanov, Y.V.; Shapeev, A.V. Automated calculation of thermal rate coefficients using ring polymer molecular dynamics and machine-learning interatomic potentials with active learning. Phys. Chem. Chem. Phys. 2018, 20, 29503–29512. [Google Scholar] [CrossRef] [PubMed]
- Rosenbrock, C.W.; Gubaev, K.; Shapeev, A.V.; Pártay, L.B.; Bernstein, N.; Csányi, G.; Hart, G.L. Machine-learned interatomic potentials for alloys and alloy phase diagrams. NPJ Comput. Mater. 2021, 7, 24. [Google Scholar] [CrossRef]
- Novikov, I.S.; Gubaev, K.; Podryabinkin, E.V.; Shapeev, A.V. The MLIP package: Moment tensor potentials with MPI and active learning. Mach. Learn. Sci. Technol. 2020, 2, 025002. [Google Scholar] [CrossRef]
- Podryabinkin, E.V.; Shapeev, A.V. Active learning of linearly parametrized interatomic potentials. Comput. Mater. Sci. 2017, 140, 171–180. [Google Scholar] [CrossRef]
- Plimpton, S. Fast parallel algorithms for short-range molecular dynamics. J. Comput. Phys. 1995, 117, 1–19. [Google Scholar] [CrossRef]
- Thompson, A.P.; Aktulga, H.M.; Berger, R.; Bolintineanu, D.S.; Brown, W.M.; Crozier, P.S.; in’t Veld, P.J.; Kohlmeyer, A.; Moore, S.G.; Nguyen, T.D.; et al. LAMMPS—A flexible simulation tool for particle-based materials modeling at the atomic, meso, and continuum scales. Comput. Phys. Commun. 2022, 271, 108171. [Google Scholar] [CrossRef]
- Hoover, W.G. Constant-pressure equations of motion. Phys. Rev. A 1986, 34, 2499. [Google Scholar] [CrossRef] [PubMed]
- Evans, D.J.; Holian, B.L. The nose–hoover thermostat. J. Chem. Phys. 1985, 83, 4069–4074. [Google Scholar] [CrossRef]
- Saal, J.E.; Kirklin, S.; Aykol, M.; Meredig, B.; Wolverton, C. Materials design and discovery with high-throughput density functional theory: The open quantum materials database (OQMD). Jom 2013, 65, 1501–1509. [Google Scholar] [CrossRef]
- Kirklin, S.; Saal, J.E.; Meredig, B.; Thompson, A.; Doak, J.W.; Aykol, M.; Rühl, S.; Wolverton, C. The Open Quantum Materials Database (OQMD): Assessing the accuracy of DFT formation energies. NPJ Comput. Mater. 2015, 1, 15010. [Google Scholar] [CrossRef]
- Jain, A.; Ong, S.P.; Hautier, G.; Chen, W.; Richards, W.D.; Dacek, S.; Cholia, S.; Gunter, D.; Skinner, D.; Ceder, G.; et al. Commentary: The Materials Project: A materials genome approach to accelerating materials innovation. APL Mater. 2013, 1, 011002. [Google Scholar] [CrossRef]
- Sen, R.; Johari, P. Comment on “Structure Prediction of Li-Sn and Li-Sb Intermetallics for Lithium-Ion Batteries Anodes”. arXiv 2018, arXiv:1807.10540. [Google Scholar]
- Mayo, M.; Morris, A.J. Structure prediction of Li–Sn and Li–Sb intermetallics for lithium-ion batteries anodes. Chem. Mater. 2017, 29, 5787–5795. [Google Scholar] [CrossRef]
- Fei, G.; Duan, S.; Zhang, M.; Ren, Z.; Cui, Y.; Chen, X.; Liu, Y.; Yi, W.; Liu, X. Predicted stable Li5P2 and Li4P at ambient pressure: Novel high-performance anodes for lithium-ion batteries. Phys. Chem. Chem. Phys. 2020, 22, 19172–19177. [Google Scholar] [CrossRef] [PubMed]
- Oganov, A.R.; Glass, C.W. Crystal structure prediction using ab initio evolutionary techniques: Principles and applications. He J. Chem. Phys. 2006, 124, 244704. [Google Scholar] [CrossRef] [PubMed]
- Oganov, A.R.; Lyakhov, A.O.; Valle, M. How Evolutionary Crystal Structure Prediction Works and Why. Acc. Chem. Res. 2011, 44, 227–237. [Google Scholar] [CrossRef]
- Lyakhov, A.O.; Oganov, A.R.; Stokes, H.T.; Zhu, Q. New developments in evolutionary structure prediction algorithm USPEX. Comput. Phys. Commun. 2013, 4, 1172–1182. [Google Scholar] [CrossRef]
- Dong, Y.; DiSalvo, F.J. Reinvestigation of trilithium phosphide, Li3P. Acta Crystallogr. Sect. E Struct. Rep. Online 2007, 63, i97–i98. [Google Scholar] [CrossRef]
- Hönle, W.; von Schnering, H.G. Chemistry and Structural Chemistry of Phosphides and Polyphosphides 23* Zur Struktur von LiP und KSb. Z. Krist. Cryst. Mater. 1981, 155, 307–314. [Google Scholar] [CrossRef]
- Manriquez, V.; Hönle, W.; von Schnering, H.G. Zur Chemie und Strukturchemie von Phosphiden und Polyphosphiden. 42. Trilithiumheptaphosphid Li3P7: Darstellung, Struktur und Eigenschaften. Z. Anorg. Allg. Chem. 1986, 539, 95–109. [Google Scholar] [CrossRef]
- Wichelhaus, W. Die Lithiumphosphide LiP5 und LiP7. Naturwissenschaften 1972, 59, 78–79. [Google Scholar]
- Ozisik, H.; Colakoglu, K.; Deligoz, E.; Ozisik, H. First principles study on the structural, electronic, and elastic properties of Na–As systems. Solid State Commun. 2011, 151, 1349–1354. [Google Scholar] [CrossRef]
- Wegner, F.; Kamm, F.; Pielnhofer, F.; Pfitzner, A. Li3As and Li3P revisited: DFT modelling on phase stability and ion conductivity. Z. Anorg. Allg. Chem. 2022, 648, e202100358. [Google Scholar]
- Nazri, G. Preparation, structure and ionic conductivity of lithium phosphide. Solid State Ionics 1989, 34, 97–102. [Google Scholar] [CrossRef]
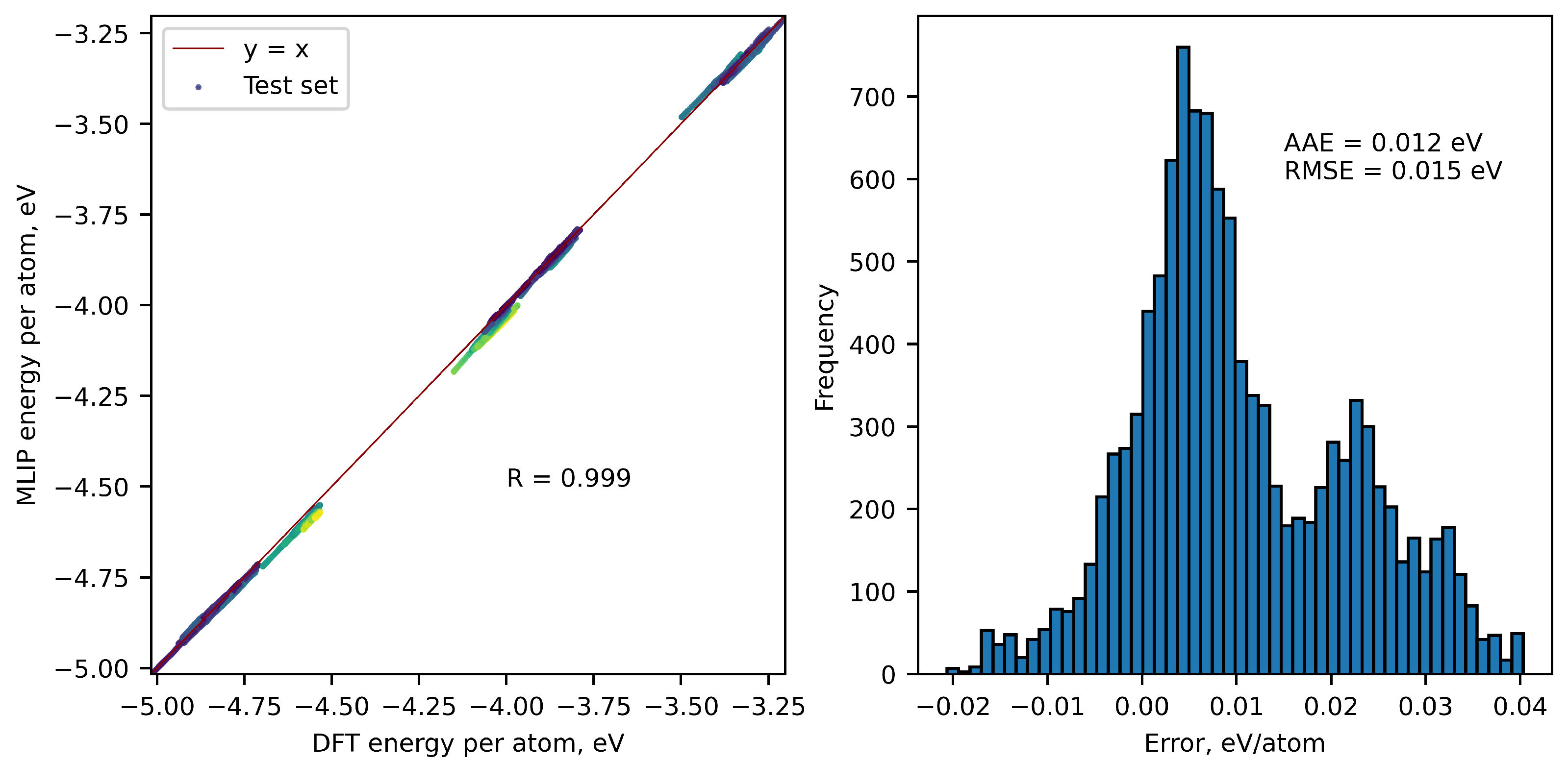

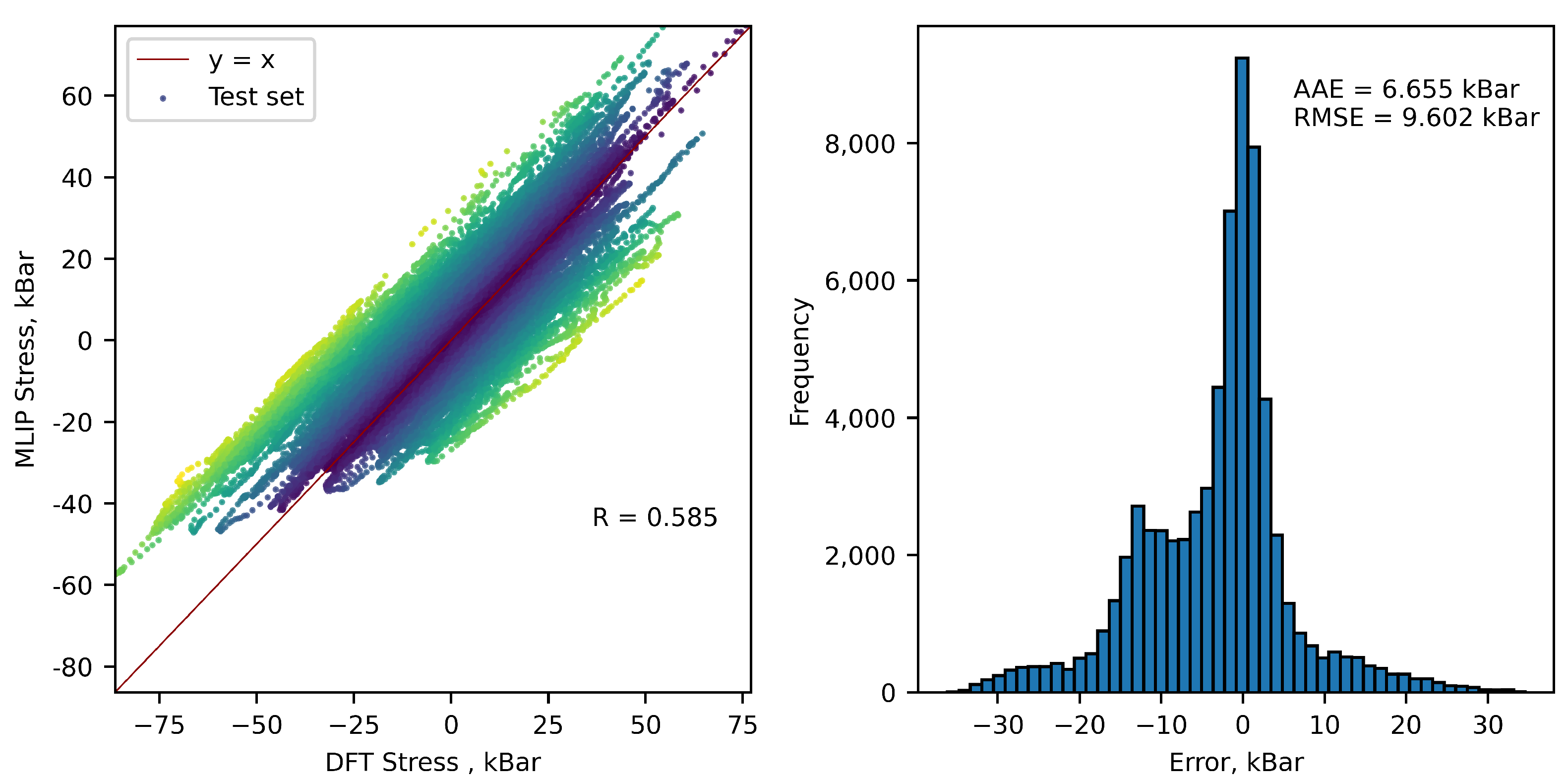


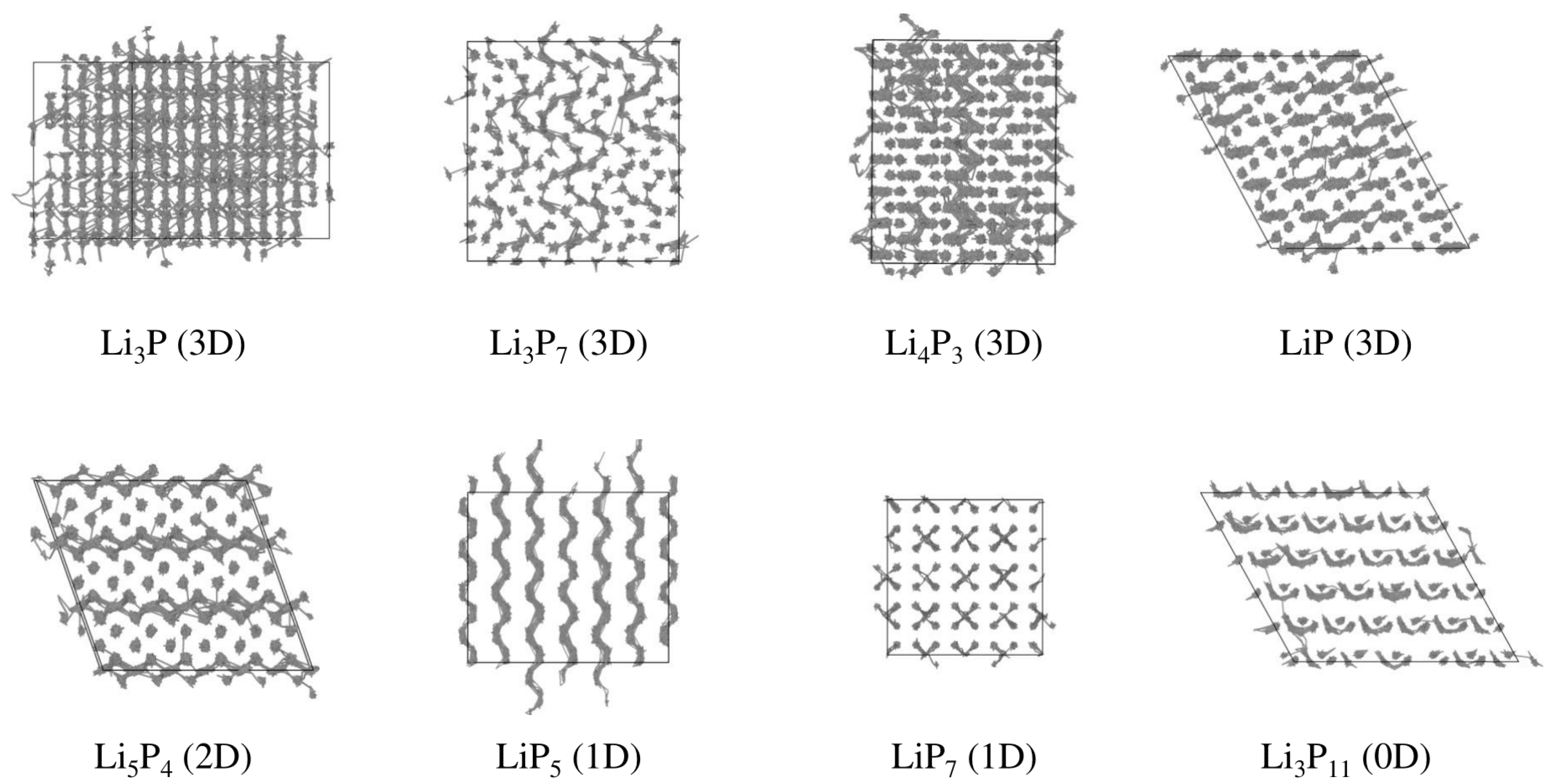
| Number of configurations | 1047 | ||
|---|---|---|---|
| MAE | AAD | RMS | |
| Errors in energy per atom, eV/atom | 0.063 | 0.0145 | 0.0185 |
| Range of energies per atom in training set, eV/atom | −5.09: −3.20 | ||
| Errors in forces, eV/ Å | 1.97 | 0.27 | 0.335 |
| Range of forces in training set, eV/ Å | −10.86: +18.18 | ||
| Errors in stresses, kBar | 37.76 | 5.576 | 8.0 |
| Range of stresses in training set, kBar | −84.1: +98.2 | ||
| Super Cell | Lattice Parameters | N | ||||||
|---|---|---|---|---|---|---|---|---|
| a, Å | b, Å | c, Å | , deg | , deg | , deg | |||
| LiP | 6 × 6 × 3 | 25.36 | 25.36 | 22.66 | 90 | 90 | 120 | 864 |
| LiP | 4 × 3 × 3 | 30.52 | 29.63 | 31.71 | 90 | 90 | 90 | 1440 |
| LiP | 3 × 5 × 5 | 39.33 | 30.21 | 33.94 | 90 | 118.95 | 90 | 1680 |
| LiP | 2 × 7 × 3 | 22.33 | 27.17 | 30.52 | 90 | 90 | 90 | 1176 |
| LiP | 3 × 7 × 3 | 26.59 | 27.84 | 25.45 | 90 | 109.37 | 90 | 1134 |
| LiP | 5 × 5 × 3 | 27.975 | 24.82 | 30.77 | 90 | 118.16 | 90 | 1200 |
| LiP | 3 × 5 × 4 | 31.45 | 33.39 | 26.7 | 90 | 90 | 90 | 1440 |
| LiP | 2 × 2 × 2 | 27.29 | 27.29 | 29.74 | 90 | 90 | 90 | 1024 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maltsev, A.P.; Chepkasov, I.V.; Kvashnin, A.G.; Oganov, A.R. Ionic Conductivity of Lithium Phosphides. Crystals 2023, 13, 756. https://doi.org/10.3390/cryst13050756
Maltsev AP, Chepkasov IV, Kvashnin AG, Oganov AR. Ionic Conductivity of Lithium Phosphides. Crystals. 2023; 13(5):756. https://doi.org/10.3390/cryst13050756
Chicago/Turabian StyleMaltsev, Alexey P., Ilya V. Chepkasov, Alexander G. Kvashnin, and Artem R. Oganov. 2023. "Ionic Conductivity of Lithium Phosphides" Crystals 13, no. 5: 756. https://doi.org/10.3390/cryst13050756
APA StyleMaltsev, A. P., Chepkasov, I. V., Kvashnin, A. G., & Oganov, A. R. (2023). Ionic Conductivity of Lithium Phosphides. Crystals, 13(5), 756. https://doi.org/10.3390/cryst13050756







