Mechanochemically Synthesized Solid Solutions La1−xCexFeO3+x/2 for Activation of Peroxydisulfate in Catalytical Reaction for Tetracycline Degradation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Mechanochemical Synthesis of the Samples
2.3. Methods for Characterization
2.4. Tetracycline Hydrochloride (TCH) Degradation
3. Results
3.1. Characterization of the Samples
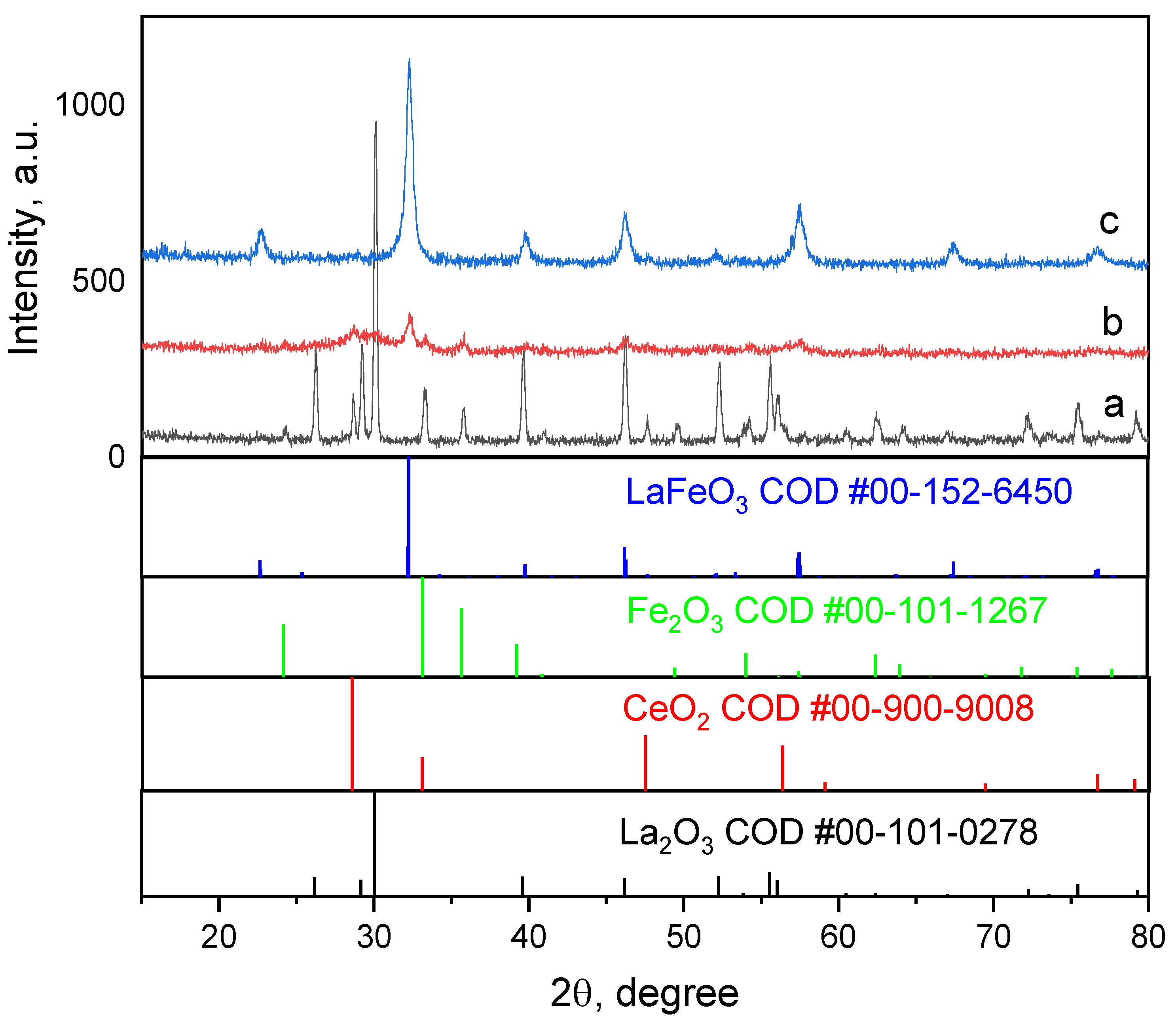
3.1.1. Phase Homogeneity
3.1.2. Morphology of the Samples
3.1.3. Textural Characteristics of the Samples
3.1.4. UV/Vis Spectroscopy
3.2. Catalytic Decomposition of TCH
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bahuguna, A.; Singh, S.K.; Bahuguna, A.; Sharma, S.; Dadarwal, B.K. Physical method of wastewater treatment—A review. J. Res. Environ. Earth Sci. 2021, 7, 29–36. [Google Scholar]
- Hasan, H.A.; Muhammad, M.H.; Ismail, N.I. A review of biological drinking water treatment technologies for contaminants removal from polluted water resources. J. Water Process Eng. 2020, 33, 101035. [Google Scholar] [CrossRef]
- Zahmatkesh, S.; Keshteli, M.H.; Bokhari, A.; Sundaramurthy, S.; Panneerselvam, B.; Rezakhani, Y. Wastewater treatment with nanomaterials for the future: A state-of-the-art review. Environ. Res. 2023, 216, 114652. [Google Scholar] [CrossRef] [PubMed]
- Ghernaout, D.; Elboughdiri, N. Advanced Oxidation Processes for Wastewater Treatment: Facts and Future Trends. Open Access Libr. J. 2020, 7, e6139. [Google Scholar] [CrossRef]
- Xiao, R.; Luo, Z.; Wei, Z.; Luo, S.; Spinney, R.; Yang, W.; Dionysiou, D.D. Activation of peroxymonosulfate/persulfate by nanomaterials for sulfate radical-based advanced oxidation technologies. Curr. Opin. Chem. Eng. 2018, 19, 51–58. [Google Scholar] [CrossRef]
- Zheng, X.; Niu, X.; Zhang, D.; Lv, M.; Ye, X.; Ma, J.; Lin, Z.; Fu, M. Metal-based catalysts for persulfate and peroxymonosulfate activation in heterogeneous ways: A review. Chem. Eng. J. 2022, 429, 132323. [Google Scholar] [CrossRef]
- Wang, J.; Wang, S. Activation of persulfate (PS) and peroxymonosulfate (PMS) and application for the degradation of emerging contaminants. Chem. Eng. J. 2018, 334, 1502–1517. [Google Scholar] [CrossRef]
- Ghanbari, F.; Moradi, M. Application of peroxymonosulfate and its activation methods for degradation of environmental organic pollutants: Review. Chem. Eng. J. 2017, 310, 41–62. [Google Scholar] [CrossRef]
- Oh, W.-D.; Lim, T.-T. Design and application of heterogeneous catalysts as peroxydisulfate activator for organics removal: An overview. Chem. Eng. J. 2019, 358, 110–133. [Google Scholar] [CrossRef]
- Arman, M.M.; El-Dek, S.I. Role of annealing temperature in tailoring Ce-doped LaFeO3 features. J. Phys. Chem. Solids 2021, 152, 109980. [Google Scholar] [CrossRef]
- Arima, T.; Tokura, Y.; Torrance, J.B. Variation of Optical Gaps in Perovskite-Type 3d Transition—Metal Oxides. Phys. Rev. B 1993, 48, 17006. [Google Scholar] [CrossRef] [PubMed]
- Tijare, S.N.; Joshi, M.V.; Padole, P.S.; Mangrulkar, P.A.; Rayalu, S.S.; Labhsetwar, N.K. Photocatalytic Hydrogen Generation Through Water Splitting on Nano-Crystalline LaFeO3 Perovskite. Int. J. Hydrogen Energy 2012, 37, 10451. [Google Scholar] [CrossRef]
- Wiranwetchayan, O.; Promnopas, S.; Phadungdhitidhada, S.; Phuruangrat, A.; Thongtem, T.; Singjai, P.; Thongtem, S. Characterization of perovskite LaFeO3 synthesized by microwave plasma method for photocatalytic applications. Ceram. Int. 2019, 45, 4802–4809. [Google Scholar] [CrossRef]
- Dumitru, R.; Negrea, S.; Ianculescu, A.; Păcurariu, C.; Vasile, B.; Surdu, A.; Manea, F. Lanthanum Ferrite Ceramic Powders: Synthesis, Characterization and Electrochemical Detection Application. Materials 2020, 13, 2061. [Google Scholar] [CrossRef] [PubMed]
- Andoulsin, R.; Horchani-Naifer, K.; Férid, M. Preparation of lanthanum ferrite powder at low temperature. Ceramica 2012, 58, 126–130. [Google Scholar] [CrossRef]
- Bhargav, K.K.; Ram, S.; Majumder, S.B. Physics of the multi-functionality of lanthanum ferrite ceramics. J. Appl. Phys. 2014, 115, 204109. [Google Scholar] [CrossRef]
- Shen, H.; Cheng, G.; Wu, A.; Xu, J.; Zhao, J. Combustion synthesis and characterization of nano-crystalline LaFeO3 powder. Phys. Status Solidi A 2009, 206, 1420–1424. [Google Scholar] [CrossRef]
- Humayun, M.; Ullah, H.; Usman, M.; Habibi-Yangjeh, A.; Tahir, A.A.; Wang, C.; Luo, W. Perovskite-type lanthanum ferrite based photocatalysts: Preparation, properties, and applications. J. Energy Chem. 2022, 66, 314–338. [Google Scholar] [CrossRef]
- Andoulsin, R.; Naifer, K.H.; Ferid, M. Electrical conductivity of La1−xCaxFeO3−δ solid solutions. Ceram. Int. 2013, 39, 6527–6531. [Google Scholar] [CrossRef]
- Rai, A.; Thakur, A.K. Influence of co-substitution driven property tailoring in lanthanum orthoferrites (LaFeO3). Ceram. Int. 2017, 43, 13828–13838. [Google Scholar] [CrossRef]
- Wen, Y.; Zhang, C.; He, H.; Yu, Y.; Teraoka, Y. Catalytic oxidation of nitrogen monoxide over La1−xCexCoO3 perovskites. Catal. Today 2007, 126, 400–405. [Google Scholar] [CrossRef]
- Saikia, N.; Chakravarty, R.; Bhattacharjee, S.; Hota, R.L.; Parida, R.K.; Parida, B.N. Synthesis and characterization of Gd-doped LaFeO3 for device application. Mater. Sci. Semicond. Process. 2022, 151, 106969. [Google Scholar] [CrossRef]
- Prasad, B.V.; Rao, B.V.; Narsaiah, K.; Rao, G.N.; Chen, J.W.; Babu, D.S. Preparation and characterization of perovskite Cu doped LaFeO3 semiconductor ceramics. IOP Conf. Ser. Mater. Sci. Eng. 2015, 73, 012129. [Google Scholar] [CrossRef]
- Hunpratub, S.; Karaphun, A.; Phokh, S.; Swatsitang, E. Optical and magnetic properties of La1−xGaxFeO3 nanoparticles synthesized by polymerization complex method. Appl. Surf. Sci. 2016, 380, 52–59. [Google Scholar] [CrossRef]
- Janbutrach, Y.; Hunpratub, S.; Swatsitang, E. Ferromagnetism and optical properties of La1 − xAlxFeO3 nanopowders. Nanoscale Res. Let. 2014, 9, 498. [Google Scholar] [CrossRef]
- Barbero, P.; Gambo, J.A.; Cadús, L.E. Synthesis and characterisation of La1−xCaxFeO3 perovskite-type oxide catalysts for total oxidation of volatile organic compounds. Appl. Catal. B Environ. 2006, 65, 21. [Google Scholar] [CrossRef]
- Bashir, B.; Warsi, M.F.; Khan, M.A.; Akhtar, M.N.; Gilani, Z.A.; Shakir, I.; Wadood, A. Rare earth Tb3+ doped LaFeO3 nanoparticles: New materials for high frequency devices fabrication. Ceram. Int. 2015, 41, 9199–9202. [Google Scholar] [CrossRef]
- Al-Mamari, R.T.; Widatallah, H.M.; Elzain, M.E.; Gismelseed, A.M.; Al-Rawas, A.D.; Al-Harthi, S.H.; Souier, T.M.; Al-Abri, M. Structural, Mössbauer, and optical studies of mechano-synthesized Ru3+-doped LaFeO3 nanoparticles. Hyperfine Interact. 2022, 243, 4. [Google Scholar] [CrossRef]
- Xiang, X.-P.; Zhao, L.-H.; Teng, B.-T.; Lang, J.-J.; Hu, X.; Li, T.; Fang, Y.-A.; Luo, M.-F.; Lin, J.-J. Catalytic combustion of methane on La1−xCexFeO3 oxides. Appl. Surf. Sci. 2013, 276, 328–332. [Google Scholar] [CrossRef]
- Shikha, P.; Kang, T.S.; Randhawa, B.S. Effect of different synthetic routes on the structural, morphological and magnetic properties of Ce doped LaFeO3 nanoparticles. J. Alloys Compd. 2015, 625, 336–345. [Google Scholar] [CrossRef]
- Cotton, S. Lanthanide and Actinide Chemistry; John Wiley & Sons Ltd.: West Sussex, UK, 2006; p. 29. [Google Scholar]
- Nitadori, T.; Misono, M. Catalytic properties of La1-xAxFeO3 (A = Sr, Ce) and La1-xCexCoO3. J. Catal. 1985, 93, 459–466. [Google Scholar] [CrossRef]
- Zhang, Q.; Saito, F. Effect of Fe2O3 crystallite size on its mechanochemical reaction with La2O3 to form LaFeO3. J. Mater. Sci. 2001, 36, 2287–2290. [Google Scholar] [CrossRef]
- Miao, J.; Zhang, R.; Zhang, L. Photocatalytic degradations of three dyes with different chemical structures using ball-milled TiO2. Mater. Res. Bull. 2018, 97, 109–114. [Google Scholar] [CrossRef]
- Wu, D.; Li, C.; Zhang, D.; Wang, L.; Zhang, X.; Shi, Z.; Lin, Q. Photocatalytic improvement of Y3+ modified TiO2 prepared by a ball milling method and application in shrimp wastewater treatment. RSC Adv. 2019, 9, 14609–14620. [Google Scholar] [CrossRef]
- Yin, Z.; Zhang, Q.; Li, S.; Cagnetta, G.; Huang, J.; Deng, S.; Yu, G. Mechanochemical synthesis of catalysts and reagents for water decontamination: Recent advances and perspective. Sci. Total Environ. 2022, 825, 153992. [Google Scholar] [CrossRef] [PubMed]
- Thomas, N.; Dionysiou, D.D.; Pillai, S.C. Heterogeneous Fenton catalysts: A review of recent advances. J. Hazard. Mater. 2021, 404, 124082. [Google Scholar] [CrossRef]
- Liu, J.; Zhou, B.; Zhang, H.; Ma, J.; Mu, B.; Zhang, W. A novel Biochar modified by Chitosan-Fe/S for tetracycline adsorption and studies on site energy distribution. Bioresour. Technol. 2019, 294, 122152. [Google Scholar] [CrossRef]
- Danner, M.-C.; Robertson, A.; Behrends, V.; Reis, J. Antibiotic pollution in surface fresh waters: Occurrence and effects. Sci. Total Environ. 2019, 664, 793–804. [Google Scholar] [CrossRef]
- Xiong, H.; Dong, S.; Zhang, J.; Zhou, D.; Rittmann, B.E. Roles of an easily biodegradable co-substrate in enhancing tetracycline treatment in an intimately coupled photocatalytic-biological reactor. Water Res. 2018, 136, 75–83. [Google Scholar] [CrossRef]
- Xiong, H.; Zou, D.; Zhou, D.; Dong, S.; Wang, J.; Rittmann, B.E. Enhancing degradation and mineralization of tetracycline using intimately coupled photocatalysis and biodegradation (ICPB). Chem. Eng. J. 2017, 316, 7–14. [Google Scholar] [CrossRef]
- Rodriguez-Carvajal, J. Recent developments of the program fullprof. In Newsletter in Commission on Powder Diffraction; IUCr: Chester, UK, 2001; Volume 26, pp. 12–19. [Google Scholar]
- Tauc, J.; Grigorovici, R.; Vancu, A. Optical properties and electronic structure of amorphous germanium. Phys. Status Solidi 1966, 15, 627–637. [Google Scholar] [CrossRef]
- Shanon, R.D. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Cryst. 1976, A32, 751–767. [Google Scholar] [CrossRef]
- Gowri, G.; Saravanan, R.; Sasikumar, S.; Banu, I.B.S. Exchange bias effect, ferroelectric property, primary bonding and charge density analysis of La1-xCexFeO3 multiferroics. Mater. Res. Bull. 2019, 118, 110512. [Google Scholar] [CrossRef]
- Beranek, R. (Photo)electrochemical methods for the determination of the band edge positions of TiO2-based nanomaterials. Adv. Phys. Chem. 2011, 2011, 786759. [Google Scholar] [CrossRef]
- Dimitrov, V.; Sakka, S. Electronic Oxide Polarizability and Optical Basicity of Simple Oxide. J. Appl. Phys. 1996, 79, 1736–1740. [Google Scholar] [CrossRef]
- Feng, Q.; Zhou, J.; Luo, W.; Ding, L.; Cai, W. Photo-Fenton removal of tetracycline hydrochloride using LaFeO3 as a persulfate activator under visible light. Ecotoxicol. Environ. Saf. 2020, 198, 110661. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Wang, Z.; Li, F.; Wang, J.; Xu, N.; Jia, Y.; Gao, S.; Tian, T.; Shen, W. Degradation of tetracycline by activated peroxodisulfate using CuFe2O4-loaded biochar. J. Mol. Liq. 2022, 368 Part A, 120622. [Google Scholar] [CrossRef]
- Zhong, Q.; Lin, Q.; Huang, R.; Fu, H.; Zhang, X.; Luo, H.; Xiao, R. Oxidative degradation of tetracycline using persulfate activated by N and Cu codoped biochar. Chem. Eng. J. 2020, 380, 122608. [Google Scholar] [CrossRef]
- Cheng, G.; Yuan, C.; Ruan, W.; Ma, B.; Zhang, X.; Yuan, X.; Li, Z.; Wang, D.; Teng, F. Visible light enhanced persulfate activation for degradation of tetracycline via boosting adsorption of persulfate by ligand-deficient MIL-101(Fe) icosahedron. Chemosphere 2023, 317, 137857. [Google Scholar] [CrossRef]
- Ge, X.; Meng, G.; Liu, B. Efficient degradation of antibiotics by oxygen vacancy-LaFeO3/polystyrene-driven photo-Fenton system: Highlight the impacts of molecular structures. J. Water Process Eng. 2023, 51, 103428. [Google Scholar] [CrossRef]
- Gao, J.; Sun, Y.; Xiong, R.; Ma, Y.; Wang, L.; Qiao, S.; Zhang, J.; Ji, W.; Li, Y. Strategy for oxygen vacancy enriched CoMn spinel oxide catalyst activated peroxodisulfate for tetracycline degradation: Process, mechanism, and toxicity analysis. RSC Adv. 2023, 13, 11472–11479. [Google Scholar] [CrossRef]
- Schneider, J.T.; Scheres Firak, D.; Ribeiro, R.R.; Peralta-Zamora, P. Use of scavenger agents in heterogeneous photocatalysis: Truths, half-truths, and misinterpretations. Phys. Chem. Chem. Phys. 2020, 22, 15723–15733. [Google Scholar] [CrossRef] [PubMed]
- Rodrıguez, E.M.; Marquez, G.; Tena, M.; Alvarez, P.M.; Beltran, F.J. Determination of main species involved in the first steps of TiO2 photocatalytic degradation of organics with the use of scavengers: The case of ofloxacin. Appl. Catal. B Environ. 2015, 178, 44–53. [Google Scholar] [CrossRef]
- Nimse, S.B.; Pal, D. Free radicals, natural antioxidants, and their reaction mechanisms. RSC Adv. 2015, 5, 27986–28006. [Google Scholar] [CrossRef]
- Wexler, R.B.; Gautam, G.S.; Stechel, E.B.; Carter, E.A. Factors Governing Oxygen Vacancy Formation in Oxide Perovskites. J. Am. Chem. Soc. 2021, 143, 13212–13227. [Google Scholar] [CrossRef] [PubMed]
- Kato, T.; Iwamoto, M.; Tokoro, C. Investigation of cerium reduction efficiency by grinding with microwave irradiation in mechanochemical processing. Minerals 2022, 12, 189. [Google Scholar] [CrossRef]
- Mutter, D.; Schierholz, R.; Urban, D.F.; Heuer, S.A.; Ohlerth, T.; Kungl, H.; Elsässer, C.; Eichel, R.-A. Defects and Phase Formation in Non-Stoichiometric LaFeO3: A Combined Theoretical and Experimental Study. Chem. Mater. 2021, 33, 9473–9485. [Google Scholar] [CrossRef]








| Sample | Unit Cell Parameters, Å | Unit Cell Volume, Å3 | Crystallite Size, nm | Microstrains, ×10−3 a.u. | Dislocation Density, ×10−3 nm−2 | Rwp, % | GOF * |
|---|---|---|---|---|---|---|---|
| LaFeO3 | a = 5.552(4) b = 5.577(4) c = 7.843(5) | 242.9(3) | 14.4(4) | 1.1 | 4.8 | 12.0 | 1.14 |
| La0.99Ce0.01FeO3+x/2 | a = 5.550(4) b = 5.578(5) c = 7.843(5) | 242.8(3) | 14.9(3) | 0.9 | 4.5 | 12.1 | 1.12 |
| La0.97Ce0.03FeO3+x/2 | a = 5.551(3) b = 5.578(3) c = 7.844(4) | 242.9(3) | 15.0(2) | 0.8 | 4.4 | 12.3 | 1.17 |
| La0.95Ce0.05FeO3+x/2 | a = 5.550(5) b = 5.575(7) c = 7.846(9) | 242.8(5) | 16.8(4) | 0.8 | 3.5 | 11.9 | 1.16 |
| La0.93Ce0.07FeO3+x/2 | a = 5.549(5) b = 5.576(6) c = 7.848(7) | 242.8(4) | 16.9(5) | 0.7 | 3.5 | 12.6 | 1.21 |
| Sample | Specific Surface Area SBET, m2/g | Total Pore Volume Vt, cm3/g | Average Pore Size Dav, nm |
|---|---|---|---|
| LaFeO3 | 9 | 0.04 | 19 |
| La0.99Ce0.01FeO3+x/2 | 9 | 0.04 | 16 |
| La0.97Ce0.03FeO3+x/2 | 8 | 0.04 | 18 |
| La0.95Ce0.05FeO3+x/2 | 8 | 0.04 | 19 |
| La0.93Ce0.07FeO3+x/2 | 7 | 0.03 | 15 |
| Sample | Eg, eV/λ, nm | Refractive Index | EVB, eV | ECB, eV |
|---|---|---|---|---|
| LaFeO3 | 2.32/533 | 2.61 | 2.21 | −0.11 |
| La0.99Ce0.01FeO3+x/2 | 2.38/520 | 2.59 | 2.24 | −0.14 |
| La0.97Ce0.03FeO3+x/2 | 2.37/522 | 2.59 | 2.23 | −0.14 |
| La0.95Ce0.05FeO3+x/2 | 2.39/518 | 2.58 | 2.24 | −0.15 |
| La0.93Ce0.07FeO3+x/2 | 2.36/524 | 2.59 | 2.23 | −0.13 |
| Catalyst | Rate Constant, ×10−3 min−1 | R2 | Degradation, % | TOC Removal, % |
|---|---|---|---|---|
| LaFeO3 | 16.4 ± 0.9 | 0.972 | 62.0 | 41.2 ± 0.3 |
| La0.99Ce0.01FeO+x/23 | 18.8 ± 0.6 | 0.989 | 64.6 | 45.1 ± 0.7 |
| La0.97Ce0.03FeO3+x/2 | 20.0 ± 1.1 | 0.975 | 67.3 | 47.0 ± 0.5 |
| La0.95Ce0.05FeO3+x/2 | 22.6 ± 1.4 | 0.969 | 69.1 | 51.2 ± 1.1 |
| La0.93Ce0.07FeO3+x/2 | 29.2 ± 2.5 | 0.946 | 72.9 | 61.0 ± 0.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsvetkov, M.; Encheva, E.; Petrova, S.; Spassova, I.; Milanova, M. Mechanochemically Synthesized Solid Solutions La1−xCexFeO3+x/2 for Activation of Peroxydisulfate in Catalytical Reaction for Tetracycline Degradation. Crystals 2023, 13, 769. https://doi.org/10.3390/cryst13050769
Tsvetkov M, Encheva E, Petrova S, Spassova I, Milanova M. Mechanochemically Synthesized Solid Solutions La1−xCexFeO3+x/2 for Activation of Peroxydisulfate in Catalytical Reaction for Tetracycline Degradation. Crystals. 2023; 13(5):769. https://doi.org/10.3390/cryst13050769
Chicago/Turabian StyleTsvetkov, Martin, Elzhana Encheva, Stefani Petrova, Ivanka Spassova, and Maria Milanova. 2023. "Mechanochemically Synthesized Solid Solutions La1−xCexFeO3+x/2 for Activation of Peroxydisulfate in Catalytical Reaction for Tetracycline Degradation" Crystals 13, no. 5: 769. https://doi.org/10.3390/cryst13050769






