Preparation of Thiadiazole Modified UiO-68-CdS Composites for RhB Degradation under Visible Light Irradiation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and General Methods
2.2. Synthesis of the Complexes
3. Results and Discussion
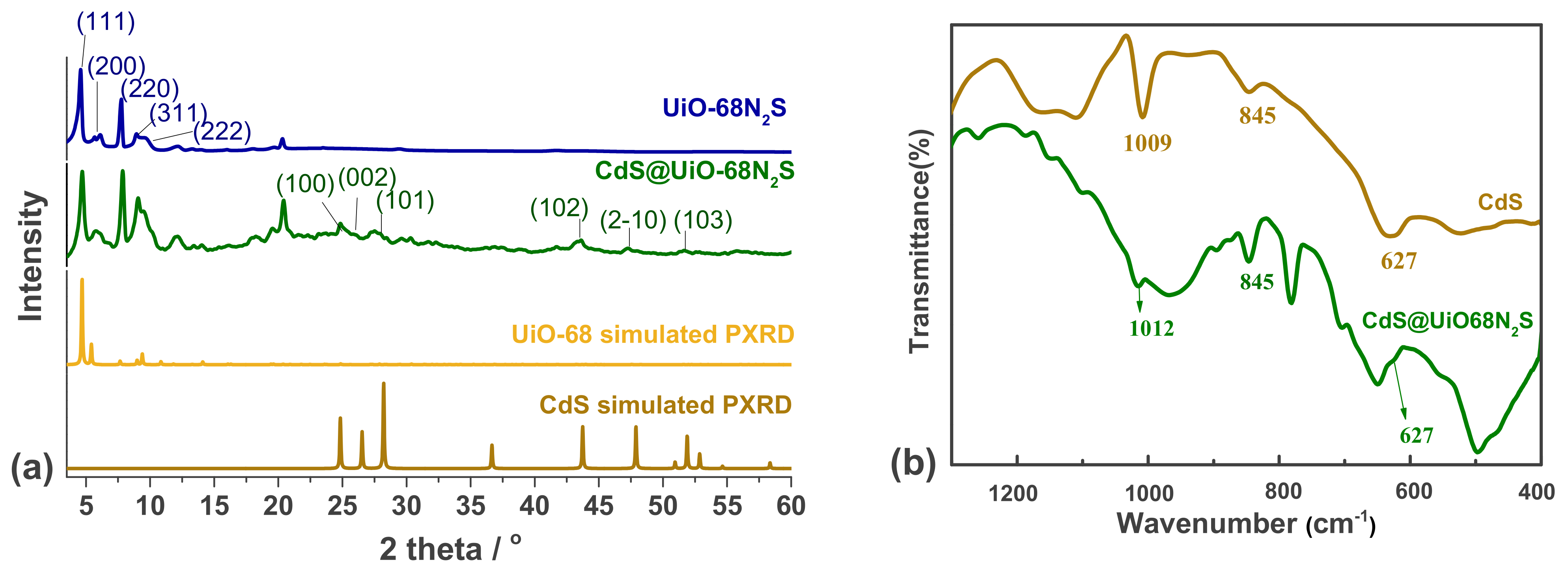
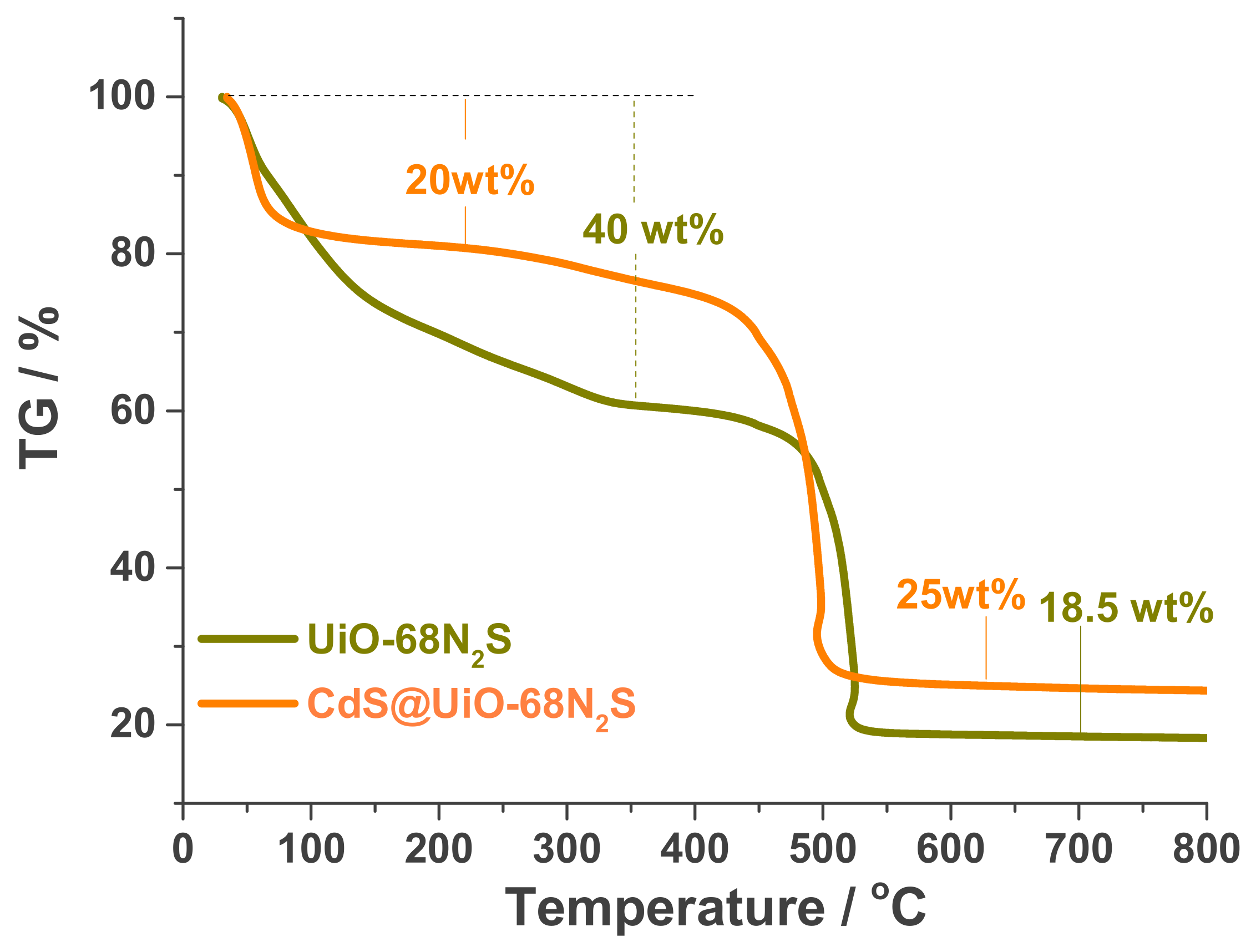
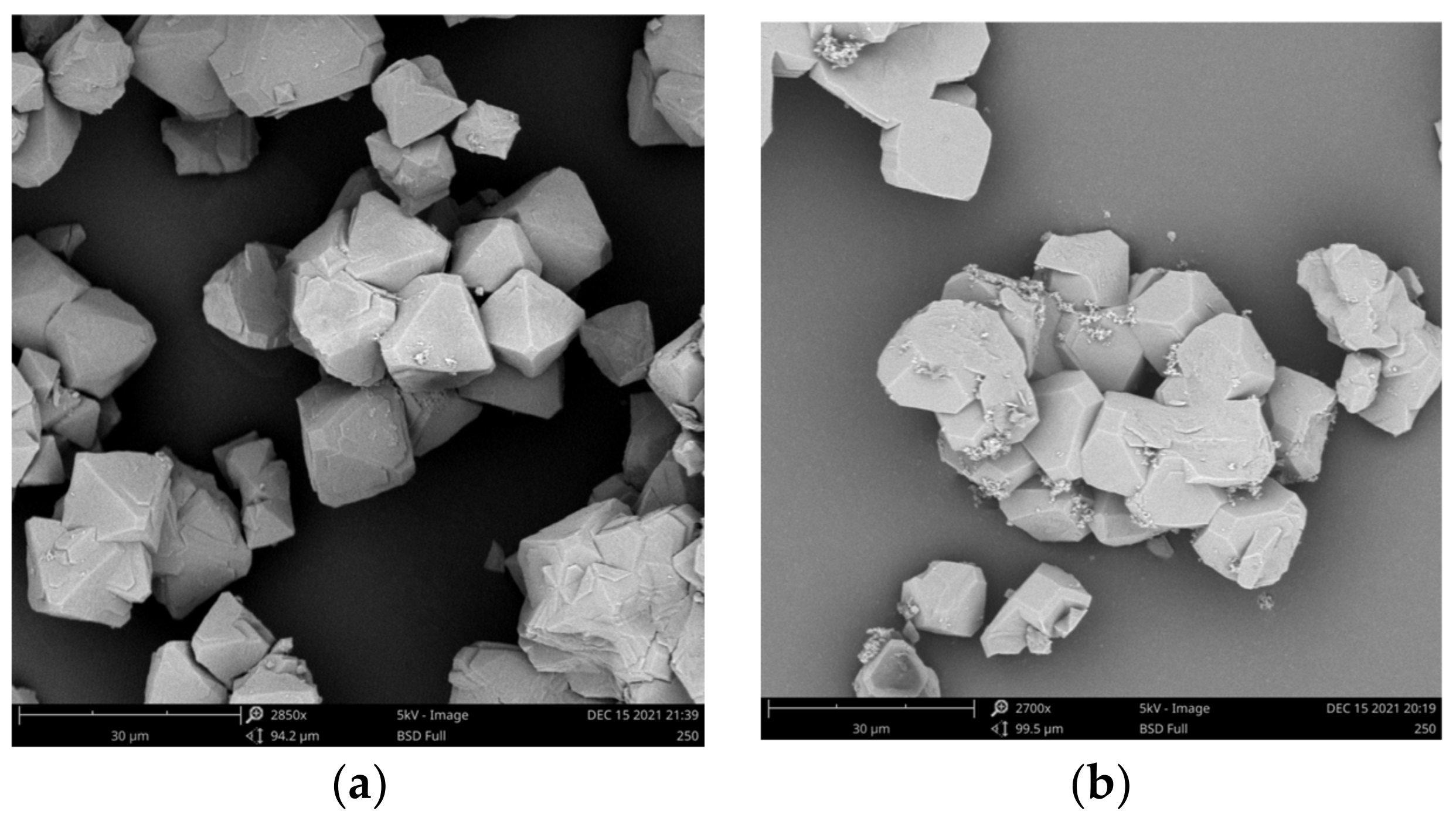
3.1. Characterization
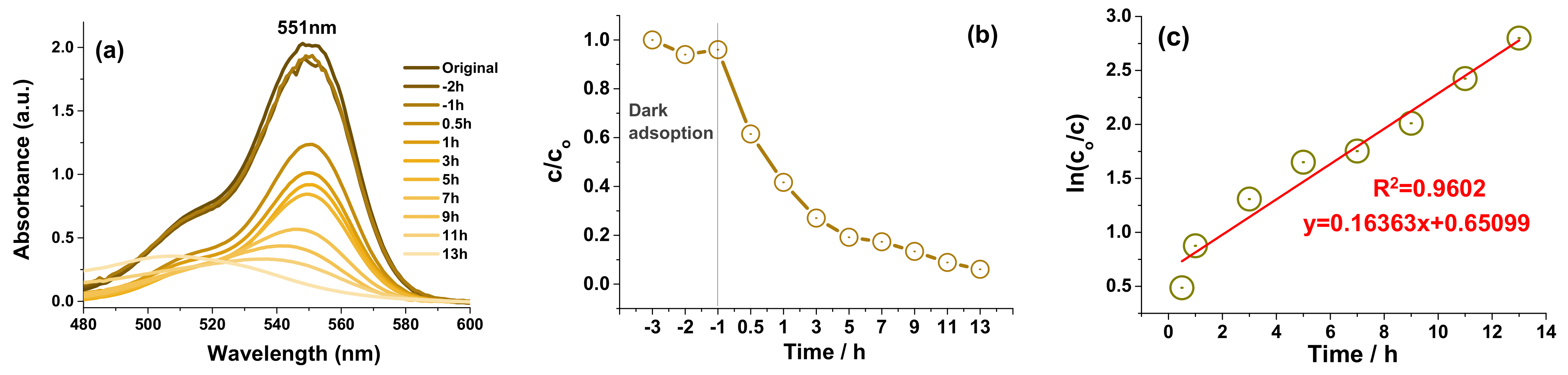
3.2. Catalytic Experiments
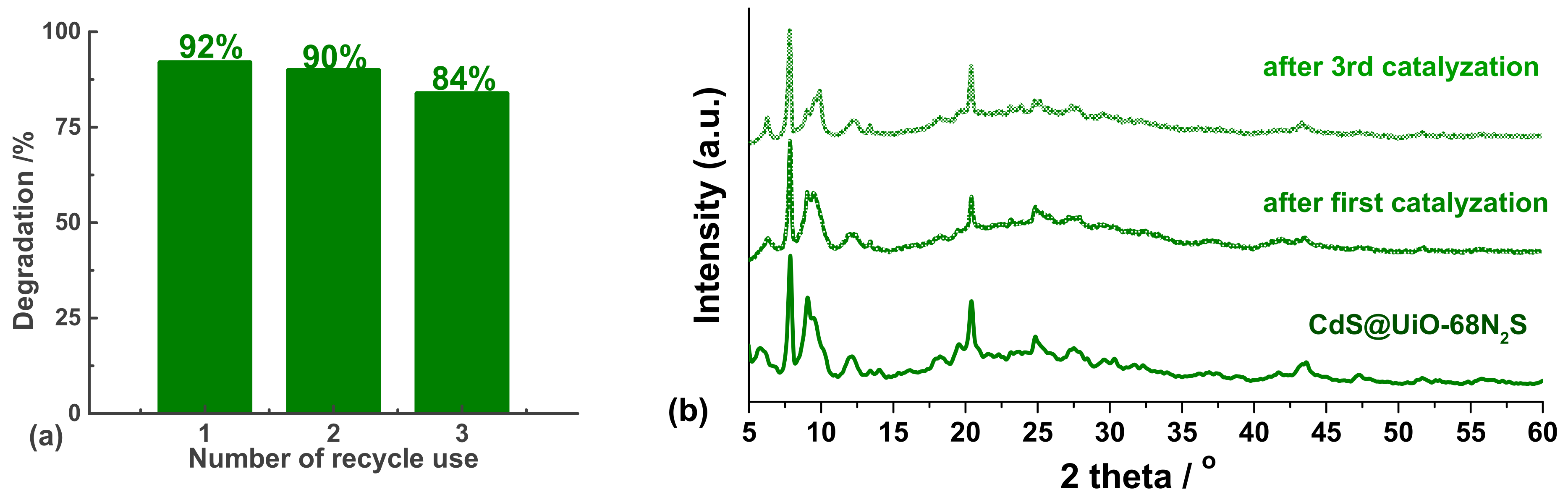
3.3. Photocatalytic Recycle Experiments
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Forgacs, E.; Cserhati, T.; Oros, G. Removal of synthetic dyes from wastewaters: A review. Environ. Int. 2004, 30, 953–971. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Ma, W.; Zhao, J. Semiconductor-mediated photo degradation of pollutants under visible-light irradiation. Chem. Soc. Rev. 2010, 39, 4206–4219. [Google Scholar] [CrossRef] [PubMed]
- Basavarajappa, P.S.; Patil, S.B.; Ganganagappa, N.; Reddy, K.R.; Raghu, A.V.; Reddy, C.V. Recent progress in metal-doped TiO2, non-metal doped/codoped TiO2 and TiO2 nanostructured hybrids for enhanced photocatalysis. Int. J. Hydrogen Energy 2020, 45, 7764–7778. [Google Scholar] [CrossRef]
- Ma, L.L.; Sun, H.Z.; Zhang, Y.G.; Lin, Y.L.; Li, J.L.; Wang, E.K.; Yu, Y.; Tan, M.; Wang, J.B. Preparation, characterization and photocatalytic properties of CdS nanoparticles dotted on the surface of carbon nanotubes. Nanotechnology 2008, 19, 8575–8581. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.W.; Cao, H.Q.; Yin, J.F. Generation and photocatalytic activities of Bi@Bi2O3 microspheres. Nano Res. 2011, 4, 470–482. [Google Scholar] [CrossRef]
- Zhang, A.Y.; Wang, W.K.; Pei, D.N.; Yu, H.Q. Degradation of refractory pollutants under solar light irradiation by a robust and self-protected ZnO/CdS/TiO2 hybrid photo catalyst. Water Res. 2016, 92, 78–86. [Google Scholar] [CrossRef]
- Wang, C.; Cao, M.; Wang, P.; Ao, Y. Preparation, characterization of CdS-deposited graphene-carbon nanotubes hybrid photocatalysts with enhanced photocatalytic activity. Mater. Lett. 2013, 108, 336–339. [Google Scholar] [CrossRef]
- Wang, Y.J.; Zhang, Y.N.; Jiang, Z.Q.; Jiang, G.Y.; Zhao, Z.; Wu, Q.H.; Liu, Y.; Xu, Q.; Duan, A.J.; Xu, C.M. Controlled fabrication and enhanced visible-light photocatalytic hydrogen production of Au@CdS/MIL-101 heterostructure. Appl. Catal. B 2016, 185, 307–314. [Google Scholar] [CrossRef]
- Meissner, D.; Memming, R.; Kastening, B. Photoelectrochemistry of cadmium sulfide. Reanalysis of photocorrosion and flat-band potential. J. Phys. Chem. 1988, 92, 3476–3483. [Google Scholar] [CrossRef]
- Persano, L.; Camposeo, A.; Di Benedetto, F.; Stabile, R.; Laera, A.M.; Piscopiello, E.; Tapfer, L.; Pisignano, D. CdS–Polymer Nanocomposites and Light-Emitting Fibers by In Situ Electron-Beam Synthesis and Lithography. Adv. Mater. 2012, 24, 5320–5326. [Google Scholar] [CrossRef]
- Hoffman, A.; Mills, G.; Yee, H.; Hoffmann, M.R. Q-sized cadmium sulfide: Synthesis, characterization, and efficiency of photo initiation of polymerization of several vinylic monomers. J. Phys. Chem. 1992, 96, 5546–5552. [Google Scholar] [CrossRef]
- Harris, R.D.; Homan, S.B.; Kodaimati, M.; He, C.; Nepomnyashchii, A.B.; Swenson, N.K.; Lian, S.; Calzada, R.; Weiss, E.A. Electronic Processes within Quantum Dot-Molecule Complexes. Chem. Rev. 2016, 116, 12865–12919. [Google Scholar] [CrossRef] [PubMed]
- Majeed, I.; Nadeem, M.A.; Al-Oufi, M.; Nadeem, M.A.; Waterhouse, G.I.N.; Badshah, A.; Metson, J.B.; Idriss, H. On the role of metal particle size and surface coverage for photo-catalytic hydrogen production: A case study of the Au/CdS system. Appl. Catal. B 2016, 182, 266–276. [Google Scholar] [CrossRef]
- Feng, J.; An, C.; Dai, L.; Liu, J.; Wei, G.; Bai, S.; Zhang, J.; Xiong, Y. Long-term production of H2 over Pt/CdS nanoplates under sunlight illumination. Chem. Eng. J. 2016, 283, 351–357. [Google Scholar] [CrossRef]
- Zheng, N.-C.; Ouyang, T.; Chen, Y.; Wang, Z.; Chen, D.-Y.; Liu, Z.-Q. Ultrathin CdS shell-sensitized hollow S-doped CeO2 spheres for efficient visible-light photocatalysis. Catal. Sci. Technol. 2019, 9, 1357–1364. [Google Scholar] [CrossRef]
- Zhang, P.; Liu, Y.; Tian, B.; Luo, Y.; Zhang, J. Synthesis of core-shell structured CdS@CeO2 and CdS@TiO2 composites and comparison of their photocatalytic activities for the selective oxidation of benzyl alcohol to benzaldehyde. Catal. Today 2017, 281, 181–188. [Google Scholar] [CrossRef]
- Fang, S.S.; Sun, M.Y.; Zhou, Y.W.; Liang, Q.; Li, Z.Y.; Xu, S. Solvothermal synthesis of CdS QDs/MWCNTs nano composites with high efficient photo catalytic activity under visible light irradiation. J. Alloys Compd. 2016, 656, 771–776. [Google Scholar] [CrossRef]
- Zhou, H.C.; Long, J.R.; Yaghi, O.M. Introduction to metal-organic frameworks. Chem. Rev. 2012, 112, 673–674. [Google Scholar] [CrossRef]
- Cai, G.; Yan, P.; Zhang, L.; Zhou, H.-C.; Jiang, H.-L. Metal–Organic Framework-Based Hierarchically Porous Materials: Synthesis and Applications. Chem. Rev. 2021, 121, 12278–12326. [Google Scholar] [CrossRef]
- Liang, W.; Wied, P.; Carraro, F.; Sumby, C.J.; Nidetzky, B.; Tsung, C.-K.; Falcaro, P.; Doonan, C.J. Metal–Organic Framework-Based Enzyme Biocomposites. Chem. Rev. 2021, 121, 1077–1129. [Google Scholar] [CrossRef]
- Zhang, W.-X.; Liao, P.-Q.; Lin, R.-B.; Wei, Y.-S.; Zeng, M.-H.; Chen, X.-M. Metal Cluster-based Functional Porous Coordination Polymers. Coord. Chem. Rev. 2015, 263, 293–294. [Google Scholar] [CrossRef]
- Yin, Z.; Zhou, Y.-L.; Zeng, M.-H.; Kurmoo, M. The Concept of Mixed Organic Ligands in Metal-Organic Frameworks: Design, Tuning and Functions. Dalton Trans. 2015, 44, 5258–5275. [Google Scholar] [CrossRef] [PubMed]
- Peng, H.-J.; Zhu, L.; Wang, Y.-L.; Chao, H.-Y.; Jiang, L.; Qiao, Z.-P. CdS/ZIF-67 nanocomposites with enhanced performance for visible light CO2 photo reduction. Inorg. Chem. Comm. 2020, 117, 107943. [Google Scholar] [CrossRef]
- Shen, L.; Liang, S.; Wu, W.; Liang, R.; Wu, L. CdS-decorated UiO-66(NH2) nano composites fabricated by a facile photo deposition process: An efficient and stable visible-light-driven photocatalyst for selective oxidation of alcohols. J. Mater. Chem. A 2013, 1, 11473–11482. [Google Scholar] [CrossRef]
- Chaturvedi, G.; Kaur, A.; Kansal, S.K. CdS-Decorated MIL-53(Fe) Microrods with Enhanced Visible Light Photocatalytic Performance for the Degradation of Ketorolac Tromethamine and Mechanism Insight. J. Phys. Chem. C 2019, 123, 16857–16867. [Google Scholar] [CrossRef]
- Zhang, R.; Li, G.; Zhang, Y. Photochemical synthesis of CdS-MIL-125(Ti) with enhanced visible light photocatalytic performance for the selective oxidation of benzyl alcohol to benzaldehyde. Photochem. Photobiol. Sci. 2017, 16, 996. [Google Scholar] [CrossRef]
- Liang, R.; Jing, F.; Yan, G.; Wu, L. Synthesis of CdS-decorated MIL-68(Fe) nanocomposites: Efficient andstable visible light photocatalysts for the selective reduction of 4-nitroaniline to p-phenylenediamine in water. Appl. Catal. B-Environ. 2017, 218, 452–459. [Google Scholar] [CrossRef]
- Jing, C.; Zhang, Y.; Zheng, J.; Ge, S.; Lin, J.; Pan, D.; Naik, N.; Guo, Z. In-situ constructing visible light CdS/Cd-MOF photo catalyst with enhanced photo degradation of methylene blue. Particuology 2022, 69, 111–122. [Google Scholar] [CrossRef]
- Li, S.-R.; Ren, F.-D.; Wang, L.; Chen, Y.-Z. Photocatalytic cascade reactions and dye degradation over CdS–metal–organic framework hybrids. RSC Adv. 2021, 11, 35326. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Xu, Q.; Jiang, H.-L. Metal–organic frameworks meet metal nanoparticles: Synergistic effect for enhanced catalysis. Chem. Soc. Rev. 2017, 46, 4774–4808. [Google Scholar] [CrossRef]
- Dutta, S.; Lee, I.S. Metal-organic framework based catalytic nanoreactors: Synthetic challenges and applications. Mater. Chem. Front. 2021, 5, 3986–4021. [Google Scholar] [CrossRef]
- Bai, Y.; Dou, Y.; Xie, L.-H.; Rutledge, W.; Li, J.-R.; Zhou, H.-C. Zr-based metal-organic frameworks: Design, synthesis, structure, and applications. Chem. Soc. Rev. 2016, 45, 2327–2367. [Google Scholar] [CrossRef] [PubMed]
- Cavka, J.H.; Jakobsen, S.; Olsbye, U.; Guillou, N.; Lamberti, C.; Bordiga, S.; Lillerud, K.P. A New Zirconium Inorganic Building Brick Forming Metal Organic Frameworks with Exceptional Stability. J. Am. Chem. Soc. 2008, 130, 13850–13851. [Google Scholar] [CrossRef] [PubMed]
- Goswami, S.; Miller, C.E.; Logsdon, J.L.; Buru, C.T.; Wu, Y.-L.; Bowman, D.N.; Islamoglu, T.; Asiri, A.M.; Cramer, C.J.; Wasielewski, M.R.; et al. Atomistic Approach toward Selective Photocatalytic Oxidation of a Mustard-Gas Simulant: A Case Study with Heavy-Chalcogen-Containing PCN-57 Analogues. ACS Appl. Mater. Interfaces 2017, 9, 19535–19540. [Google Scholar] [CrossRef] [PubMed]
- Jia, J.; Gutiérrez-Arzaluz, L.; Shekhah, O.; Alsadun, N.; Czaban-Jóźwiak, J.; Zhou, S.; Bakr, O.M.; Mohammed, O.F.; Eddaoudi, M. Access to Highly Efficient Energy Transfer in Metal–Organic Frameworks via Mixed Linkers Approach. J. Am. Chem. Soc. 2020, 142, 8580–8584. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Ren, D.; Zhou, K.; Xia, H.-L.; Liu, X.-Y.; Wang, X.; Li, J. Linker Engineering toward Full-Color Emission of UiO-68 Type Metal–Organic Frameworks. J. Am. Chem. Soc. 2021, 143, 10547–10552. [Google Scholar] [CrossRef] [PubMed]
- Mallick, A.; El-Zohry, A.M.; Shekhah, O.; Yin, J.; Jia, J.; Aggarwal, H.; Emwas, A.-H.; Mohammed, O.F.; Eddaoudi, M. Unprecedented Ultralow Detection Limit of Amines using a Thiadiazole-Functionalized Zr(IV)-Based Metal–Organic Framework. J. Am. Chem. Soc. 2019, 141, 7245–7249. [Google Scholar] [CrossRef]
- Mercuri, G.; Giambastiani, G.; Rossin, A. Thiazole- and Thiadiazole-Based Metal–Organic Frameworks and Coordination Polymers for Luminescent Applications. Inorganics 2019, 7, 144. [Google Scholar] [CrossRef]
- Wei, N.; Zhang, Y.-R.; Han, Z.-B. Thiadiazole-functional porous metal–organic framework as luminescent probe for Cd2+. CrystEngComm 2013, 15, 8883–8886. [Google Scholar] [CrossRef]
- Ge, L.; Zuo, F.; Liu, J.K.; Ma, Q.; Wang, C.; Sun, D.Z.; Bartels, L.; Feng, P.Y. Synthesis and efficient visible light photocatalytic hydrogen evolution of polymeric g-C3N4 coupled with CdS quantum dots. J. Phys. Chem. C 2012, 116, 13708–13714. [Google Scholar] [CrossRef]
- Wei, L.-Q.; Ye, B.-H. Cyclometalated Ir–Zr Metal–Organic Frameworks as Recyclable Visible-Light Photocatalysts for Sulfide Oxidation into Sulfoxide in Water. ACS Appl. Mater. Interfaces 2019, 11, 41448–41457. [Google Scholar] [CrossRef] [PubMed]
- Low, J.; Dai, B.; Tong, T.; Jiang, C.; Yu, J. In Situ Irradiated X-Ray Photoelectron Spectroscopy Investigation on a Direct Z-Scheme TiO2/CdS Composite Film Photocatalyst. Adv. Mater. 2018, 31, 1802981. [Google Scholar] [CrossRef] [PubMed]
- Long, J.; Wang, S.; Ding, Z.; Wang, S.; Zhou, Y.; Huang, L.; Wang, X. Amine-functionalized zirconium metal-organic framework as efficient visible-light photo catalyst for aerobic organic transformations. Chem. Commun. 2012, 48, 11656–11658. [Google Scholar] [CrossRef] [PubMed]
- Mahmoodi, N.M.; Taghizadeh, A.; Taghizadeh, M.; Abdi, J. In situ deposition of Ag/AgCl on the surface of magnetic metal-organic framework nanocomposite and its application for the visible-light photocatalytic degradation of Rhodamine dye. J. Hazard. Mate. 2019, 378, 120741. [Google Scholar] [CrossRef] [PubMed]
- Bonesi, S.M.; Manet, I.; Freccero, M.; Fagnoni, M.; Albini, A. Photosensitized Oxidation of Sulfides: Discriminating between the Singlet-Oxygen Mechanism and Electron Transfer Involving Superoxide Anion or Molecular Oxygen. Chem.-Eur. J. 2006, 12, 4844–4857. [Google Scholar] [CrossRef]
- Xia, Q.S.; Yu, X.D.; Zhao, H.M.; Wang, S.P.; Wang, H.; Guo, Z.F.; Xing, H.Z. Syntheses of Novel Lanthanide Metal–Organic Frameworks for Highly Efficient Visible-Light-Driven Dye Degradation. J. Am. Chem. Soc. 2017, 17, 4189–4195. [Google Scholar] [CrossRef]
- Xu, J.; Hu, C.; Xi, Y.; Wan, B.; Zhang, C.; Zhang, Y. Synthesis and visible light photocatalytic activity of β-AgVO3 nanowires. Solid State Sci. 2012, 14, 535–539. [Google Scholar] [CrossRef]
- Hu, L.; Deng, G.; Lu, W.; Pang, S.; Hu, X. Deposition of CdS nanoparticles on MIL-53(Fe) metal-organic framework with enhanced photocatalytic degradation of RhB under visible light irradiation. Appl. Surf. Sci. 2017, 410, 401–413. [Google Scholar] [CrossRef]
- Xu, M.-L.; Jiang, X.-J.; Li, J.-R.; Wang, F.-J.; Li, K.; Cheng, X. Self-Assembly of a 3D Hollow BiOBr@Bi-MOF Heterostructure with Enhanced Photocatalytic Degradation of Dyes. ACS Appl. Mater. Interfaces 2021, 13, 56171–56180. [Google Scholar] [CrossRef]
- Jin, J.-C.; Wang, J.; Guo, J.; Yan, M.-H.; Wang, J.; Srivastava, D.; Kumar, A.; Sakiyama, H.; Muddassir, M.; Pan, Y. A 3D rare cubane-like tetramer Cu(II)-based MOF with 4-fold dia topology as an efficient photocatalyst for dye degradation. Colloids Surf. A Physicochem. Eng. Asp. 2023, 656, 130475. [Google Scholar] [CrossRef]
- Chen, Y.; Zhai, B.; Liang, Y.; Li, Y.; Li, J. Preparation of CdS/ g-C3N4/ MOF composite with enhanced visible-light photocatalytic activity for dye degradation. J. Solid State Chem. 2019, 274, 32–39. [Google Scholar] [CrossRef]




| Entry | Catalyst | Additive | Light | Degradation |
|---|---|---|---|---|
| 1 | CdS@UiO-68N2S | N.D. | Blue light | 94% |
| 2 | N.D. | N.D. | Blue light | 1% |
| 3 | UiO-68N2S | N.D. | Blue light | 39% |
| 4 | CdS b | N.D. | Blue light | 6% |
| 5 | CdS@UiO-68N2S | Benoquinone c | Blue light | 4% |
| 6 | CdS@UiO-68N2S | tert-butanol d | Blue light | 86% |
| Catalyst | Catalyst Dosage | RhB Concentration | Light Source | Oxygen Source | Catalytic Activity | k a/min−1 |
|---|---|---|---|---|---|---|
| Ag/AgCl@CFNMT b [44] | 0.08 g/L | 20 mg/L | Visible–light LED (100 W) | H2O2 (20 uL) | 98.8% (10 min) | 0.103 |
| 1.5-CdS/MIL53 [48] | 1.0 g/L | 10 mg/L | Xenon lamp (500 W) | Air | 86% (1.5 h) | 0.0158 |
| BiOBr@Bi-MOF [49] | 0.6 g/L | 20 mg/L | Xenon lamp (300 W) | Air | 99.4% (60 min) | 0.07009 |
| Cu(II)-based MOF [50] | 30 mg Cat. for 40 ppm RhB | UV light | Air | 80% (100 min) | 0.01358 | |
| CdS/g-C3N4/MIL125(Ti) [51] | N.D. | N.D. | Xenon lamp (300 W, λ > 420 nm) | Air | 90.2% (90 min) | 0.0414 |
| CdS@UiO-68N2S [this work] | 1.0 g/L | 25 mg/L | Blue light LED (80 W, λ = 480 nm) | Air | 94% (14 h) | 0.0027 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wei, L.-Q.; Wei, J.-B.; Yang, F.; Li, Z.-W.; Lai, H.-F. Preparation of Thiadiazole Modified UiO-68-CdS Composites for RhB Degradation under Visible Light Irradiation. Crystals 2023, 13, 785. https://doi.org/10.3390/cryst13050785
Wei L-Q, Wei J-B, Yang F, Li Z-W, Lai H-F. Preparation of Thiadiazole Modified UiO-68-CdS Composites for RhB Degradation under Visible Light Irradiation. Crystals. 2023; 13(5):785. https://doi.org/10.3390/cryst13050785
Chicago/Turabian StyleWei, Lian-Qiang, Jiu-Bin Wei, Fei Yang, Zhi-Wei Li, and Hong-Fang Lai. 2023. "Preparation of Thiadiazole Modified UiO-68-CdS Composites for RhB Degradation under Visible Light Irradiation" Crystals 13, no. 5: 785. https://doi.org/10.3390/cryst13050785
APA StyleWei, L.-Q., Wei, J.-B., Yang, F., Li, Z.-W., & Lai, H.-F. (2023). Preparation of Thiadiazole Modified UiO-68-CdS Composites for RhB Degradation under Visible Light Irradiation. Crystals, 13(5), 785. https://doi.org/10.3390/cryst13050785








