Schiff-Based Modified Bentonite Clay Composites for Wastewater Treatment: Experimental and DFT-Based Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Schiff Base (SB)
2.3. Purification of Bentonite Clay
2.4. Synthesis of Bentonite Clay Composite (Bentonite–SB)
2.5. Characterization
2.6. Batch Adsorption Study
2.7. Adsorption Isotherm
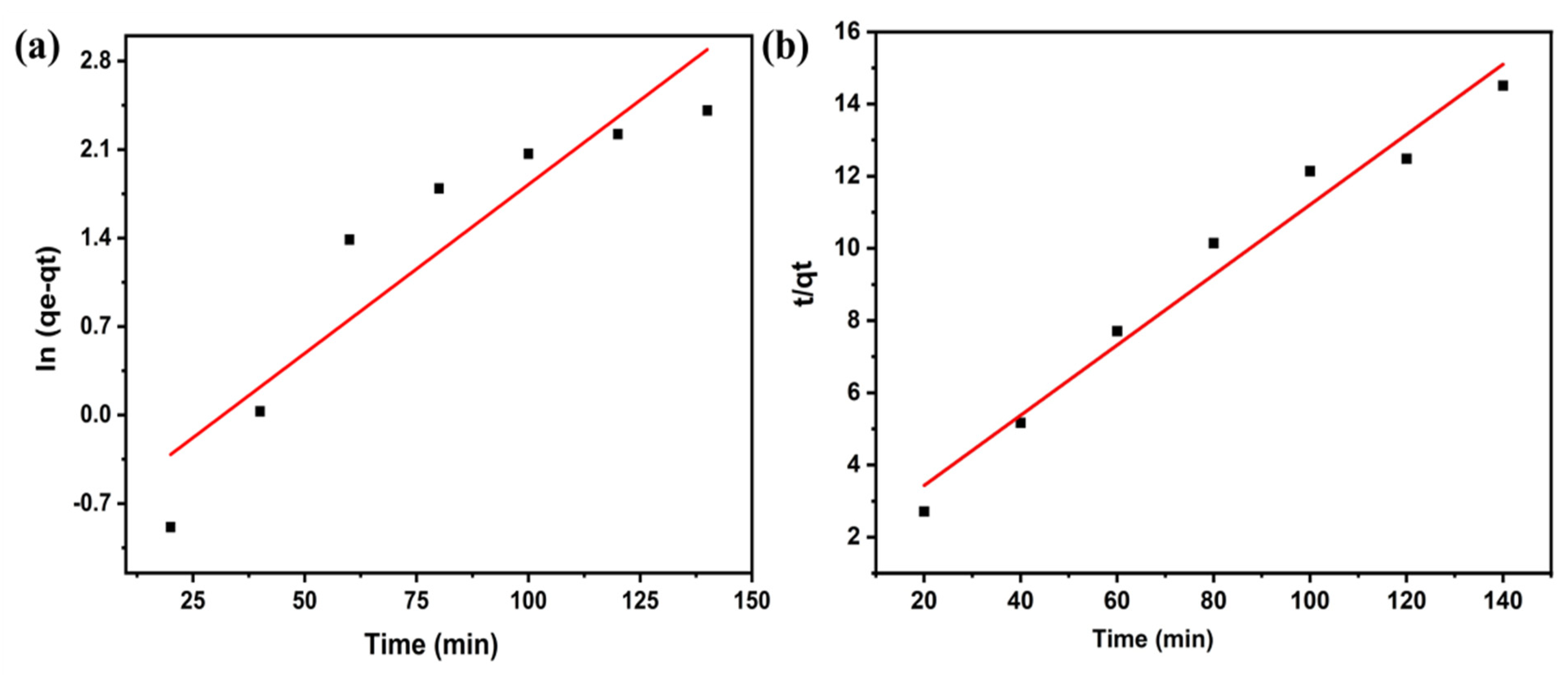
2.8. Adsorption Kinetics
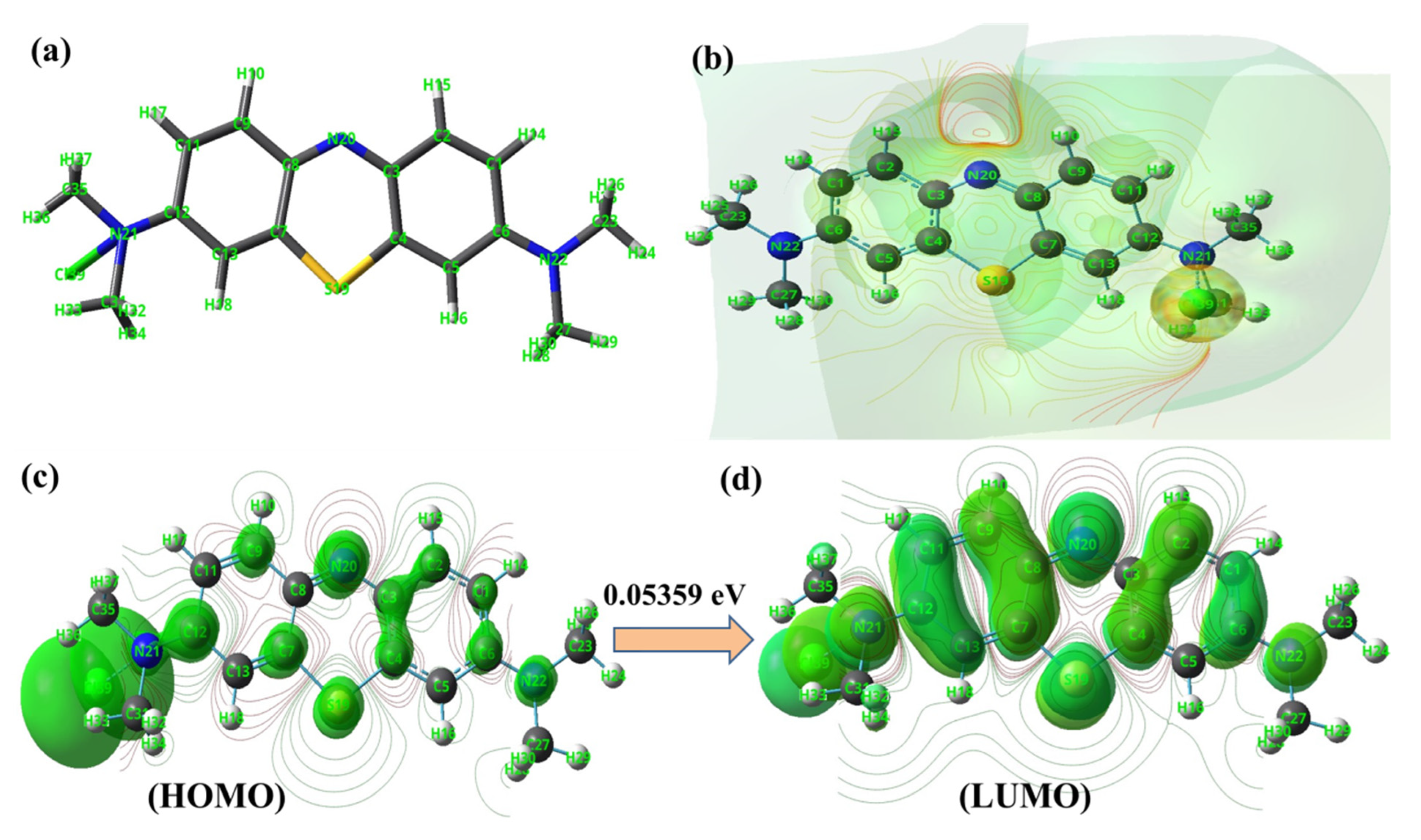
2.9. Density Functional Theory (DFT) Analysis of MB
3. Results
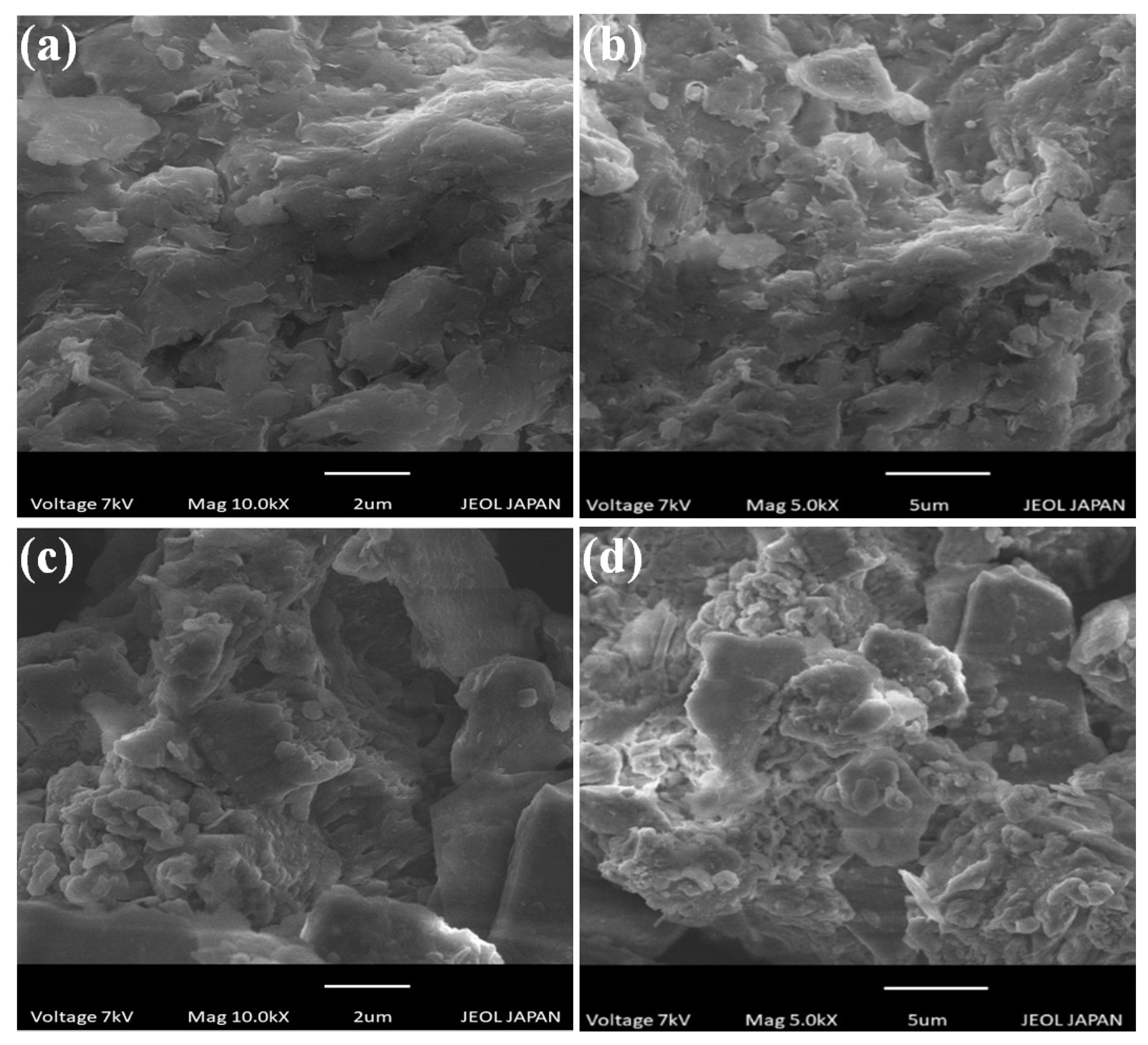
3.1. SEM Analysis
3.2. XRD Analysis
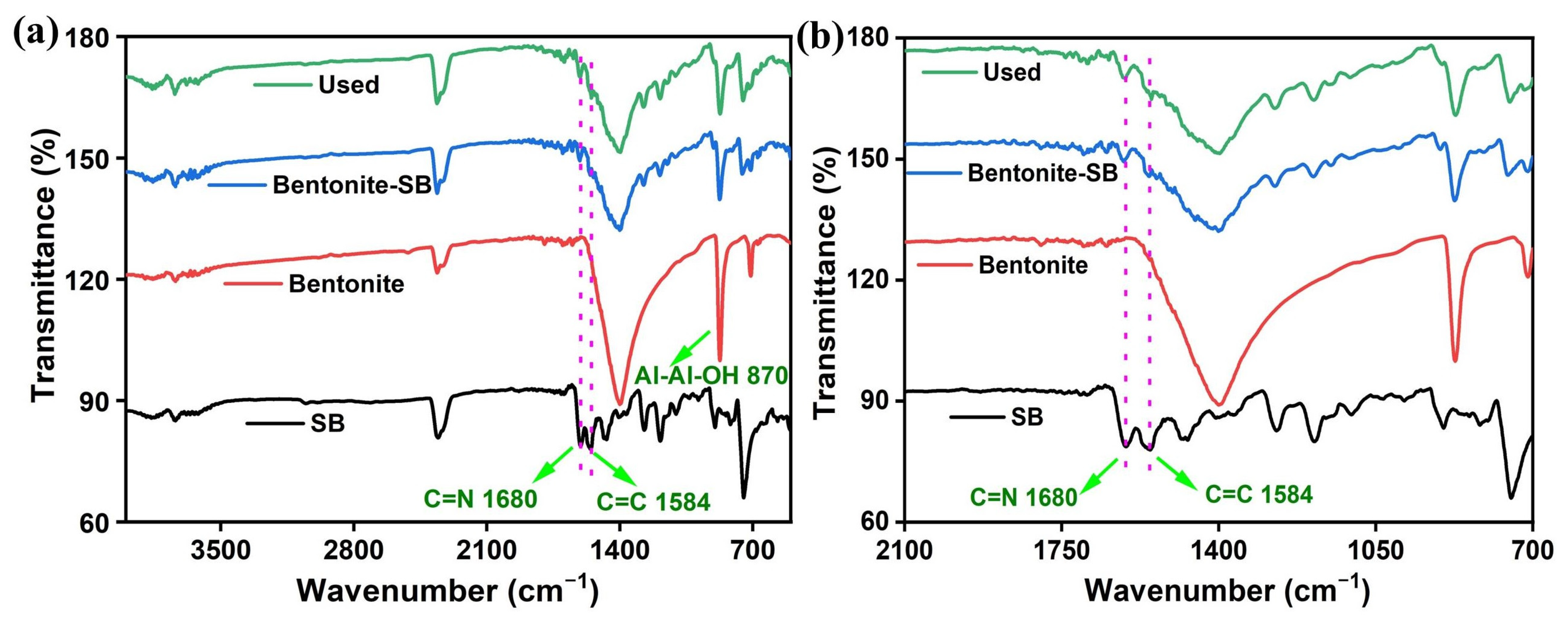
3.3. FTIR
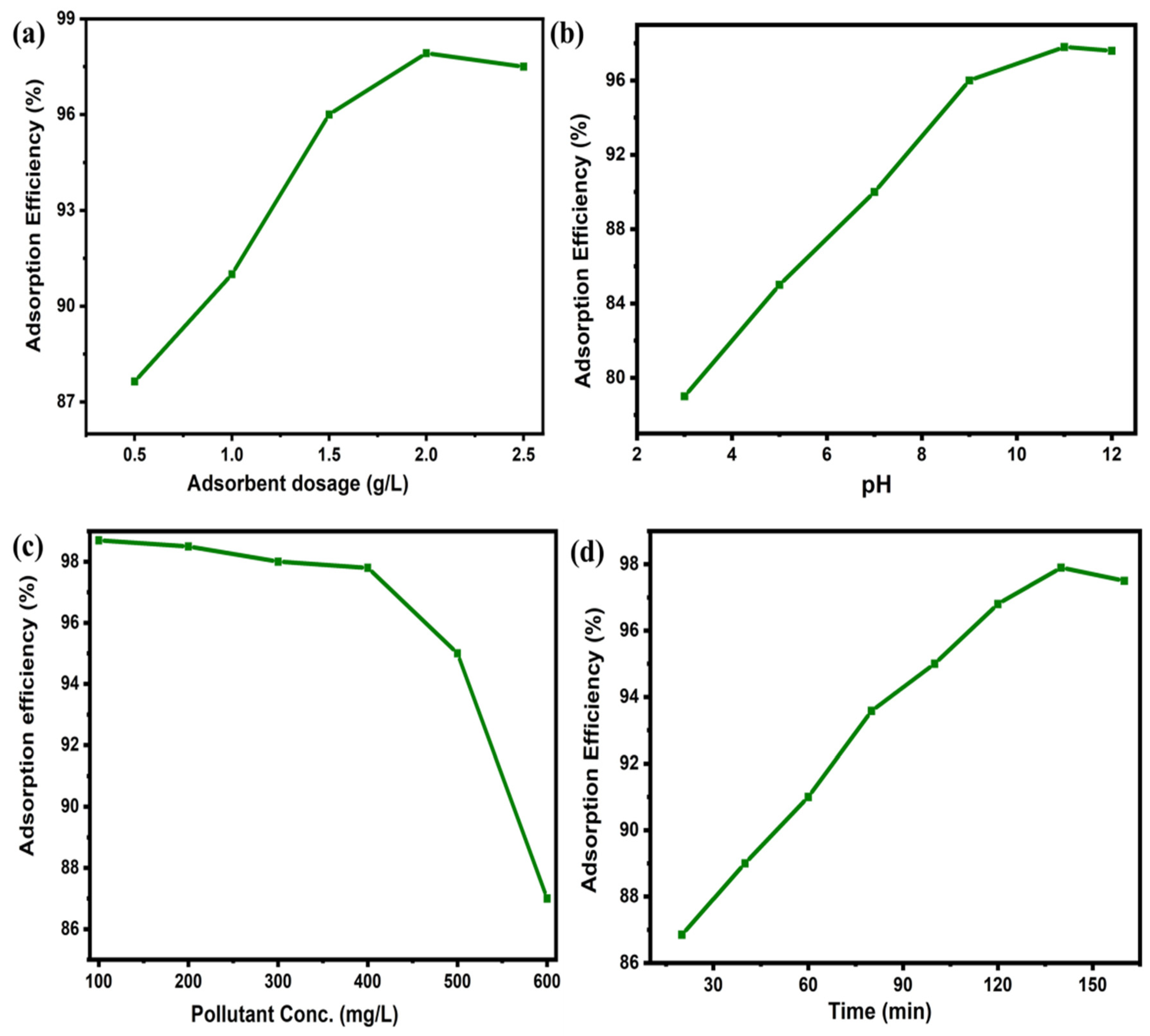
3.4. Effect of Various Parameters on Adsorption
3.4.1. Effect of Adsorbent Dosage
3.4.2. Effect of pH
3.4.3. Effect of Initial Concentration of Dye
3.4.4. Effect of Contact Time
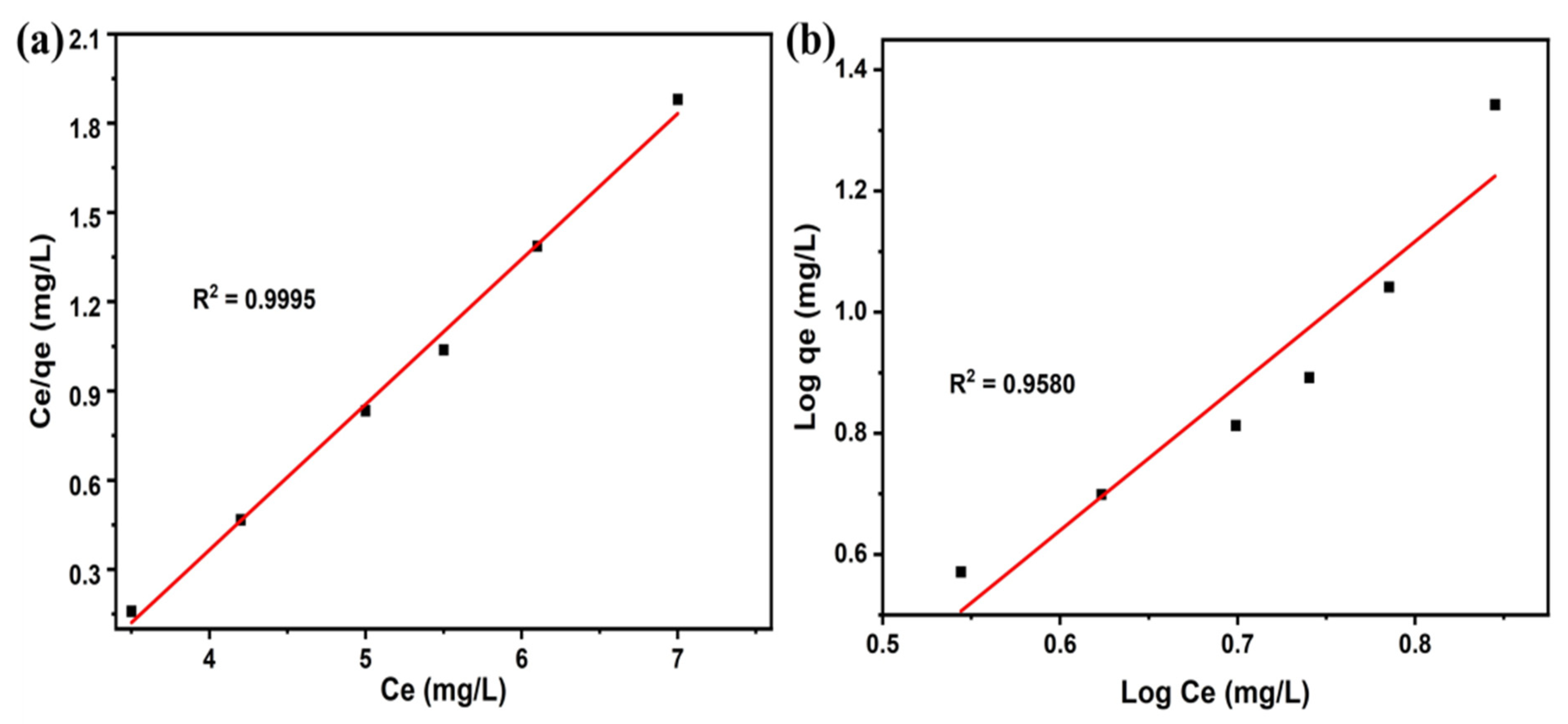
3.5. Adsorption Isotherm
3.6. Adsorption Kinetics
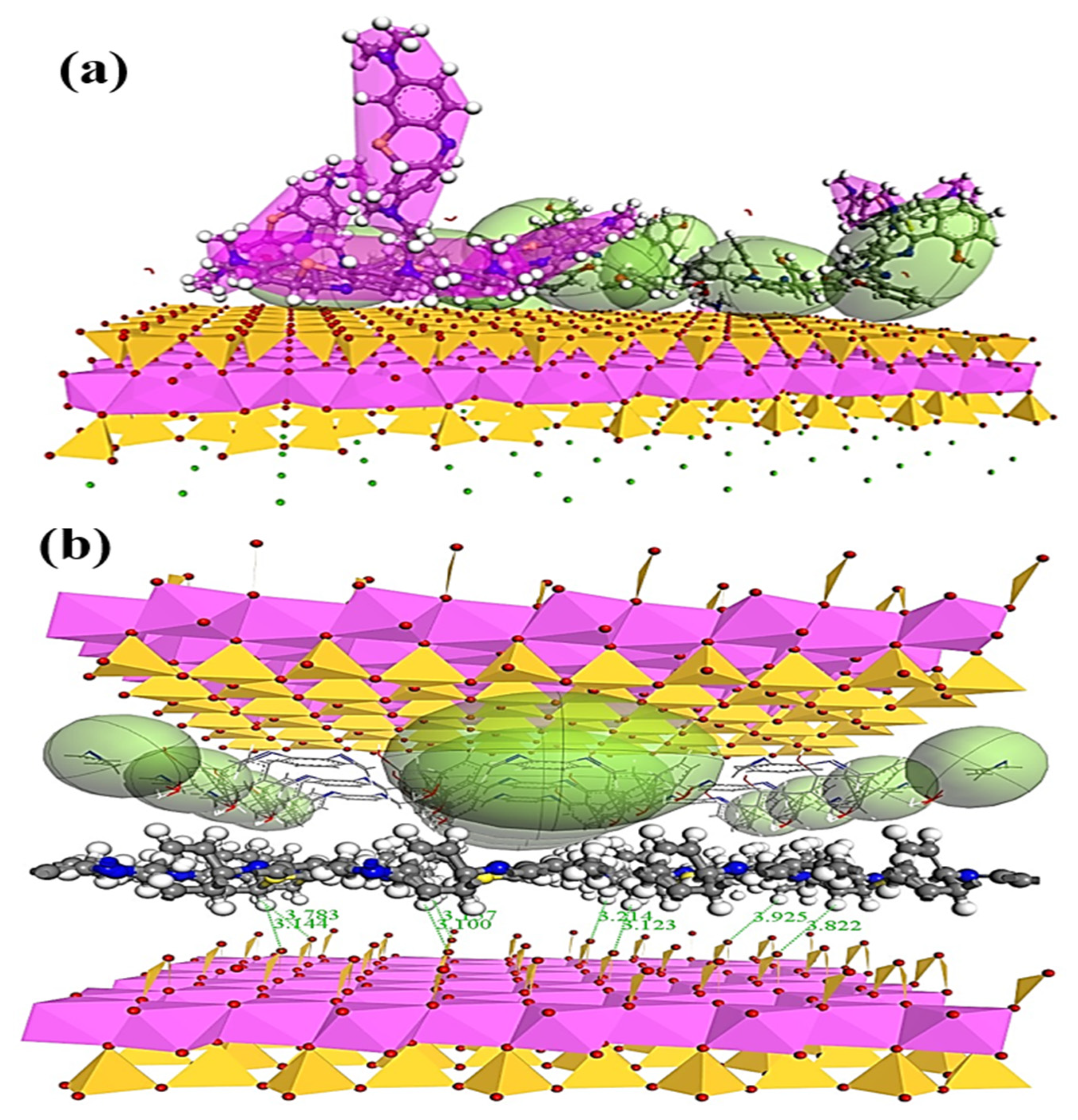
3.7. Adsorption Mechanism
3.8. Examining the Adsorption Mechanism Using DFT Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhou, L.; Liu, Z.; Liu, J.; Huang, Q. Adsorption of Hg (II) from aqueous solution by ethylenediamine-modified magnetic crosslinking chitosan microspheres. Desalination 2010, 258, 41–47. [Google Scholar] [CrossRef]
- Naushad, M.; Ahamad, T.; Alothman, Z.A.; Shar, M.A.; AlHokbany, N.S.; Alshehri, S.M. Synthesis, characterization and application of curcumin formaldehyde resin for the removal of Cd2+ from wastewater: Kinetics, isotherms and thermodynamic studies. J. Ind. Eng. Chem. 2015, 29, 78–86. [Google Scholar] [CrossRef]
- Hokkanen, S.; Bhatnagar, A.; Sillanpää, M. A review on modification methods to cellulose-based adsorbents to improve adsorption capacity. Water Res. 2016, 91, 156–173. [Google Scholar] [CrossRef] [PubMed]
- Niu, H.; Luo, P.; Zhang, M.; Zhang, L.; Hao, L.; Luo, J.; Bai, X.; Wang, W. Multifunctional, photochromic, acidichromic, electrochromic molecular switch: Novel aromatic poly (azomehine) s containing triphenylamine Group. Eur. Polym. J. 2009, 45, 3058–3071. [Google Scholar] [CrossRef]
- Naushad, M.; Ahamad, T.; Sharma, G.; Ala’a, H.; Albadarin, A.B.; Alam, M.M.; ALOthman, Z.A.; Alshehri, S.M.; Ghfar, A.A. Synthesis and characterization of a new starch/SnO2 nanocomposite for efficient adsorption of toxic Hg2+ metal ion. Chem. Eng. J. 2016, 300, 306–316. [Google Scholar] [CrossRef]
- Wu, D.L.; Wang, W.; Zhang, J.H.; Fu, H.; Lv, X.S.; Xu, X.H. Preparation of mulberry branch biomass char and its usage in wastewater treatment. Water Environ. Res. 2012, 84, 2060–2069. [Google Scholar] [CrossRef]
- Aarab, N.; Hsini, A.; Essekri, A.; Laabd, M.; Lakhmiri, R.; Albourine, A. Removal of an emerging pharmaceutical pollutant (metronidazole) using PPY-PANi copolymer: Kinetics, equilibrium and DFT identification of adsorption mechanism. Groundw. Sustain. Dev. 2020, 11, 100416. [Google Scholar] [CrossRef]
- Bouazza, D.; Miloudi, H.; Adjdir, M.; Tayeb, A.; Boos, A. Competitive adsorption of Cu (II) and Zn (II) on impregnate raw Algerian bentonite and efficiency of extraction. Appl. Clay Sci. 2018, 151, 118–123. [Google Scholar] [CrossRef]
- Ayari, F.; Srasra, E.; Trabelsi-Ayadi, M. Retention of lead from an aqueous solution by use of bentonite as adsorbent for reducing leaching from industrial effluents. Desalination 2007, 206, 270–278. [Google Scholar] [CrossRef]
- Chen, C.; Liu, H.; Chen, T.; Chen, D.; Frost, R.L. An insight into the removal of Pb (II), Cu (II), Co (II), Cd (II), Zn (II), Ag (I), Hg (I), Cr (VI) by Na (I)-montmorillonite and Ca (II)-montmorillonite. Appl. Clay Sci. 2015, 118, 239–247. [Google Scholar] [CrossRef]
- Qu, J.; Tian, X.; Jiang, Z.; Cao, B.; Akindolie, M.S.; Hu, Q.; Feng, C.; Feng, Y.; Meng, X.; Zhang, Y. Multi-component adsorption of Pb (II), Cd (II) and Ni (II) onto microwave-functionalized cellulose: Kinetics, isotherms, thermodynamics, mechanisms and application for electroplating wastewater purification. J. Hazard. Mater. 2020, 387, 121718. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.; Huang, Y.; Zhu, Z.; Dong, L.; Zha, J.; Yu, M. Enhanced PbCl2 adsorption capacity of modified kaolin in the furnace using a combined method of thermal pre-activation and acid impregnation. Chem. Eng. J. 2021, 414, 128672. [Google Scholar] [CrossRef]
- Murugesan, A.; Loganathan, M.; Kumar, P.S.; Vo, D.-V.N. Cobalt and nickel oxides supported activated carbon as an effective photocatalysts for the degradation Methylene Blue dye from aquatic environment. Sustain. Chem. Pharm. 2021, 21, 100406. [Google Scholar] [CrossRef]
- Huang, J.; Huang, D.; Zeng, F.; Ma, L.; Wang, Z. Photocatalytic MOF fibrous membranes for cyclic adsorption and degradation of dyes. J. Mater. Sci. 2021, 56, 3127–3139. [Google Scholar] [CrossRef]
- Zhu, S.; Asim Khan, M.; Wang, F.; Bano, Z.; Xia, M. Rapid removal of toxic metals Cu2+ and Pb2+ by amino trimethylene phosphonic acid intercalated layered double hydroxide: A combined experimental and DFT study. Chem. Eng. J. 2020, 392, 123711. [Google Scholar] [CrossRef]
- Zhu, S.; Khan, M.A.; Wang, F.; Bano, Z.; Xia, M. Exploration of adsorption mechanism of 2-phosphonobutane-1,2,4-tricarboxylic acid onto kaolinite and montmorillonite via batch experiment and theoretical studies. J. Hazard. Mater. 2021, 403, 123810. [Google Scholar] [CrossRef]
- Zhu, S.; Khan, M.A.; Kameda, T.; Xu, H.; Wang, F.; Xia, M.; Yoshioka, T. New insights into the capture performance and mechanism of hazardous metals Cr3+ and Cd2+ onto an effective layered double hydroxide based material. J. Hazard. Mater. 2022, 426, 128062. [Google Scholar] [CrossRef]
- Xu, Y.; Khan, M.A.; Wang, F.; Xia, M.; Lei, W. Novel multi amine-containing Gemini surfactant modified montmorillonite as adsorbents for removal of phenols. Appl. Clay Sci. 2018, 162, 204–213. [Google Scholar] [CrossRef]
- Zhang, X.; Huang, Q.; Liu, M.; Tian, J.; Zeng, G.; Li, Z.; Wang, K.; Zhang, Q.; Wan, Q.; Deng, F. Preparation of amine functionalized carbon nanotubes via a bioinspired strategy and their application in Cu2+ removal. Appl. Surf. Sci. 2015, 343, 19–27. [Google Scholar] [CrossRef]
- Chakrabarty, K.; Saha, P.; Ghoshal, A.K. Separation of mercury from its aqueous solution through supported liquid membrane using environmentally benign diluent. J. Membr. Sci. 2010, 350, 395–401. [Google Scholar] [CrossRef]
- Ding, F.; Gao, M.; Shen, T.; Zeng, H.; Xiang, Y. Comparative study of organo-vermiculite, organo-montmorillonite and organo-silica nanosheets functionalized by an ether-spacer-containing Gemini surfactant: Congo red adsorption and wettability. Chem. Eng. J. 2018, 349, 388–396. [Google Scholar] [CrossRef]
- Tabassam, N.; Mutahir, S.; Khan, M.A.; Khan, I.U.; Habiba, U.; Refat, M.S. Facile synthesis of cinnamic acid sensitized rice husk biochar for removal of organic dyes from wastewaters: Batch experimental and theoretical studies. Mater. Chem. Phys. 2022, 288, 126327. [Google Scholar] [CrossRef]
- Habiba, U.; Mutahir, S.; Khan, M.A.; Humayun, M.; Refat, M.S.; Munawar, K.S. Effective Removal of Refractory Pollutants through Cinnamic Acid-Modified Wheat Husk Biochar: Experimental and DFT-Based Analysis. Catalysts 2022, 12, 1063. [Google Scholar] [CrossRef]
- Saha, D.; Barakat, S.; Van Bramer, S.E.; Nelson, K.A.; Hensley, D.K.; Chen, J. Noncompetitive and competitive adsorption of heavy metals in sulfur-functionalized ordered mesoporous carbon. ACS Appl. Mater. Interfaces 2016, 8, 34132–34142. [Google Scholar] [CrossRef]
- Yek, S.M.-G.; Baran, T.; Nasrollahzadeh, M.; Bakhshali-Dehkordi, R.; Baran, N.Y.; Shokouhimehr, M. Synthesis and characterization of Pd (0) Schiff base complex supported on halloysite nanoclay as a reusable catalyst for treating wastewater contaminants in aqueous media. Optik 2021, 238, 166672. [Google Scholar] [CrossRef]
- Hassan, R.; Arida, H.; Montasser, M.; Abdel Latif, N. Synthesis of new Schiff base from natural products for remediation of water pollution with heavy metals in industrial areas. J. Chem. 2013, 2013, 240568. [Google Scholar] [CrossRef]
- Cegłowski, M.; Schroeder, G. Preparation of porous resin with Schiff base chelating groups for removal of heavy metal ions from aqueous solutions. Chem. Eng. J. 2015, 263, 402–411. [Google Scholar] [CrossRef]
- Wang, S.; Dong, Y.; He, M.; Chen, L.; Yu, X. Characterization of GMZ bentonite and its application in the adsorption of Pb (II) from aqueous solutions. Appl. Clay Sci. 2009, 43, 164–171. [Google Scholar] [CrossRef]
- Liu, L.; Zhang, B.; Zhang, Y.; He, Y.; Huang, L.; Tan, S.; Cai, X. Simultaneous removal of cationic and anionic dyes from environmental water using montmorillonite-pillared graphene oxide. J. Chem. Eng. Data 2015, 60, 1270–1278. [Google Scholar] [CrossRef]
- Alhokbany, N.; Ahamad, T.; Naushad, M.; Alshehri, S.M. Feasibility of toxic metal removal from aqueous medium using Schiff-base based highly porous nanocomposite: Adsorption characteristics and post characterization. J. Mol. Liq. 2019, 294, 111598. [Google Scholar] [CrossRef]
- Jourvand, M.; Shams Khorramabadi, G.; Omidi Khaniabadi, Y.; Godini, H.; Nourmoradi, H. Removal of methylene blue from aqueous solutions using modified clay. J. Basic Res. Med. Sci. 2015, 2, 32–41. [Google Scholar]
- Guediri, A.; Bouguettoucha, A.; Chebli, D.; Chafai, N.; Amrane, A. Molecular dynamic simulation and DFT computational studies on the adsorption performances of methylene blue in aqueous solutions by orange peel-modified phosphoric acid. J. Mol. Struct. 2020, 1202, 127290. [Google Scholar] [CrossRef]
- Munir, M.; Nazar, M.F.; Zafar, M.N.; Zubair, M.; Ashfaq, M.; Hosseini-Bandegharaei, A.; Khan, S.U.-D.; Ahmad, A. Effective adsorptive removal of methylene blue from water by didodecyldimethylammonium bromide-modified Brown clay. ACS Omega 2020, 5, 16711–16721. [Google Scholar] [CrossRef] [PubMed]
- Mouni, L.; Belkhiri, L.; Bollinger, J.-C.; Bouzaza, A.; Assadi, A.; Tirri, A.; Dahmoune, F.; Madani, K.; Remini, H. Removal of Methylene Blue from aqueous solutions by adsorption on Kaolin: Kinetic and equilibrium studies. Appl. Clay Sci. 2018, 153, 38–45. [Google Scholar] [CrossRef]
- Fabryanty, R.; Valencia, C.; Soetaredjo, F.E.; Putro, J.N.; Santoso, S.P.; Kurniawan, A.; Ju, Y.-H.; Ismadji, S. Removal of crystal violet dye by adsorption using bentonite–alginate composite. J. Environ. Chem. Eng. 2017, 5, 5677–5687. [Google Scholar] [CrossRef]
- Mutahir, S.; Irfan, T.; Nadeem, N.; Humayun, M.; Khan, M.A.; Refat, M.S.; Wang, C.; Sheikh, T.A. Synthesis and Micromechanistic Studies of Sensitized Bentonite for Methyl Orange and Rhodamine-B Adsorption from Wastewater: Experimental and DFT-Based Analysis. Molecules 2022, 27, 5567. [Google Scholar] [CrossRef]
- Zhirong, L.; Uddin, M.A.; Zhanxue, S. FT-IR and XRD analysis of natural Na-bentonite and Cu (II)-loaded Na-bentonite. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2011, 79, 1013–1016. [Google Scholar] [CrossRef]
- Frenkel, D.; Smit, B.; Tobochnik, J.; McKay, S.R.; Christian, W. Understanding molecular simulation. Comput. Phys. 1997, 11, 351–354. [Google Scholar] [CrossRef]
- Chu, Y.; Khan, M.A.; Zhu, S.; Xia, M.; Lei, W.; Wang, F.; Xu, Y. Microstructural modification of organo-montmorillonite with Gemini surfactant containing four ammonium cations: Molecular dynamics (MD) simulations and adsorption capacity for copper ions. J. Chem. Technol. Biotechnol. 2019, 94, 3585–3594. [Google Scholar] [CrossRef]



| Pseudo-First Order Model | Co (mg/L) | QE (mg/g) (exp) | QE (mg/g) (cal) | K1 (min−1) | R2 |
| 100 | 89.214 | 333.461 | 0.001972 | 0.82878 | |
| Pseudo-Second Order Model | Co (mg/L) | QE (mg/g) (exp) | QE (mg/g) (cal) | K2 (g·mg−1min−1) | R2 |
| 100 | 89.214 | 102.300 | 0.006369 | 0.96543 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mutahir, S.; Yaqoob, F.; Khan, M.A.; Alsuhaibani, A.M.; Abouzied, A.S.; Refat, M.S.; Huwaimel, B. Schiff-Based Modified Bentonite Clay Composites for Wastewater Treatment: Experimental and DFT-Based Analysis. Crystals 2023, 13, 806. https://doi.org/10.3390/cryst13050806
Mutahir S, Yaqoob F, Khan MA, Alsuhaibani AM, Abouzied AS, Refat MS, Huwaimel B. Schiff-Based Modified Bentonite Clay Composites for Wastewater Treatment: Experimental and DFT-Based Analysis. Crystals. 2023; 13(5):806. https://doi.org/10.3390/cryst13050806
Chicago/Turabian StyleMutahir, Sadaf, Fakhira Yaqoob, Muhammad Asim Khan, Amnah Mohammed Alsuhaibani, Amr S. Abouzied, Moamen S. Refat, and Bader Huwaimel. 2023. "Schiff-Based Modified Bentonite Clay Composites for Wastewater Treatment: Experimental and DFT-Based Analysis" Crystals 13, no. 5: 806. https://doi.org/10.3390/cryst13050806







