Corrosion Performance of Commercial Alloys and Refractory Metals in Conditions for Electrorefining of Spent Nuclear Fuels
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. Corrosion in Molten LiCl-KCl Eutectic
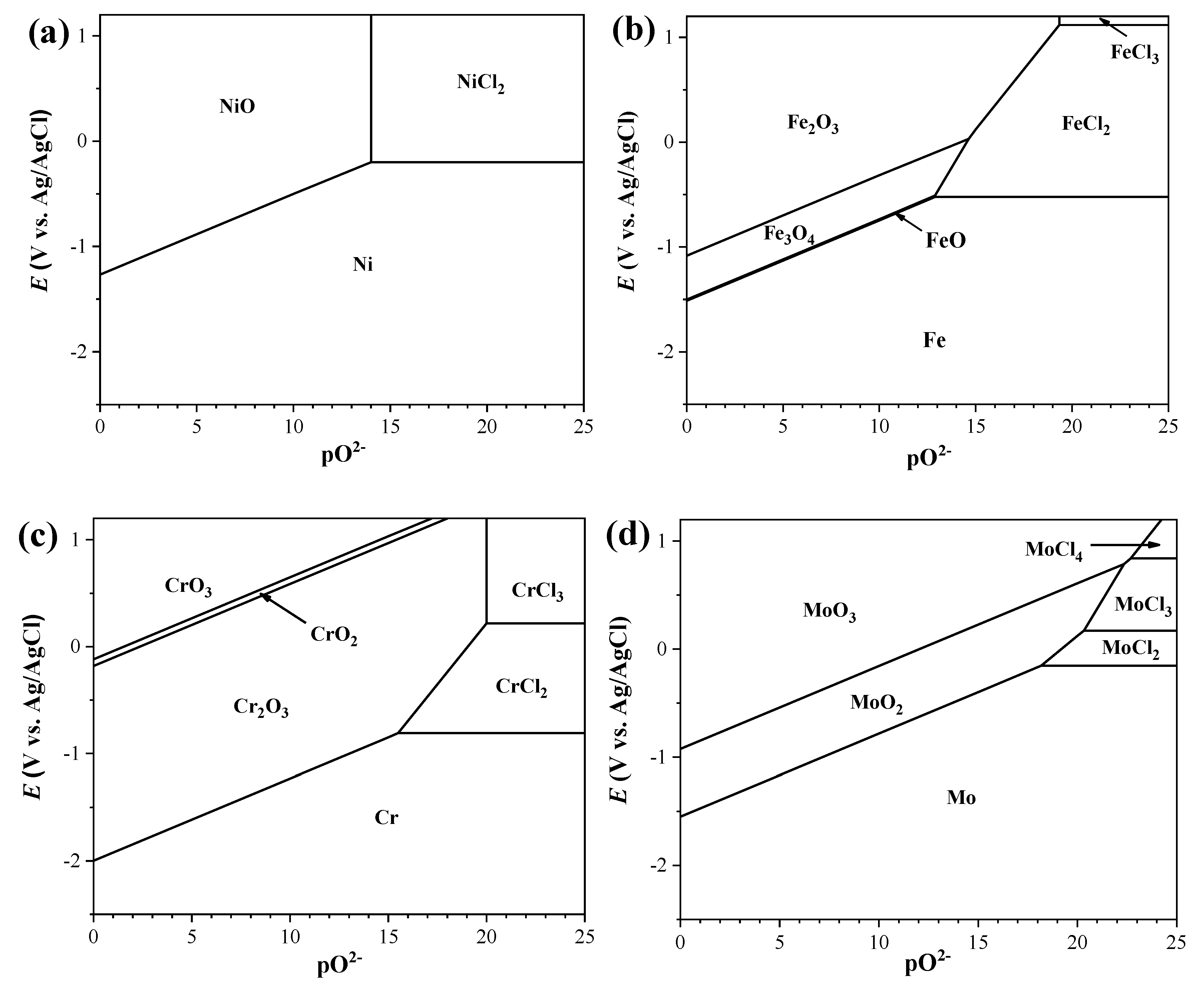
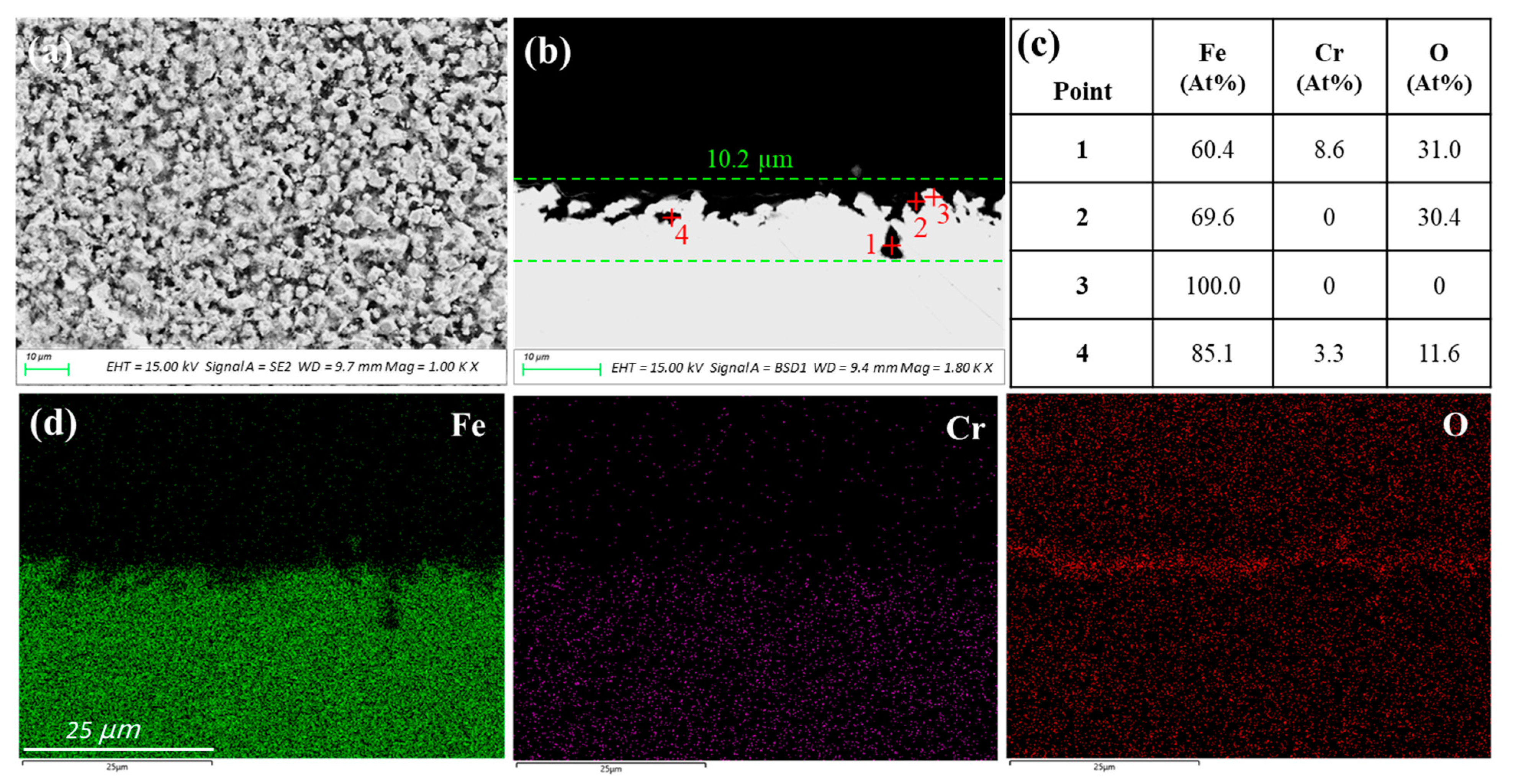
3.1.1. 42CrMo Steel
3.1.2. AISI 316L
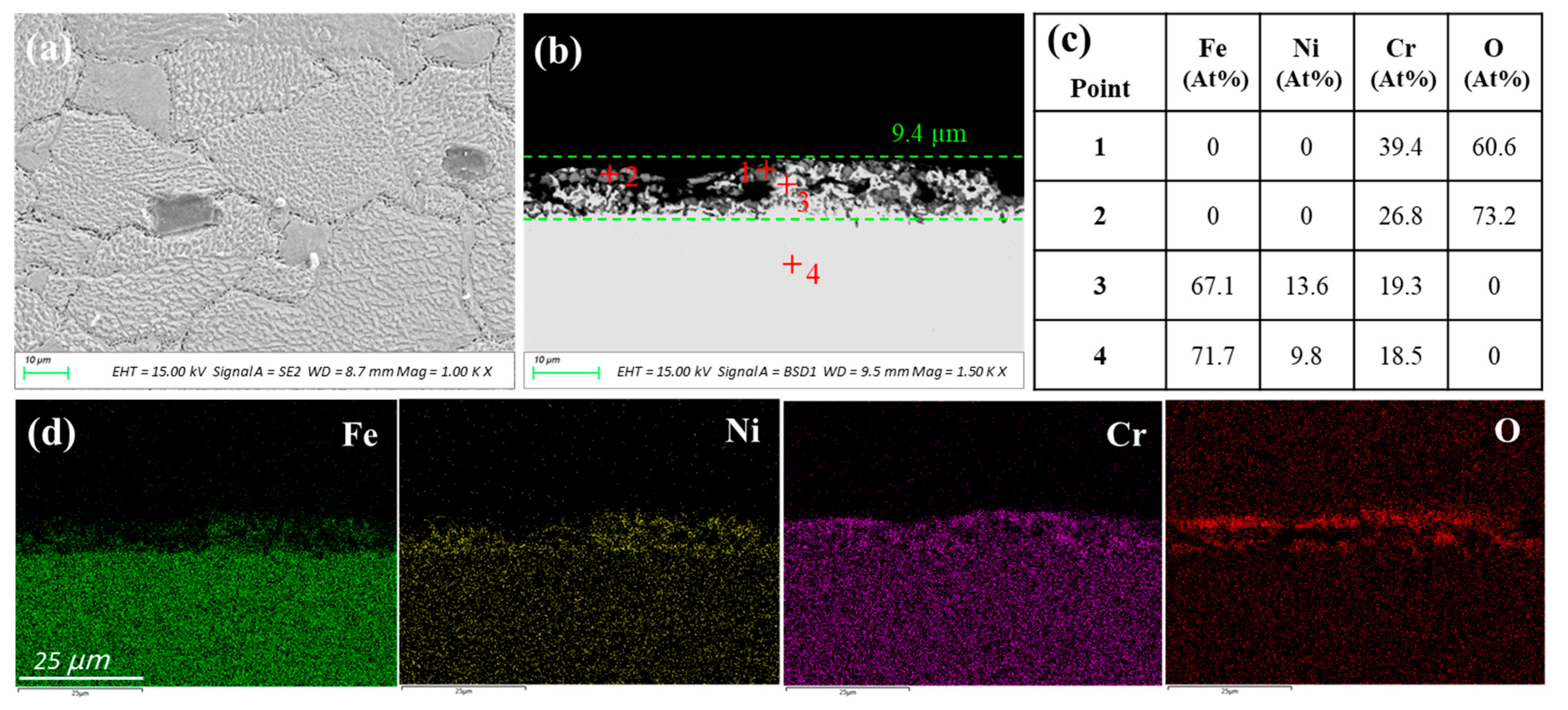
3.1.3. Inconel 600
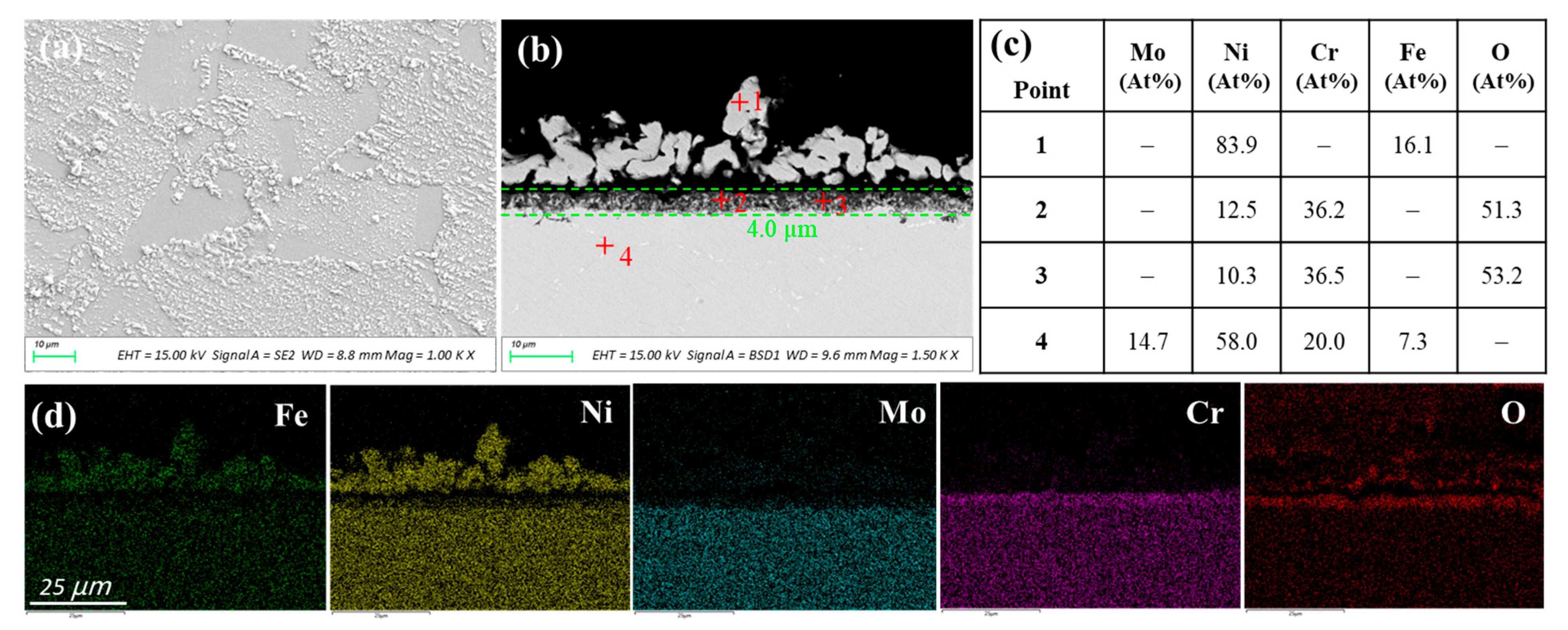
3.1.4. Haynes C276
3.2. Corrosion in Liquid Cadmium
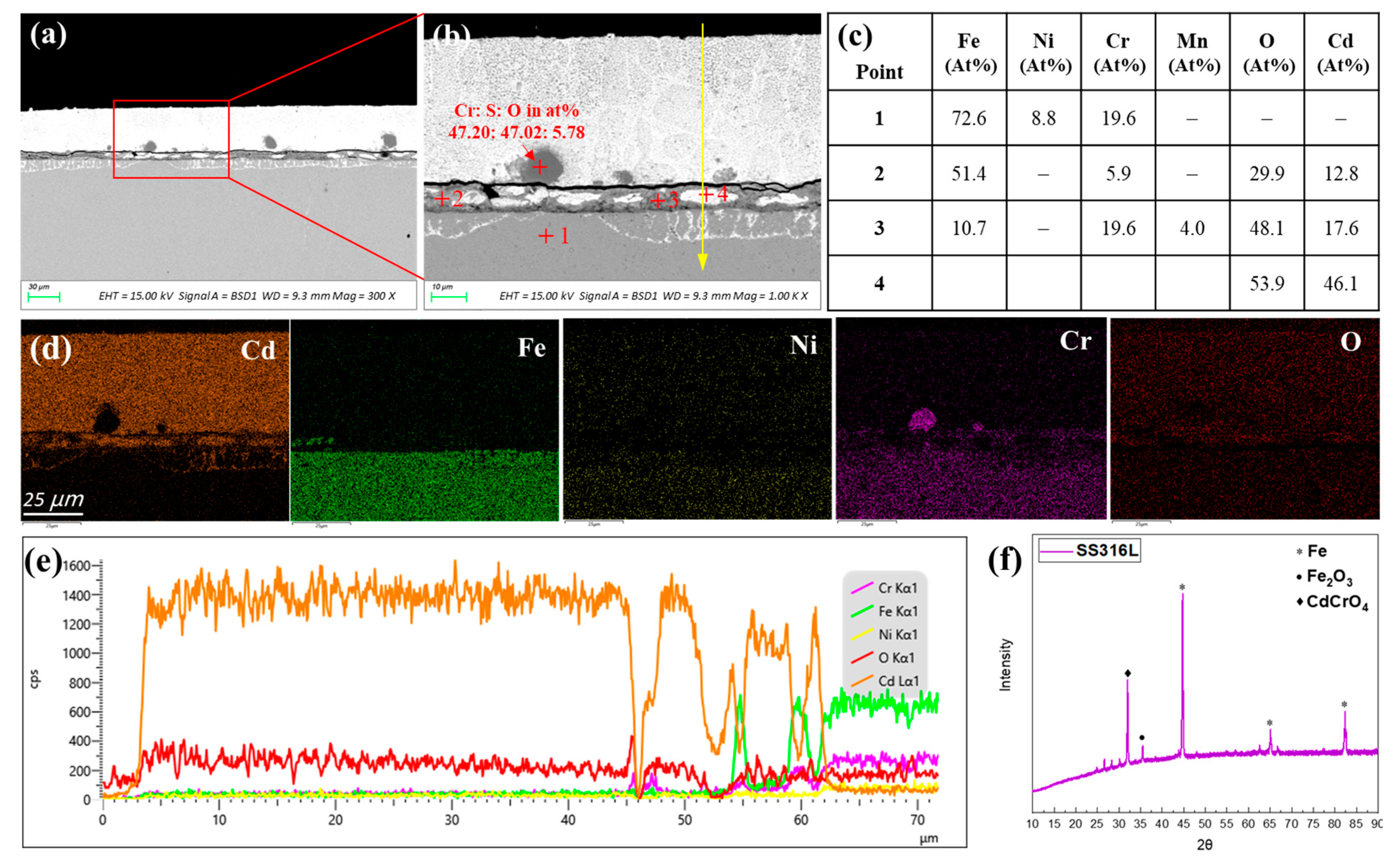
3.2.1. AISI 316L
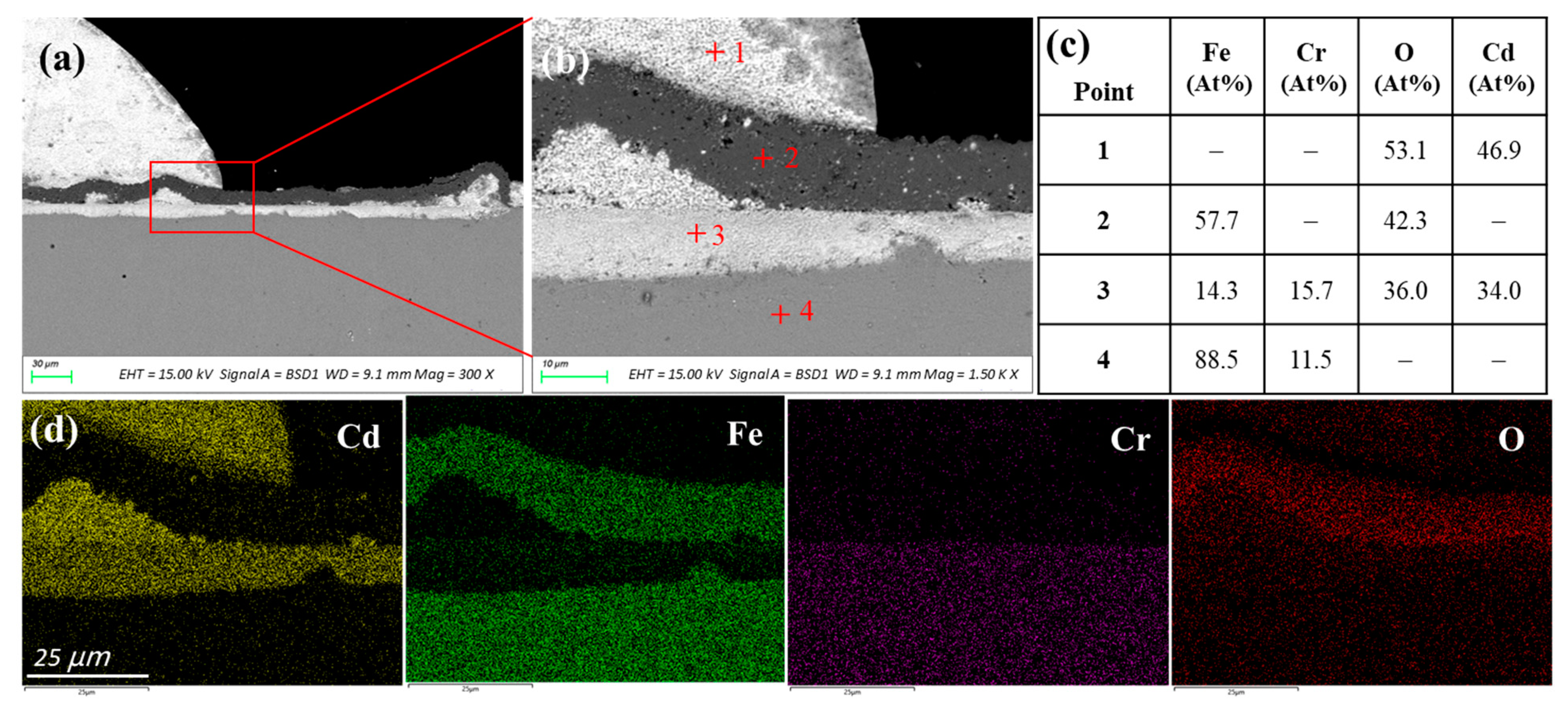
3.2.2. T91 Stainless Steel
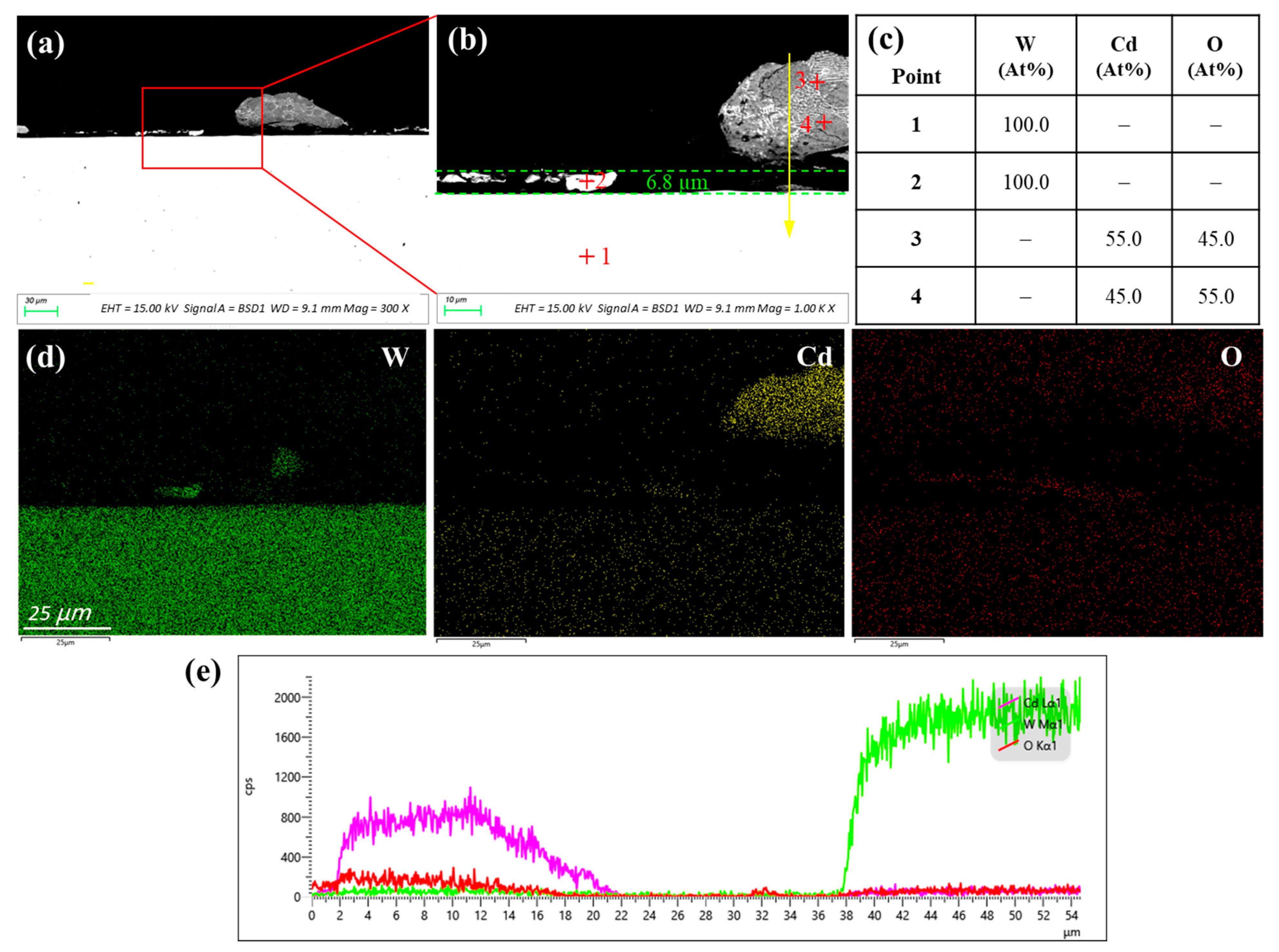
3.2.3. Tungsten
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Williamson, M.A.; Willit, J.L. Pyroprocessing flowsheets for recycling used nuclear fuel. Nucl. Eng. Technol. 2011, 43, 329–334. [Google Scholar] [CrossRef]
- Guo, S.; Zhang, J.; Wu, W.; Zhou, W. Corrosion in the Molten Fluoride and Chloride Salts and Materials Development for Nuclear Applications. Prog. Mater. Sci. 2018, 97, 448–487. [Google Scholar] [CrossRef]
- Chang, Y.; Benedict, R.W.; Bucknor, M.D.; Figueroa, J.; Herceg, J.E.; Johnson, T.R. Conceptual Design of a Pilot-Scale Pyroprocessing Facility. Nucl. Technol. 2019, 205, 708–726. [Google Scholar] [CrossRef]
- Argonne National Laboratory, Merrick and Company, Conceptual Design of a Pilot-Scale Pyroprocessing Facility. Nucl. Technol. 2018, 205, 708–726.
- Kawahara, Y. High temperature corrosion mechanisms and effect of alloying elements for materials used in waste incineration environment. Corros. Sci. 2002, 44, 223–245. [Google Scholar] [CrossRef]
- Ishitsuka, T.; Nose, K. Stability of protective oxide films in waste incineration environment-solubility measurement of oxides in molten chlorides. Corros. Sci. 2002, 44, 247–263. [Google Scholar] [CrossRef]
- Zahrani, F.M.; Alfantazi, A.M. Hot corrosion of Inconel 625 wrought alloy and weld overlay on carbon steel by gas metal arc welding in 47 PbSO4-23 ZnO-13 Pb3O4-7 PbCl2-5 CdO-5 Fe2O3 molten salt mixture. Corros. Sci. 2021, 183, 109348. [Google Scholar] [CrossRef]
- Phillips, W.; Chidambaram, D. Corrosion of stainless steel 316L in molten LiCl-Li2O-Li. J. Nucl. Mater. 2019, 517, 241–253. [Google Scholar] [CrossRef]
- Liu, R.Y.; Wang, X.; Zhang, J.S.; Wang, X.M. Corrosion of nickel in molten LiCl–Li2O at 750 °C. J. Nucl. Mater. 2004, 327, 194–201. [Google Scholar] [CrossRef]
- Ding, W.; Bonk, A.; Bauer, T. Molten chloride salts for next generation CSP plants: Selection of promising chloride salts & study on corrosion of alloys in molten chloride salts. AIP Conf. Proc. 2019, 2126, 200014. [Google Scholar]
- Grégoire, B.; Oskay, C.; Meißner, T.M.; Galetz, M.C. Corrosion mechanisms of ferritic-martensitic P91 steel and Inconel 600 nickel-based alloy in molten chlorides. Part II: NaCl-KCl-MgCl2 ternary system. Sol. Energ. Mat. Sol. 2020, 216, 110675. [Google Scholar] [CrossRef]
- Ding, W.; Shi, H.; Xiu, Y.; Bonk, A.; Weisenburger, A.; Jianu, A.; Bauer, T. Hot corrosion behavior of commercial alloys in thermal energy storage material of molten MgCl2/KCl/NaCl under inert atmosphere. Sol. Energ. Mat. Sol. 2018, 184, 22–30. [Google Scholar] [CrossRef]
- Nikitina, E.V.; Kazakovtseva, N.A.; Karfidov, E.A. Corrosion of 16Cr12MoWSVNbB (EP-823) Steel in the LiSl-KCl Melt Containing CeCl3, NdCl3 and UCl3. J. Alloys Compd. 2019, 811, 152032. [Google Scholar] [CrossRef]
- Karfidov, E.A.; Nikitina, E.V. Corrosion Electrochemical Behavior of Nickel in the LiCl-KCl Melt Containing Lanthanum Trichloride. Russ. Metall. 2019, 8, 820–824. [Google Scholar] [CrossRef]
- Guo, S.; Zhuo, W.; Wang, Y.; Zhang, J. Europium Induced Alloy Corrosion and Cracking in Molten Chloride Media for Nuclear Applications. Corros. Sci. 2020, 163, 108279. [Google Scholar] [CrossRef]
- Shankar, A.R.; Mathiya, S.; Thyagarajan, K.; Mudali, U.K. Corrosion and Microstructure Correlation in Molten LiCl-KCl Medium. Metall. Mat. Trans. A 2010, 41, 1815–1825. [Google Scholar] [CrossRef]
- Shankar, A.R.; Kanagasundar, A.; Mudali, U.K. Corrosion of Nickel-Containing Alloys in Molten LiCl-KCl Medium. Corrosion 2013, 69, 48–57. [Google Scholar] [CrossRef]
- Shankar, A.R.; Thyagarajan, K.; Mudali, U.K. Corrosion Behavior of Candidate Materials in Molten LiCl-KCl Salt under Argon Atmosphere. Corrosion 2013, 69, 655–665. [Google Scholar] [CrossRef]
- Rao, C.J.; Shankar, A.R.; Ajikumar, P.K.; Kamruddin, M.; Mallika, C.; Mudali, U.K. Corrosion Behavior of Structural Materials in LiCl-KCl Molten Salt by Thermogravimetric Study. Corrosion 2015, 71, 502–509. [Google Scholar] [CrossRef]
- Chang, S.; Jia, Y.; Du, X.; Guo, S. Corrosion Behavior of Commercial Alloys in LiCl–KCl Molten Salt Containing EuCl3. Front. Mater. 2022, 9, 958296. [Google Scholar] [CrossRef]
- Predel, B. Cd-Ni (Cadmium-Nickel), In Phase Equilibria, Crystallographic and Thermodynamic Data of Binary Alloys · Ca-Cd—Co-Zr; Madelung, O., Ed.; SpringerMaterials: Berlin/Heidelberg, Germany, 1993; pp. 1–3. [Google Scholar]
- Zhang, J.; Li, N. Review of the studies on fundamental issues in LBE corrosion. J. Nucl. Mater. 2008, 373, 351–377. [Google Scholar] [CrossRef]
- Zhang, J. A review of steel corrosion by liquid lead and lead–bismuth. Corros. Sci. 2009, 51, 1207–1227. [Google Scholar] [CrossRef]
- Gong, X.; Short, M.P.; Auger, T.; Charalampopoulou, E.; Lambrinou, K. Environmental degradation of structural materials in liquid lead- and lead-bismuth eutectic-cooled reactors. Prog. Mater. Sci. 2022, 126, 100920. [Google Scholar] [CrossRef]
- Hosoya, Y.; Terai, T.; Yoneoka, T.; Tanaka, S. Compatibility of structural materials with molten chloride mixture at high temperature. J. Nucl. Mater. 1997, 248, 348–353. [Google Scholar] [CrossRef]
- Colom, F.; Bodalo, A. Corrosion of Iron (ARMCO) in KCl-LiCl Melts. Corros. Sci. 1973, 12, 731–7388. [Google Scholar] [CrossRef]
- Nishikata, A.; Numata, H.; Tsuru, T. Electrochemistry of molten salt corrosion. Mater. Sci. Eng. A 1991, 146, 15–31. [Google Scholar] [CrossRef]
- Fusselman, S.P.; Roy, J.J.; Grimmett, S.L. Thermodynamic Properties for Rare Earths and Americium in Pyropartitioning Process Solvents. J. Electrochem. Soc. 1999, 146, 2573–2580. [Google Scholar] [CrossRef]
- Feng, X.K.; Melendres, C.A. Anodic Corrosion and Passivation Behavior of Some Metals in Molten LiCl-KCl Containing Oxide Ions. J. Electrochem. Soc. 1982, 129, 1245–1249. [Google Scholar] [CrossRef]
- Polovov, I.B.; Abramov, A.V.; Rebrin, O.I.; Volkovich, V.A.; Denisov, E.I.; Griffiths, T.R.; May, I.; Kinoshita, H. Corrosion of Stainless Steel in NaCl-KCl Based Melts. ECS Trans. 2010, 33, 321–327. [Google Scholar] [CrossRef]
- Chasanov, M.G.; Hunt, P.D.; Johnson, I.; Feder, H.M. Solubility of 3-d transition metals in liquid cadmium. Trans. Met. Soc. AIME 1962, 224, 935. [Google Scholar]
- Muller, O.; Roy, R.; White, W. Phase Equilibria in the Systems NiO-Cr2O3-O2, MgO-Cr2O3-O2, and CdO-Cr2,O3-O2 at High Oxygen Pressures. J. Am. Ceram. 1968, 51, 693–699. [Google Scholar] [CrossRef]
- Yeliseyeva, O.; Tsisar, V.; Benamati, G. Influence of temperature on the interaction mode of T91 and AISI 316L steels with Pb–Bi melt saturated by oxygen. Corros. Sci. 2008, 50, 1672–1683. [Google Scholar] [CrossRef]
- Schroer, C.; Wedemeyer, O.; Novotny, J.; Skrypnik, A.; Konys, J. Selective leaching of nickel and chromium from Type 316 austenitic steel in oxygen-containing lead–bismuth eutectic (LBE). Corros. Sci. 2014, 84, 113–124. [Google Scholar] [CrossRef]
- Hosemann, P.; Frazer, D.; Stergar, E.; Lambrinou, K. Twin boundary-accelerated ferritization of austenitic stainless steels in liquid lead–bismuth eutectic. Scr. Mater. 2016, 118, 37–40. [Google Scholar] [CrossRef]
- Jang, J.; Han, S.; Kim, T.-J.; Kim, G.-Y.; Lee, C.H.; Lee, S.-J. Stability of Tungsten Crucible against Uranium, Rare Earth, Cadmium, and Chlorides for Cathode Process in Pyroprocessing. Sci. Technol. Nucl. Install. 2019, 2019, 4121285. [Google Scholar] [CrossRef]


| Materials | Cr | Fe | Ni | Mo | Si | W | Mn | Al | V | Cu | C | Others |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Haynes C276 | 16.17 | 6.22 | Bal. | 16.08 | – | 3.21 | – | 0.28 | 0.081 | 0.079 | – | Co 0.067 |
| Inconel 600 | 15.72 | 9.90 | Bal. | – | 0.118 | – | 0.396 | 0.11 | 0.024 | – | ||
| AISI 316L | 16.3 | Bal. | 10.15 | 2.57 | 0.48 | – | 0.97 | 0.015 | – | |||
| T91 | 9.47 | Bal. | 0.073 | 0.96 | 0.27 | – | 0.42 | 0.015 | 0.21 | 0.1 | ≤0.105 | |
| 42CrMo | 1.0 | Bal. | 0.2 | 0.25 | – | 0.80 | 0.42 | – |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jia, Y.; Chang, S.; Du, X.; Guo, S. Corrosion Performance of Commercial Alloys and Refractory Metals in Conditions for Electrorefining of Spent Nuclear Fuels. Crystals 2023, 13, 817. https://doi.org/10.3390/cryst13050817
Jia Y, Chang S, Du X, Guo S. Corrosion Performance of Commercial Alloys and Refractory Metals in Conditions for Electrorefining of Spent Nuclear Fuels. Crystals. 2023; 13(5):817. https://doi.org/10.3390/cryst13050817
Chicago/Turabian StyleJia, Yanhong, Shuangshuang Chang, Xin Du, and Shaoqiang Guo. 2023. "Corrosion Performance of Commercial Alloys and Refractory Metals in Conditions for Electrorefining of Spent Nuclear Fuels" Crystals 13, no. 5: 817. https://doi.org/10.3390/cryst13050817





