Thermodynamic Analysis and Experimental Optimization for the Purification of Ni-Co-Mn Mixed Sulfate Solution from the Recovery Process of Lithium-Ion Batteries
Abstract
:1. Introduction
2. Experimental
- η: the removal rate of impurity ions;
- c0: the initial concentration of impurity ions in the leaching solution;
- V0: the corresponding initial volume of the leaching solution in a single impurity removal experiment;
- c1: the concentration of impurity ions in the solution after impurity removal;
- V1: the solution volume of the solution after impurity removal.
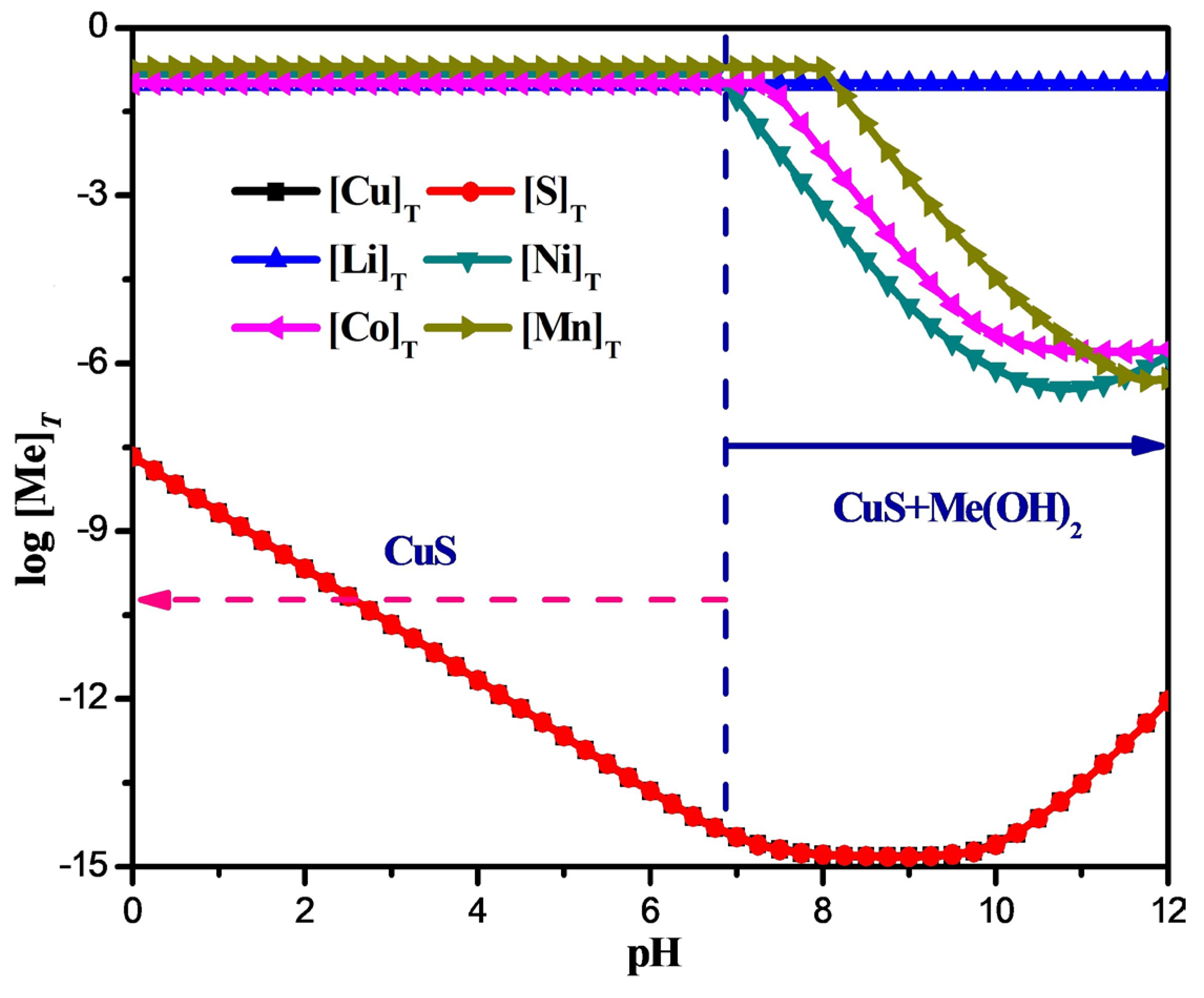
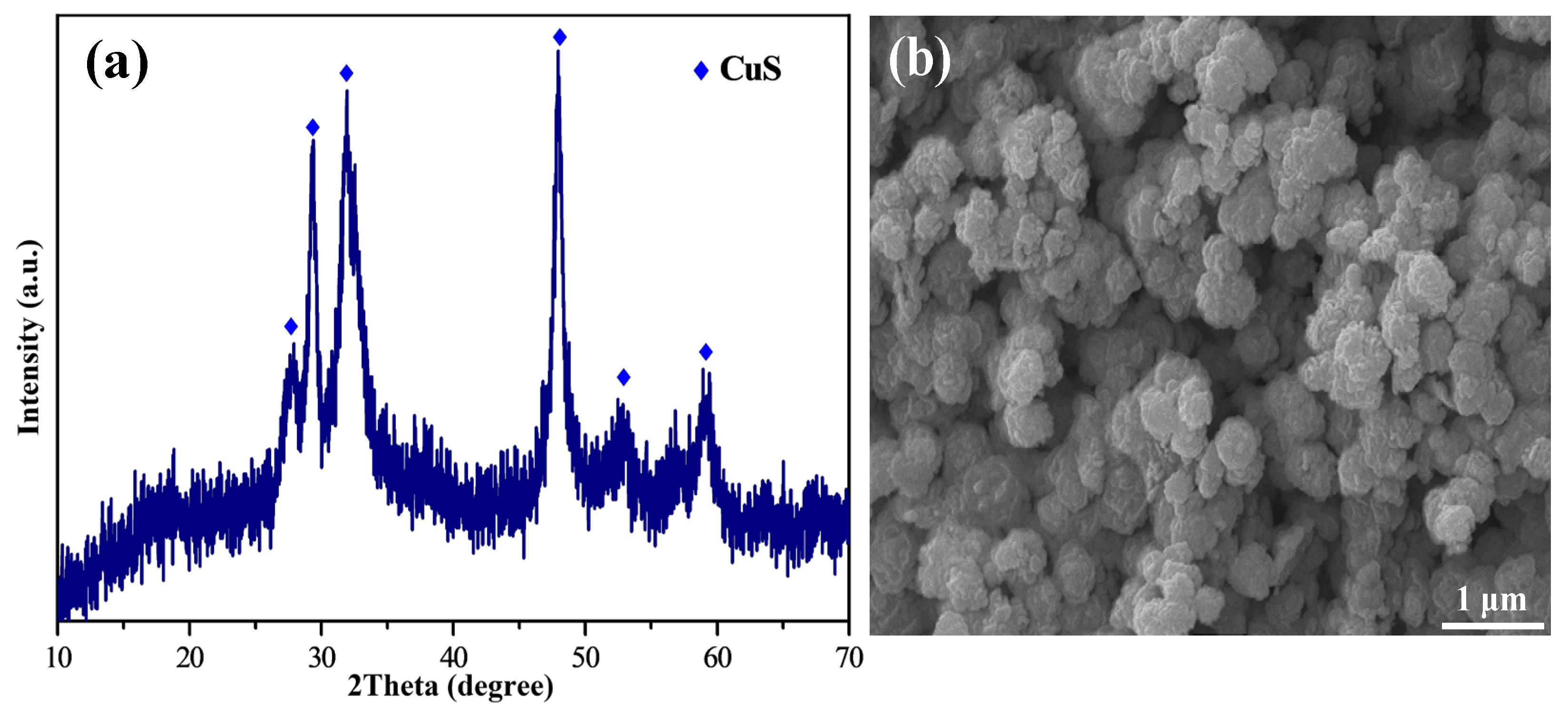
2.1. Removal of Cu2+ by Na2S2O3
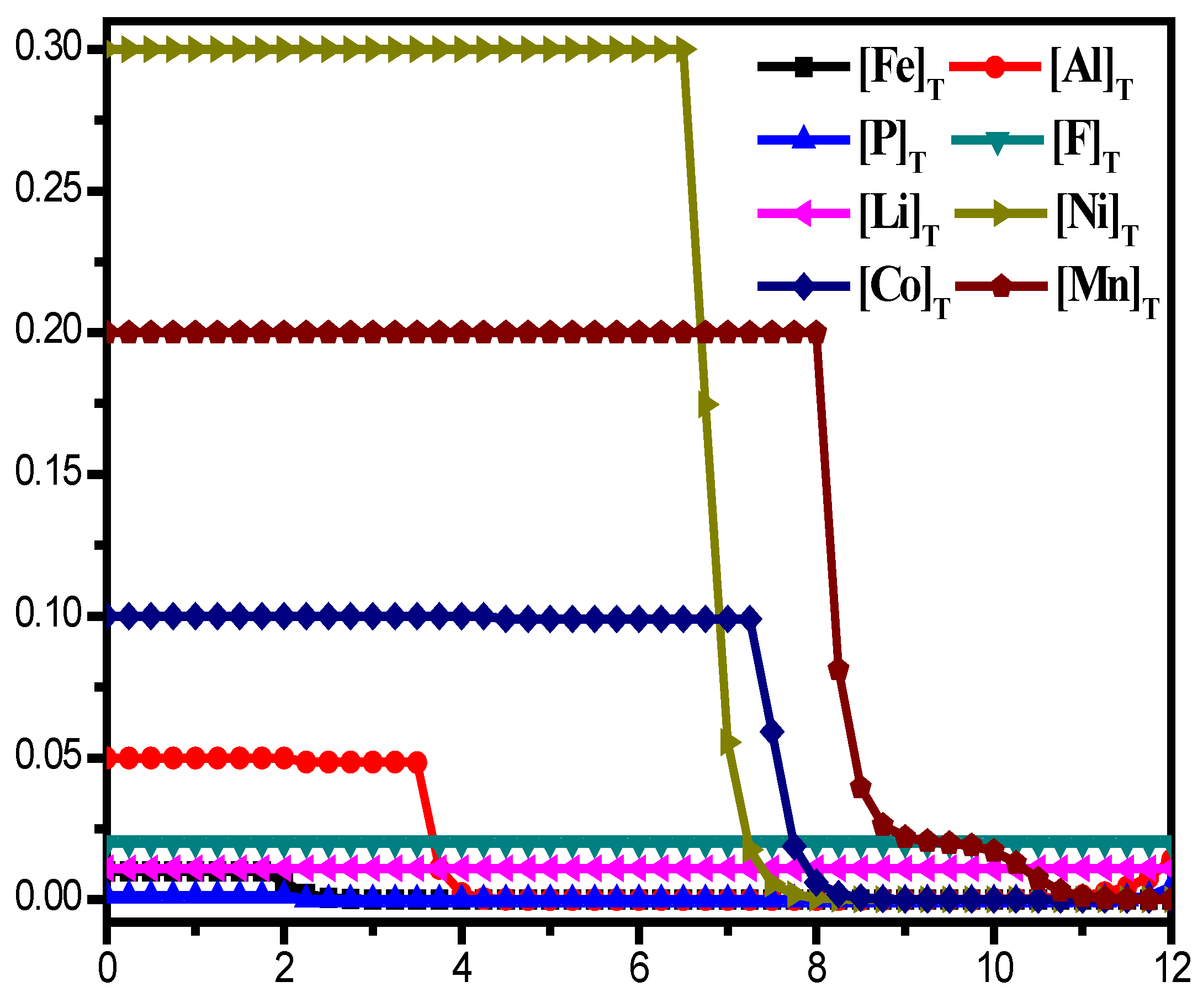
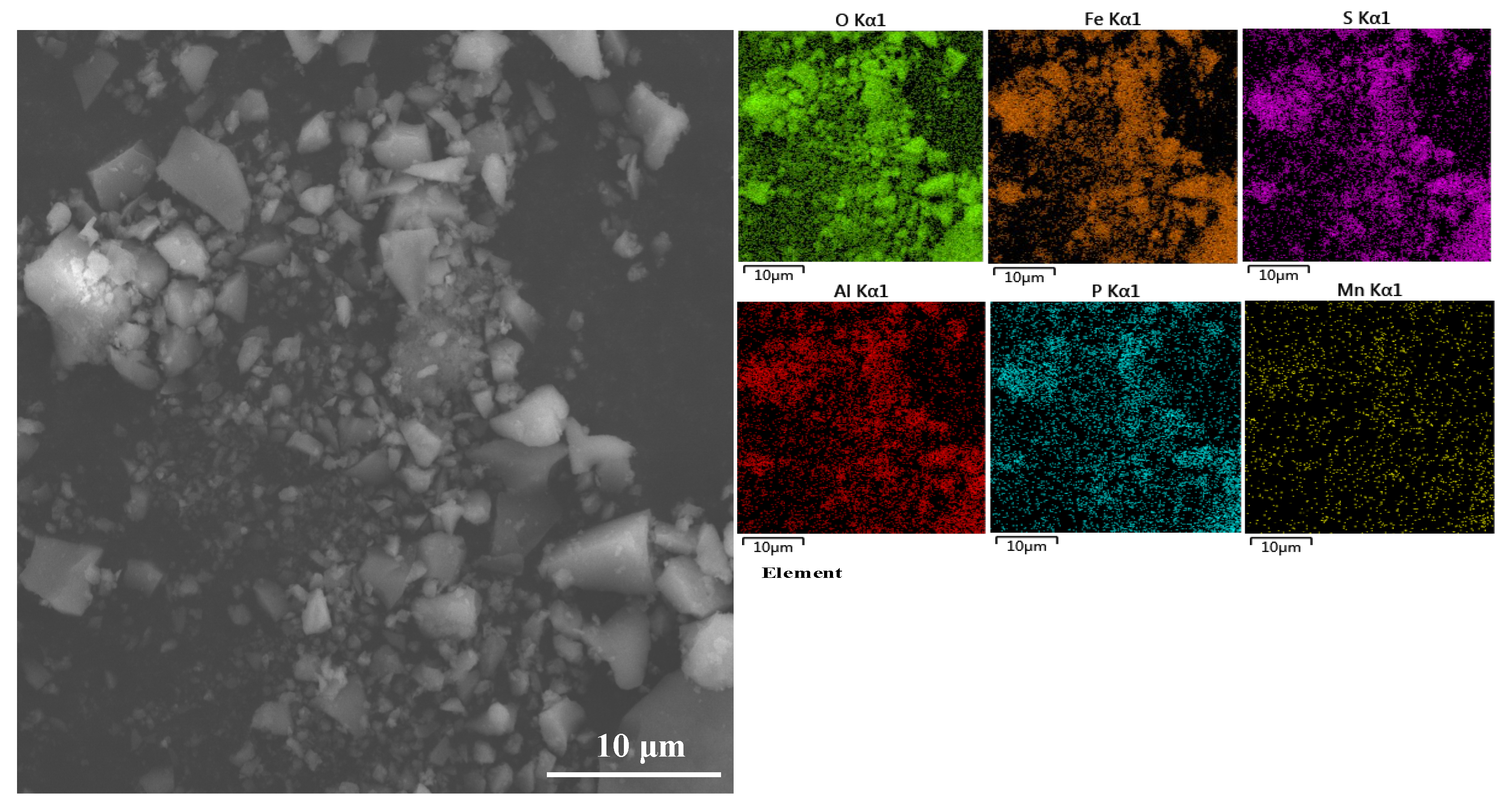
2.2. Removal of Fe3+ and PO43−
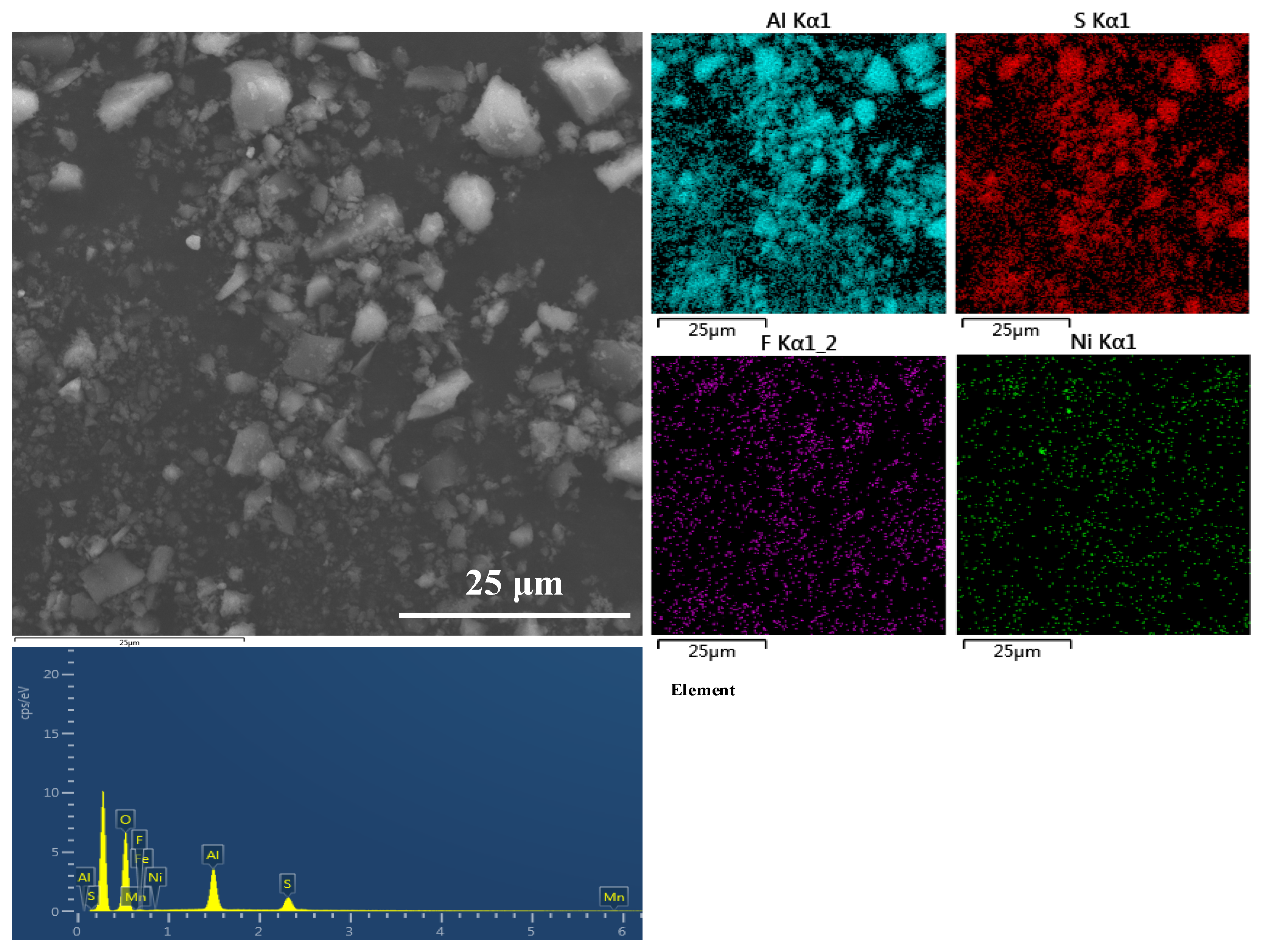
2.3. Removal of Al3+
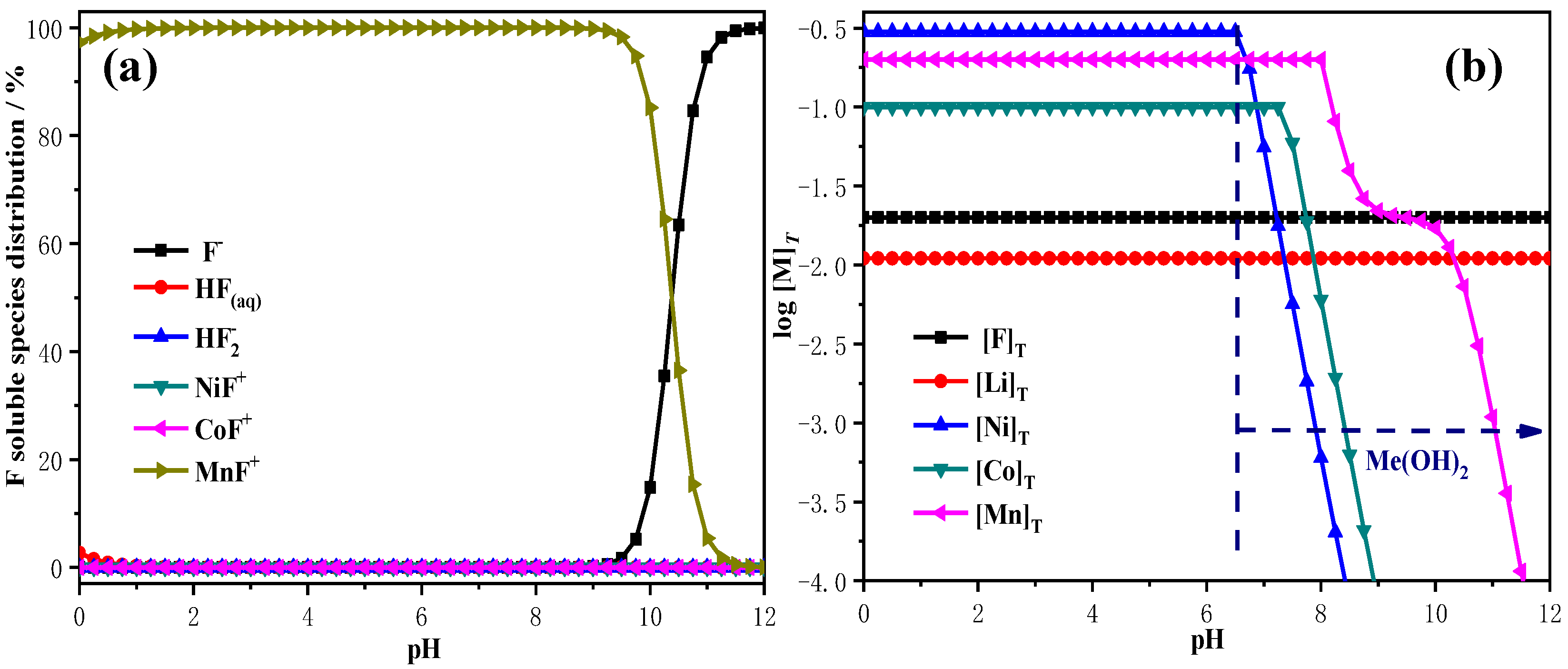
2.4. Removal of F−
3. Experimental Optimization
3.1. Optimization of Cu2+ Removal Process
3.2. Optimization of Fe3+ and PO43− Removal Process
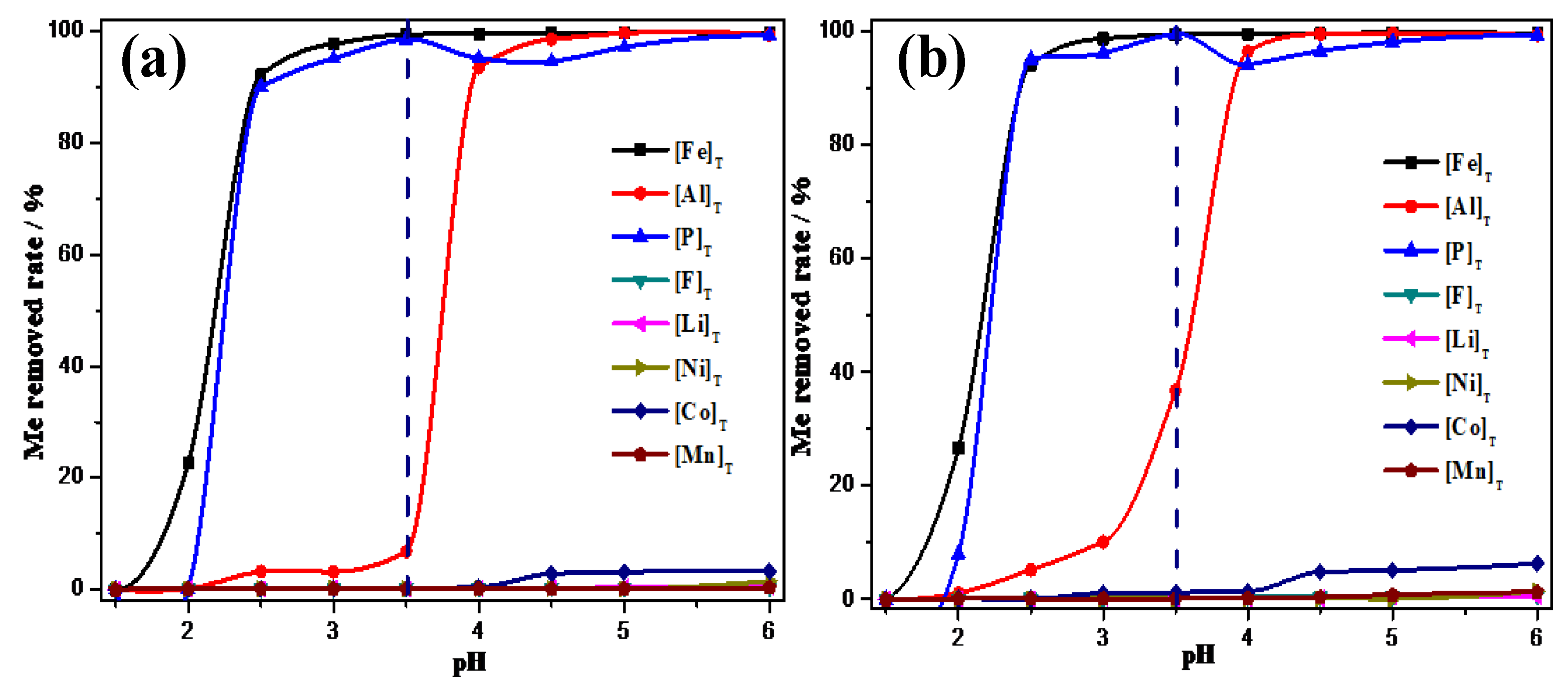
3.3. Optimization of Al3+ Removal Process
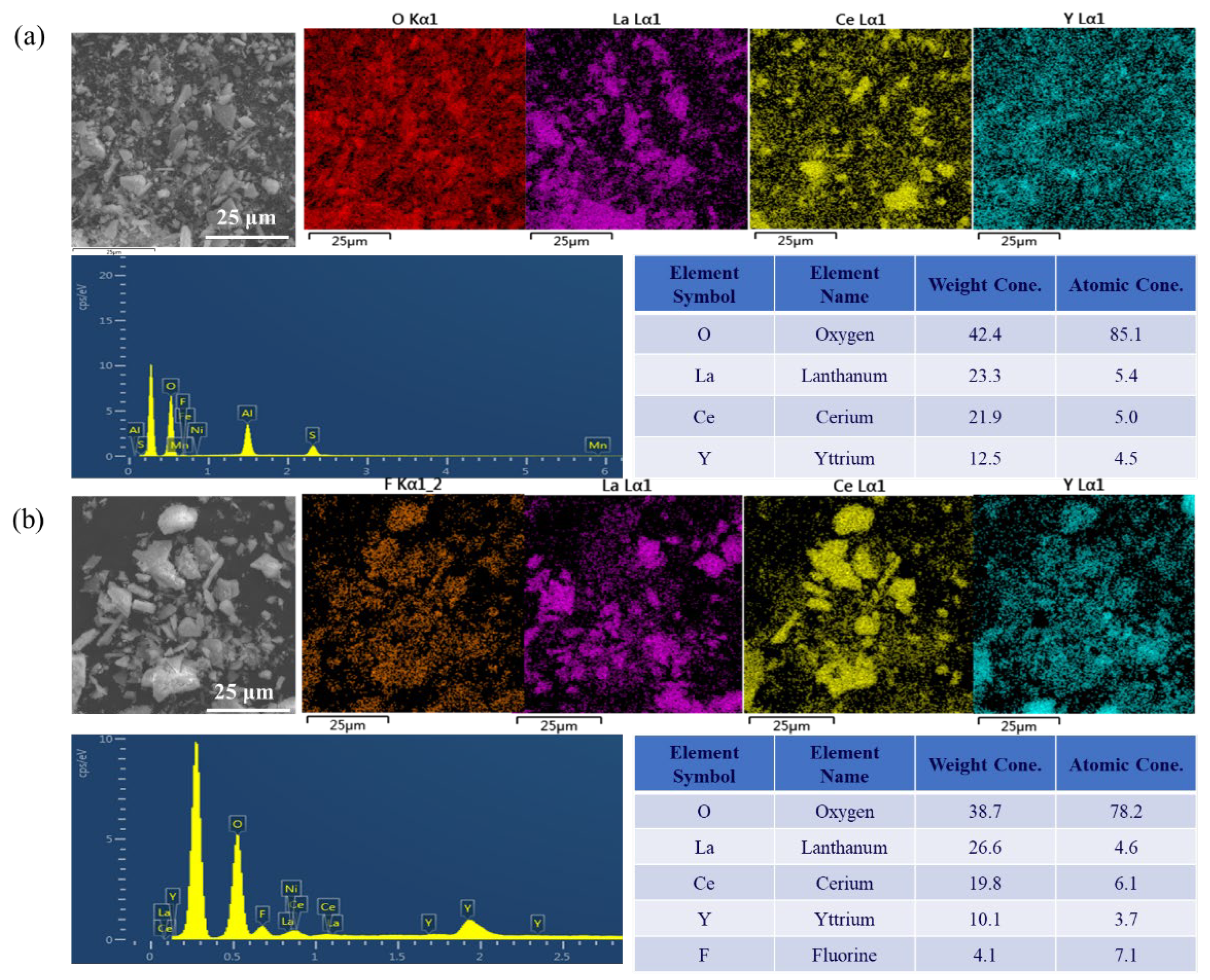
3.4. Optimization of the F− Removal Process
4. Conclusions
- (1)
- For solution systems containing multiple impurity ions at the same time, a corresponding thermodynamic model for solid-phase precipitation can be constructed to infer the types of precipitation that can be generated under corresponding pH conditions. On the basis of theory, corresponding experiments were conducted to determine the optimal process parameters for impurity ion removal, ultimately achieving the removal of impurity ions.
- (2)
- On the basis of theoretical calculations, in this study Na2S2O3 was first added to the leaching solution to precipitate and remove Cu2+ in the form of CuS. Then, the pH value of the solution system was adjusted according to the coprecipitation principle, so that Fe3+, PO43− and Al3+ were precipitated and removed in the form of Fe(OH)3, AlPO4, and Al(OH)3, respectively. Finally, rare earth oxides were used as defluorination agents for F− removal work.
- (3)
- The optimal removal conditions for Cu2+ are as follows: the acidity of the solution system is 0.1 mol/L H2SO4, 75 ℃, 120 min, and the addition of the amount of Na2S2O3 was 3 times the molar amount of Cu2+. Under these conditions, Cu2+ can be removed in the form of CuS, with a removal rate of 99.8% for Cu2+ and a loss rate of main metals below 0.2%.
- (4)
- The optimal removal conditions for Fe3+ and PO43− are the pH of the solution system is 3.5 and the temperature is 25 ℃. Under optimal conditions, Fe3+ and PO43− can be precipitated and removed in the form of FePO4, with a removal rate of 99.8% for Fe3+ and 97.8% for PO43−;
- (5)
- The optimal removal conditions for Al3+ are the pH of the solution system is 4.5 and the temperature is 25 ℃. Under optimal conditions, the removal rate of aluminum is close to 99%, and the concentration of Al3+ in the solution is less than 3 ppm.
- (6)
- The optimal removal conditions for F− are as follows: the pH of the solution system is 6.0, 25 ℃, and the dose of the dilution agent is 6 g/L and 120 min. Under the optimal F− removal conditions, the removal rate of F− can reach 97.1%, the main metal loss rate is less than 0.6%, and the concentration of F− in the solution is less than 10 ppm.
- (7)
- Various studies have been conducted on the removal of impurity ions from the leaching solution of acid-based waste lithium-ion batteries using aluminum ash, but most methods have problems such as incomplete impurity removal, introduction of new impurity ions, high cost of impurity removal reagents, and complex impurity removal processes. Compared with the above issues, the method proposed in this article is relatively simple and can simultaneously precipitate and remove multiple ions, with a good impurity removal effect. It provides a reference for impurity removal work in solution systems where multiple impurity ions coexist.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dutta, D.; Kumari, A.; Panda, R.; Jha, S.; Gupta, D.; Goel, S.; Jha, M.K. Close loop separation process for the recovery of Co, Cu, Mn, Fe and Li from spent lithium-ion batteries. Sep. Purif. Technol. 2018, 200, 327–334. [Google Scholar] [CrossRef]
- Minsang, J.; Sanghyuk, P.; Song, J.; Kwon, K. Incorporation of Cu into Li[Ni1/3Co1/3Mn1/3]O2cathode: Elucidating its electrochemical properties and stability. J. Alloys Compd. 2018, 764, 112–121. [Google Scholar]
- Beak, M.; Park, J.; Park, S.; Jeong, S.; Kang, J.; Choi, W.; Yoon, W.; Kwon, K. Understanding the effect of nonmetallic impurities in regenerated cathode materials for lithium-ion battery recycling by tracking down impurity elements. J. Hazard. Mater. 2022, 425, 127907. [Google Scholar] [CrossRef] [PubMed]
- Natarajanm, S.; Aravindan, V. Recycling strategies for spent Li-ion battery mixed cathodes. ACS Energy Lett. 2018, 3, 2101–2103. [Google Scholar] [CrossRef] [Green Version]
- Chang, Y.; Zhai, X.; Li, B.; Fu, Y. Removal of iron from acidic leach liquor of lateritic nickel ore by goethite precipitate. Hydrometallurgy 2010, 101, 84–87. [Google Scholar] [CrossRef]
- Liu, P.F.; Zhang, Y.F.; Wang, L.; You, S.; Bo, J. Thermodynamics and nucleation mechanism of ammonium jarosite in sulfuric acid solution. J. Cryst. Growth 2017, 478, 52–57. [Google Scholar] [CrossRef]
- Silva, M.D.F.D.; Oliveira, M.R.D.S.; Santos, I.D.D.; Rouse, P.R.; Mansur, M.B. Iron Precipitation Strategies from Nickel Laterite Ore Sulfuric Acid Leach Liquor. Miner. Process. Extr. Metall. Rev. 2020, 19, 28–39. [Google Scholar]
- Das, G.K.; Li, J. Iron Removal as Goethite from Synthetic Laterite Leach Solutions. ACS Omega 2023, 8, 11931–11940. [Google Scholar] [CrossRef]
- Izadi, A.; Mohebbi, A.; Amiri, M.; Izadi, N. Removal of iron ions from industrial copper raffinate and electrowinning electrolyte solutions by chemical precipitation and ion exchange. Miner. Eng. 2017, 113, 23–35. [Google Scholar] [CrossRef]
- Ma, X.; Dang, R.; Liu, J.; Li, J.; Kang, Y.; Gong, Y.; Zhang, Z.; Ma, Y. Study on the formation process for Co2+-Ni2+-Fe3+-CO32-LDHs prepared by trisodium citrate complexing agent-assisted homogeneous precipitation method. Instrum. Sci. Technol. 2016, 19, 51–63. [Google Scholar] [CrossRef]
- Feitknecht, W.; Michaelis, W. Uber die Hydrolyse von Eisen(III) Perchlorat-Lsungen. Helv. Chim. Acta 2004, 45, 212–224. [Google Scholar] [CrossRef]
- Bian, R.; Su, T.; Chen, Y.; Qu, Z.; Liu, C.; Huo, Y.; Li, T. High-performance Al separation and Zn recovery from a simulated hazardous sludge. Arab. J. Chem. 2021, 14, 102996. [Google Scholar] [CrossRef]
- Li, H.; Kuang, G.; Hu, S.; Guo, H.; Jin, R.; Vekariya, R.L. Removal of aluminum from leaching solution of lepidolite by adding ammonium. JOM 2016, 68, 2653–2658. [Google Scholar] [CrossRef]
- Türk, O.K.; Zoungrana, A.; Çakmakci, M. Chemical precipitation and membrane distillation process for the treatment of acidic anodic oxidation wastewaters. J. Environ. Chem. Eng. 2022, 10, 108036. [Google Scholar] [CrossRef]
- Peeters, N.; Binnemans, K.; Riaño, S. Solvometallurgical recovery of cobalt from lithium-ion battery cathode materials using deep-eutectic solvents. Green Chem. 2020, 22, 4210–4221. [Google Scholar] [CrossRef]
- Aksamitowski, P.; Filipowiak, K.; Wieszczycka, K. Selective extraction of copper from Cu-Zn sulfate media by new generation extractants. Sep. Purif. Technol. 2019, 222, 22–29. [Google Scholar] [CrossRef]
- Granata, G.; Tsendorj, U.; Liu, W.; Tokoro, C. Direct recovery of copper nanoparticles from leach pad drainage by surfactant-assisted cementation with iron powder. Colloids Surf. A Physicochem. Eng. Asp. 2019, 580, 123719. [Google Scholar] [CrossRef]
- Ban, Y.; Li, L.; Liu, C.; Yan, Y.; Gao, J.; Zhang, J.; Gao, J. Enhancing sludge dewatering and heavy metal removal by bioleaching with Na2S2O3 as substrates. Water Sci. Technol. 2018, 78, 1545–1555. [Google Scholar] [CrossRef]
- Yang, R.; Wang, S.; Duan, H.; Yuan, X.H.; Huang, Z.J.; Guo, H.; Yang, X.J. Efficient separation of copper and nickel from ammonium chloride solutions through the antagonistic effect of TRPO on Acorga M5640. Hydrometallurgy 2016, 163, 18–23. [Google Scholar] [CrossRef]
- Li, J.; Li, X.; Hu, Q.; Wang, Z.; Zheng, J.; Wu, L.; Zhang, L. Study of extraction and purification of Ni, Co and Mn from spent battery material. Hydrometallurgy 2009, 99, 7–12. [Google Scholar] [CrossRef]
- Wang, X.K.; Li, Y.B.; Zhang, Z.L.; Qiao, R.R.; Li, Y.; Liu, S.B. Study on the reaction behavior of copper ions in sodium thiosulfate sulfide waste acid. J. Taiyuan Univ. Technol. 2022, 53, 27–35. [Google Scholar]
- Li, J.; Zhang, H.; Zhang, J.; Xiao, Q.; Du, X.; Qi, T. Efficient removal of fluoride by complexation extraction: Mechanism and thermodynamics. Environ. Sci. Technol. 2019, 53, 9102–9108. [Google Scholar] [CrossRef] [PubMed]
- Tian, Z.L.; Guo, W.C.; Zhang, Z.J.; Lai, Y.Q.; Ye, S.L.; Li, J. Removal of fluorine ions from industrial zinc sulfate solution by a layered aluminum-based composite. Hydrometallurgy 2017, 171, 222–227. [Google Scholar] [CrossRef]
- Ping, Q.; Zhang, B.; Zhang, Z.; Lu, X.; Li, Y. Speciation analysis and formation mechanism of iron-phosphorus compounds during chemical phosphorus removal process. Chemosphere 2023, 310, 136852. [Google Scholar] [CrossRef] [PubMed]
- Gai, W.Z.; Deng, Z.Y. A comprehensive review of adsorbents for fluoride removal from water: Performance, water quality assessment and mechanism. Environ. Sci. Water Res. Technol. 2021, 7, 1362–1386. [Google Scholar] [CrossRef]
- Barathi, M.; Kumar, A.K.; Kumar, C.U.; Rajesh, N. Graphene oxide-aluminium oxyhydroxide interaction and its application for the effective adsorption of fluoride. RSC Adv. 2014, 4, 53711–53721. [Google Scholar] [CrossRef]
- Ye, D. Practical Inorganic Compound Thermodynamics Data Book; Metallurgical Industry Press: Beijing, China, 1981. [Google Scholar]
- Hu, C.Y.; Lo, S.L.; Kuan, W.H. Effects of the molar ratio of hydroxide and fluoride to Al(III) on fluoride removal by coagulation and electrocoagulation. J. Colloid Interface Sci. 2005, 283, 472–476. [Google Scholar] [CrossRef]
- Zhang, H.Q.; Chen, G.H.; Cai, X.; Fu, J.T.; Zhang, P.F.; Yu, H. Experimental study on iron removal and copper recovery from copper leaching solution through oxidation hydrolysis. Min. Metall. Eng. 2020, 194, 123–126. [Google Scholar]


| Metal | Li+ | Ni2+ | Co2+ | Mn2+ | Cu2+ | Al3+ | Fe3+ | F− | P |
|---|---|---|---|---|---|---|---|---|---|
| concentration/mol·L−1 | 0.011 | 0.3 | 0.1 | 0.2 | 0.04 | 0.05 | 0.01 | 0.02 | 0.0015 |
| concentration/g·L−1 | 0.08 | 16.93 | 5.89 | 11.06 | 2.55 | 1.04 | 0.56 | 0.38 | 0.143 |
| Concentration (g/L) | Li | Ni | Co | Mn | Cu | Al | Fe | F | P |
|---|---|---|---|---|---|---|---|---|---|
| Leaching liquor | 0.08 | 16.93 | 5.89 | 11.06 | 2.55 | 1.04 | 0.56 | 0.38 | 0.143 |
| After the removal of Cu | 0.079 | 16.89 | 5.86 | 11.03 | 0.005 | 1.03 | 0.55 | 0.376 | 0.142 |
| After the removal of Fe, P | 0.072 | 16.83 | 5.82 | 10.97 | 0.004 | 0.94 | 0.001 | 0.357 | 0.003 |
| After the removal of Al | 0.066 | 16.65 | 5.74 | 10.76 | 0.002 | 0.001 | <0.001 | 0.314 | 0.004 |
| After the removal of F | 0.047 | 16.48 | 5.67 | 10.6 | 0.003 | <0.001 | <0.001 | 0.009 | 0.002 |
| Removal rate/loss rate (%) | 41.3 | 2.66 | 3.73 | 3.64 | 99.8 | ~100 | ~100 | 97.6 | 97.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, Y.; Yang, J.; Zhang, P.; Liu, Z.; Zhang, Z.; Jia, M.; Liu, F.; Jiang, L. Thermodynamic Analysis and Experimental Optimization for the Purification of Ni-Co-Mn Mixed Sulfate Solution from the Recovery Process of Lithium-Ion Batteries. Crystals 2023, 13, 858. https://doi.org/10.3390/cryst13060858
Zhou Y, Yang J, Zhang P, Liu Z, Zhang Z, Jia M, Liu F, Jiang L. Thermodynamic Analysis and Experimental Optimization for the Purification of Ni-Co-Mn Mixed Sulfate Solution from the Recovery Process of Lithium-Ion Batteries. Crystals. 2023; 13(6):858. https://doi.org/10.3390/cryst13060858
Chicago/Turabian StyleZhou, Yuan, Jian Yang, Peisen Zhang, Zhidong Liu, Zongliang Zhang, Ming Jia, Fangyang Liu, and Liangxing Jiang. 2023. "Thermodynamic Analysis and Experimental Optimization for the Purification of Ni-Co-Mn Mixed Sulfate Solution from the Recovery Process of Lithium-Ion Batteries" Crystals 13, no. 6: 858. https://doi.org/10.3390/cryst13060858





