Influences of Nonaqueous Slurry Components on Polishing 4H-SiC Substrate with a Fixed Abrasive Pad
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Instruments
2.2. Polishing Experiment
2.3. Characterization
3. Results
3.1. Optimization of Nonaqueous Polishing Slurry
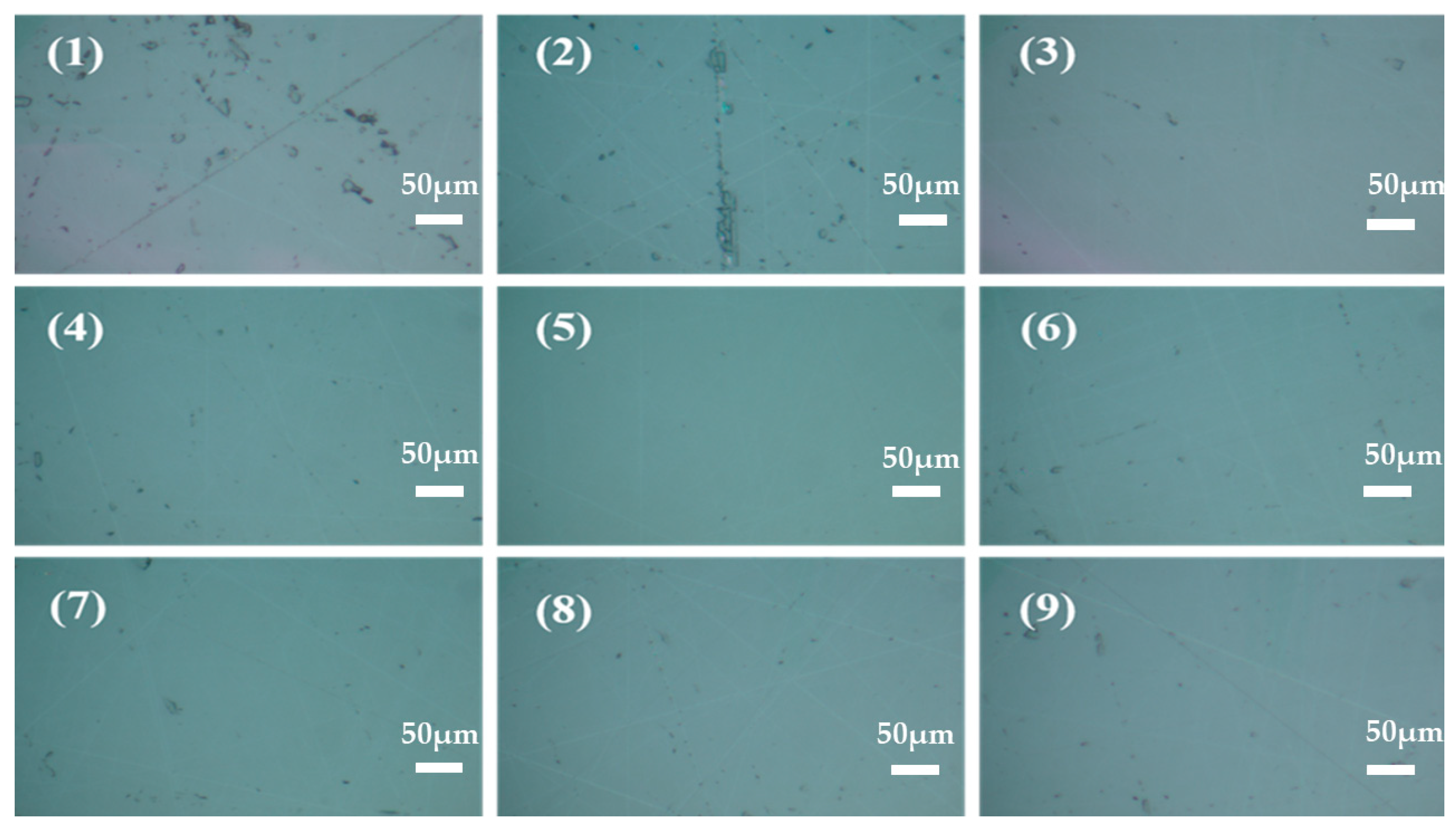
- The sum of three surface abrasion levels at level 1: K1 = 5.12 + 4.89 + 3.99 = 14 nm
- K2 = Sum of three surface abrasion levels at level 2 = 2.84, 2.23, and 3.56, or 8.63 nm
- 3.27 + 3.38 + 3.20 = 9.85 nm is the sum of the three surface roughness levels for level 3.
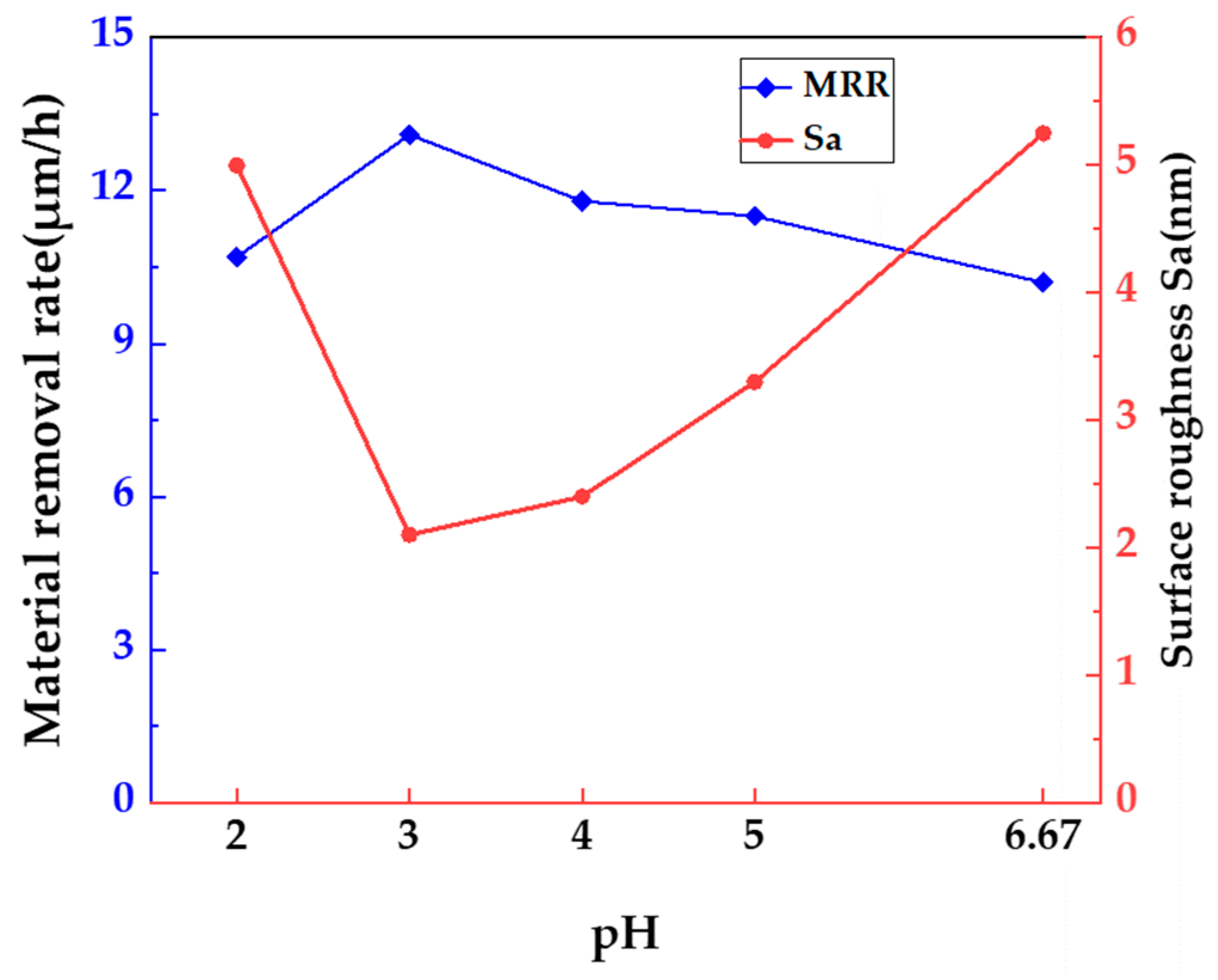
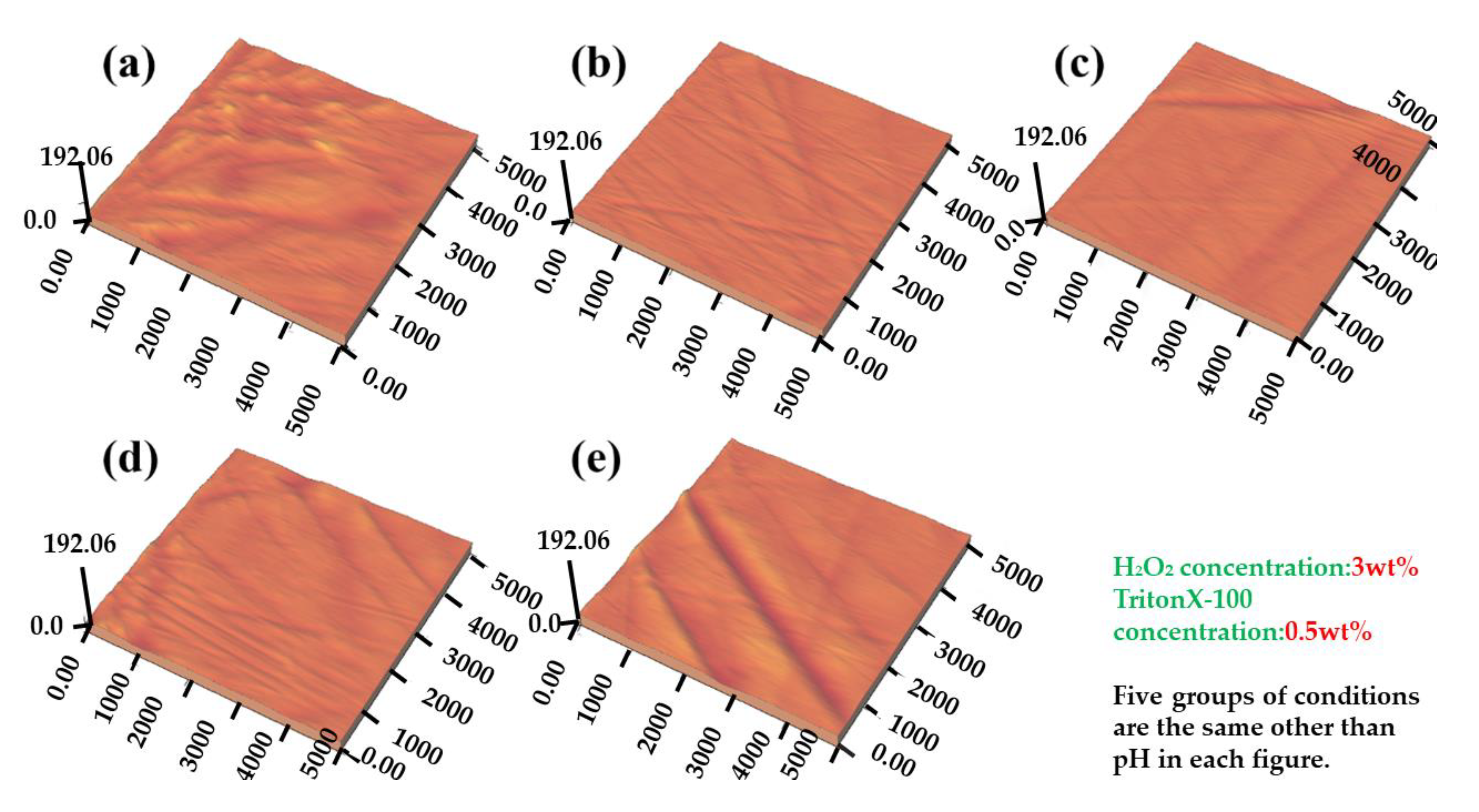
3.2. Effect of pH on Polishing 4H-SiC Substrate of Si Face
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Morris, C.A.; Anderson, M.L.; StroudIz, R.M.; MerzbacherI, C.I.; Rolison, D.R. Silica sol as a nano glue: Flexible synthesis of composite aerogels. Science 1999, 284, 622–624. [Google Scholar] [CrossRef] [PubMed]
- Kurka, D.W.; Niehues, M.; Kudruk, S.; Gerke, V.; Ravoo, B.J. Polythiolactone decorated silica particles: A versatile approach for surface functionalization, catalysis, and encapsulation. Chem. Eur. J. 2021, 27, 7667–7676. [Google Scholar] [CrossRef] [PubMed]
- Ghimire, P.P.; Jaroniec, M. Renaissance of Stöber method for synthesis of colloidal particles: New developments and opportunities. J. Colloid Interf. Sci. 2021, 584, 838–865. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Lei, H.; Liu, W. Effect of mixed-shaped silica sol abrasives on surface roughness and material removal rate of zirconia ceramic cover. Ceram. Int. 2020, 46, 23828–23833. [Google Scholar] [CrossRef]
- Sano, Y.; Arima, K.; Yamauchi, K. Planarization of SiC and GaN substrates using polishing technique utilizing catalyst surface reaction. ECS J. Solid State Sci. Technol. 2013, 2, 3028–3035. [Google Scholar] [CrossRef]
- Fury, M.A. Emerging developments in CMP for semiconductor planarization. Solid State Technol. 1995, 38, 81–86. [Google Scholar]
- Zhang, L.; Deng, H. Highly efficient and damage-free polishing of GaN (0001) by electrochemical etching-enhanced CMP process. Appl. Surf. Sci. 2020, 514, 145957. [Google Scholar] [CrossRef]
- Pan, G.; Zhou, Y.; Luo, G.; Shi, X.; Zou, C.; Gong, H. Chemical mechanical polishing (CMP) of on-axis Si-face 6H-SiC substrate for obtaining atomically flat defect-free surface. J. Mater. Sci. Mater. Electron. 2013, 24, 5040–5047. [Google Scholar] [CrossRef]
- Aida, H.; Doi, T.; Takeda, H.; Katakura, H.; Kim, S.W.; Koyama, K.; Yamazaki, T.; Uneda, M. Ultraprecision CMP for sapphire, GaN, and SiC for advanced optoelectronics materials. Curr. Appl. Phys. 2012, 12, 41–46. [Google Scholar] [CrossRef]
- Basim, G.B. Effect of slurry aging on stability and performance of chemical mechanical planarization process. Adv. Powder Technol. 2011, 22, 257–265. [Google Scholar] [CrossRef]
- Lagudu UR, K.; Babu, S. Effect of transition metal compounds on amorphous SiC removal rates. ECS J. Solid State Sci. Technol. 2014, 3, 219–225. [Google Scholar] [CrossRef] [Green Version]
- Zhang, P.; Yang, J.; Li, L. Trajectory uniformity of the double-sided mechanical polishing of SiC single crystal substrate. Mater. Sci. Semicond. Process. 2020, 107, 104814. [Google Scholar] [CrossRef]
- Lee, H.S.; Kim, D.I.; An, J.H.; Lee, H.J.; Kim, K.H.; Jeong, H. Hybrid polishing mechanism of single crystal SiC using mixed abrasive slurry (MAS). CIRP Ann. 2010, 59, 333–336. [Google Scholar] [CrossRef]
- Wang, W.; Liu, W.; Song, Z. Two-Step Chemical Mechanical Polishing of 4H-SiC (0001) Substrate. ECS J. Solid State Sci. Technol. 2021, 10, 074004. [Google Scholar] [CrossRef]
- Chen, G.; Ni, Z.; Xu, L.; Li, Q.; Zhao, Y. Performance of colloidal silica and ceria based slurries on CMP of Si-face 6H-SiC substrates. Appl. Surf. Sci. 2015, 359, 664–668. [Google Scholar] [CrossRef]
- Luo, Q.; Lu, J.; Xu, X. A comparative study on the material removal mechanisms of 6H-SiC polished by semi-fixed and fixed diamond abrasive tools. Wear 2016, 350, 99–106. [Google Scholar] [CrossRef]
- Chen, J.; Zhu, N.; Niu, F.; Peng, Y.; Su, J.; Zhu, Y. Influence of agglomerated diamond abrasive wear on sapphire material removal behavior. Diam. Relat. Mater. 2020, 108, 107965. [Google Scholar] [CrossRef]
- Chen, J.; Zhu, Y.; Wang, J.; Peng, Y.; Yao, J.; Ming, S. Relationship between mechanical properties and processing performance of agglomerated diamond abrasive compared with single diamond abrasive. Diam. Relat. Mater. 2019, 100, 107595. [Google Scholar] [CrossRef]
- Ho, J.K.; Huang, C.Y.; Tsai, M.Y.; Tsai, C.C. Investigation of Polishing Pads Impregnated with Fe and Al2O3 Particles for Single-Crystal Silicon Carbide Substrates. Appl. Sci. 2016, 6, 89. [Google Scholar] [CrossRef] [Green Version]
- Lee, E.S.; Cha, J.W.; Kim, S.H. Evaluation of the substrate polishing pad capacity and lifetime in the machining of reliable elevations. Int. J. Mach. Tools Manuf. 2013, 66, 82–94. [Google Scholar] [CrossRef]
- Kim, H.; Kim, H.; Jeong, H.; Seo, H.; Lee, S. Self-conditioning of encapsulated abrasive pad in chemical mechanical polishing. J. Mater. Process. Technol. 2003, 142, 614–618. [Google Scholar] [CrossRef]
- Chen, J.; Sun, T.; Su, J.; Li, J.; Zhou, P.; Peng, Y.; Zhu, Y. A novel agglomerated diamond abrasive with excellent micro-cutting and self-sharpening capabilities in fixed abrasive lapping processes. Wear 2021, 464, 203531. [Google Scholar] [CrossRef]
- Wang, Z.; Niu, F.; Zhu, Y.; Li, J.; Wang, J. Comparison of lapping performance between fixed agglomerated diamond pad and fixed single crystal diamond pad. Wear 2019, 432, 202963. [Google Scholar] [CrossRef]
- Yu, Y.; Hu, Z.; Wang, W.; Zhao, H.; Lu, J.; Xu, X. The double-side lapping of SiC substrates with semifixed abrasives and resin–combined plates. Int. J. Adv. Manuf. Technol. 2019, 108, 997–1006. [Google Scholar]
- Luo, Q.; Lu, J.; Xu, X. Study on the processing characteristics of SiC and sapphire substrates polished by semi-fixed and fixed abrasive tools. Tribol. Int. 2016, 104, 191–203. [Google Scholar] [CrossRef]
- Wang, H.; Niu, F.; Chen, J.; Jiang, Z.; Wang, W.; Bu, Z.; Wang, X.; Li, J.; Zhu, Y.; Sun, T. High-efficiency polishing of silicon carbide by applying reactive non-aqueous fluids to fixed abrasive pads. Ceram. Int. 2022, 48, 7273–7282. [Google Scholar] [CrossRef]
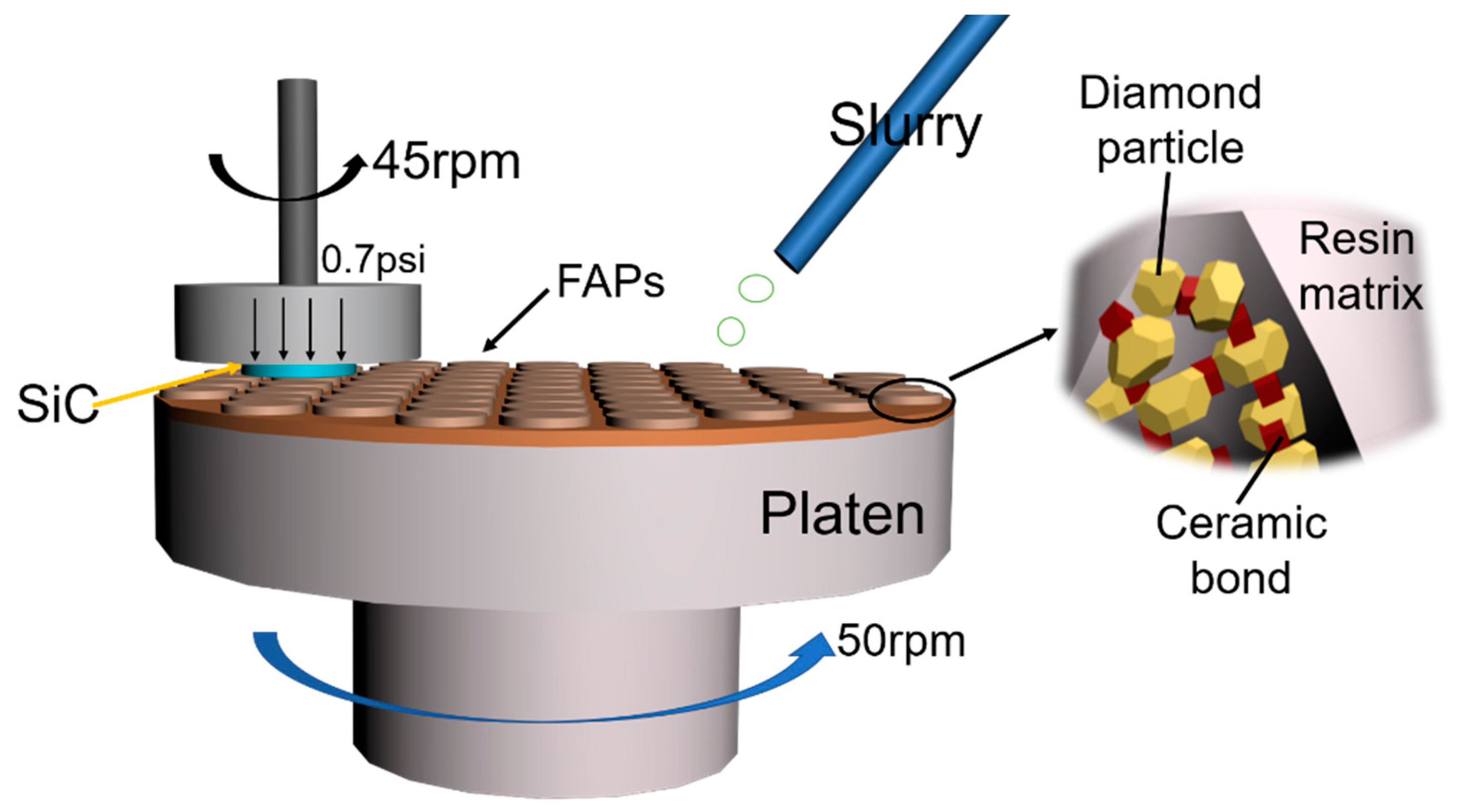

| Reagents | Specification | Manufacturers |
|---|---|---|
| 4H-SiC single-cry substrates | Φ50.8 mm | Jiangyin Lanka Crystal Materials |
| Fixed abrasive pad | Φ300 mm | Nanjing University of Aeronautics and Astronautics |
| CH3OH | (AR) | Shanghai Titan Co., Ltd. |
| Triton X-100 | (AR) | Shanghai McLean Biochemical Technology Co., Ltd. |
| H2O2 | (AR) | Shanghai Titan Co., Ltd. |
| C2H2O4 | (AR) | Shanghai Titan Co., Ltd. |
| Process Parameters | Conditions |
|---|---|
| Pressure/(psi) | 0.7 |
| Carrier speed (r/min) | 45 |
| Platen speed/(r/min) | 50 |
| Polishing rate (mL/min) | 50 |
| Polishing time (min) | 3 |
| Factors | A pH | B H2O2 Concentration (wt%) | C Triton X-100 Concentration (wt%) |
|---|---|---|---|
| Level 1 | 2 | 1 wt% | 0.1 wt% |
| Level 2 | 3 | 3 wt% | 0.3 wt% |
| Level 3 | 4 | 5 wt% | 0.5 wt% |
| Number | A pH | B H2O2 Concentration (wt%) | C Triton X-100 Concentration (wt%) | Sa (nm) |
|---|---|---|---|---|
| 1 | 1 (2) | 1 (1) | 1 (0.1) | 5.12 |
| 2 | 1 | 2 (3) | 2 (0.3) | 4.89 |
| 3 | 1 | 3 (5) | 3 (0.5) | 3.99 |
| 4 | 2 (3) | 1 | 2 | 2.84 |
| 5 | 2 | 2 | 3 | 2.23 |
| 6 | 2 | 3 | 1 | 3.56 |
| 7 | 3 (4) | 1 | 3 | 3.27 |
| 8 | 3 | 2 | 1 | 3.38 |
| 9 | 3 | 3 | 2 | 3.2 |
| K1 | 14 | 11.23 | 12.06 | |
| K2 | 8.63 | 10.5 | 10.93 | |
| K3 | 9.85 | 10.75 | 9.49 | |
| R | 5.37 | 0.73 | 2.57 |
| Number | A pH | B H2O2 Concentration (wt%) | C Triton X-100 Concentration (wt%) | Sa (nm) |
|---|---|---|---|---|
| 5 | 3 | 3 | 0.5 | 2.23 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhong, J.; Chen, J.; Wang, H.; Chen, H.; Gu, Y.; Shen, J.; Sun, T. Influences of Nonaqueous Slurry Components on Polishing 4H-SiC Substrate with a Fixed Abrasive Pad. Crystals 2023, 13, 869. https://doi.org/10.3390/cryst13060869
Zhong J, Chen J, Wang H, Chen H, Gu Y, Shen J, Sun T. Influences of Nonaqueous Slurry Components on Polishing 4H-SiC Substrate with a Fixed Abrasive Pad. Crystals. 2023; 13(6):869. https://doi.org/10.3390/cryst13060869
Chicago/Turabian StyleZhong, Jiyuan, Jiapeng Chen, Hanqiang Wang, Haibo Chen, Yunyun Gu, Juanfen Shen, and Tao Sun. 2023. "Influences of Nonaqueous Slurry Components on Polishing 4H-SiC Substrate with a Fixed Abrasive Pad" Crystals 13, no. 6: 869. https://doi.org/10.3390/cryst13060869




