Experimental Study of the Evolution of Creep-Resistant Steel’s High-Temperature Oxidation Behavior
Abstract
:1. Introduction
2. Materials and Methods
2.1. Material
2.2. Methods
3. Results and Discussion
3.1. Microstructure of the MarBN Steel in the Initial State
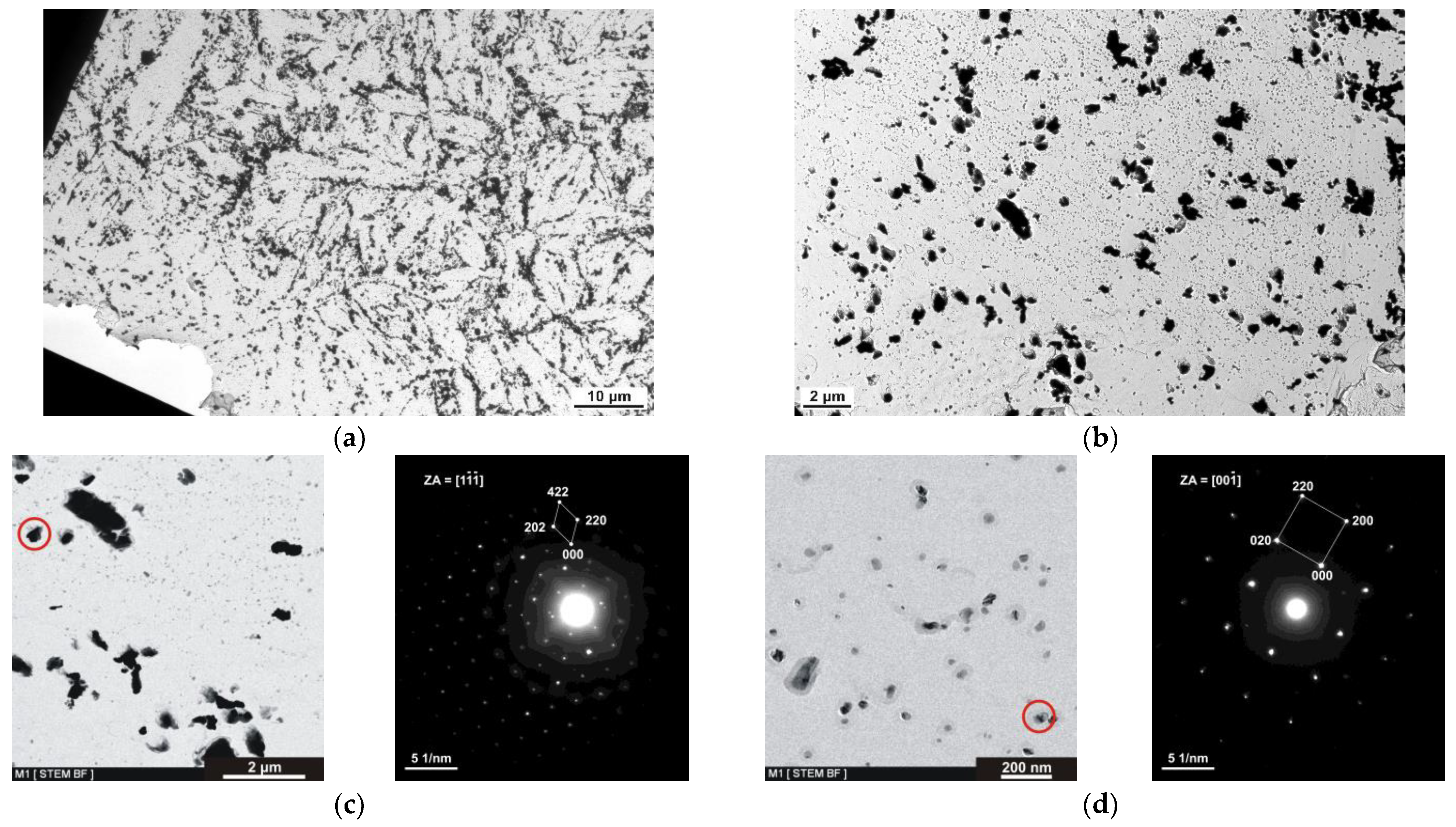
TEM Analysis of MarBN Steel in the Initial State
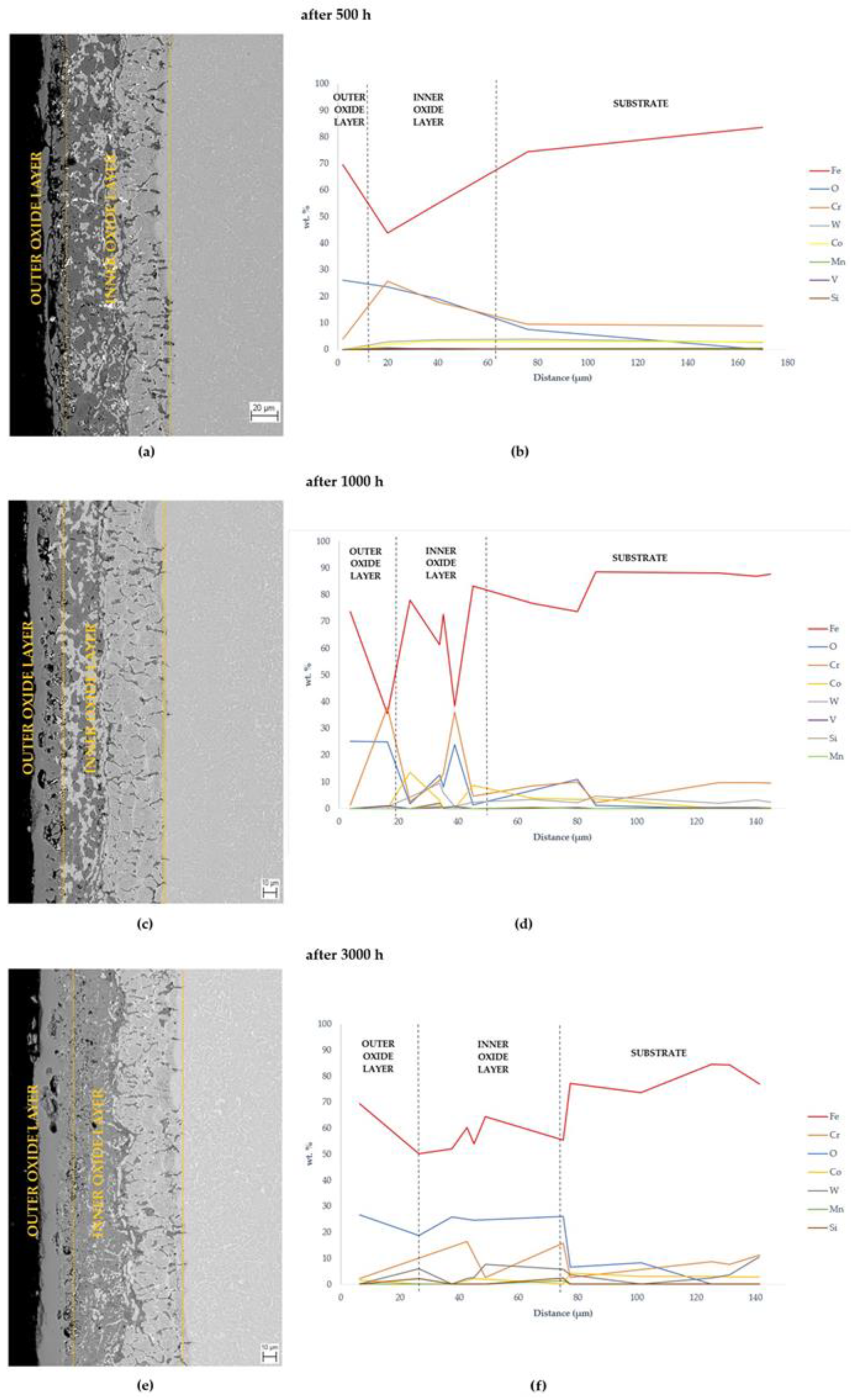
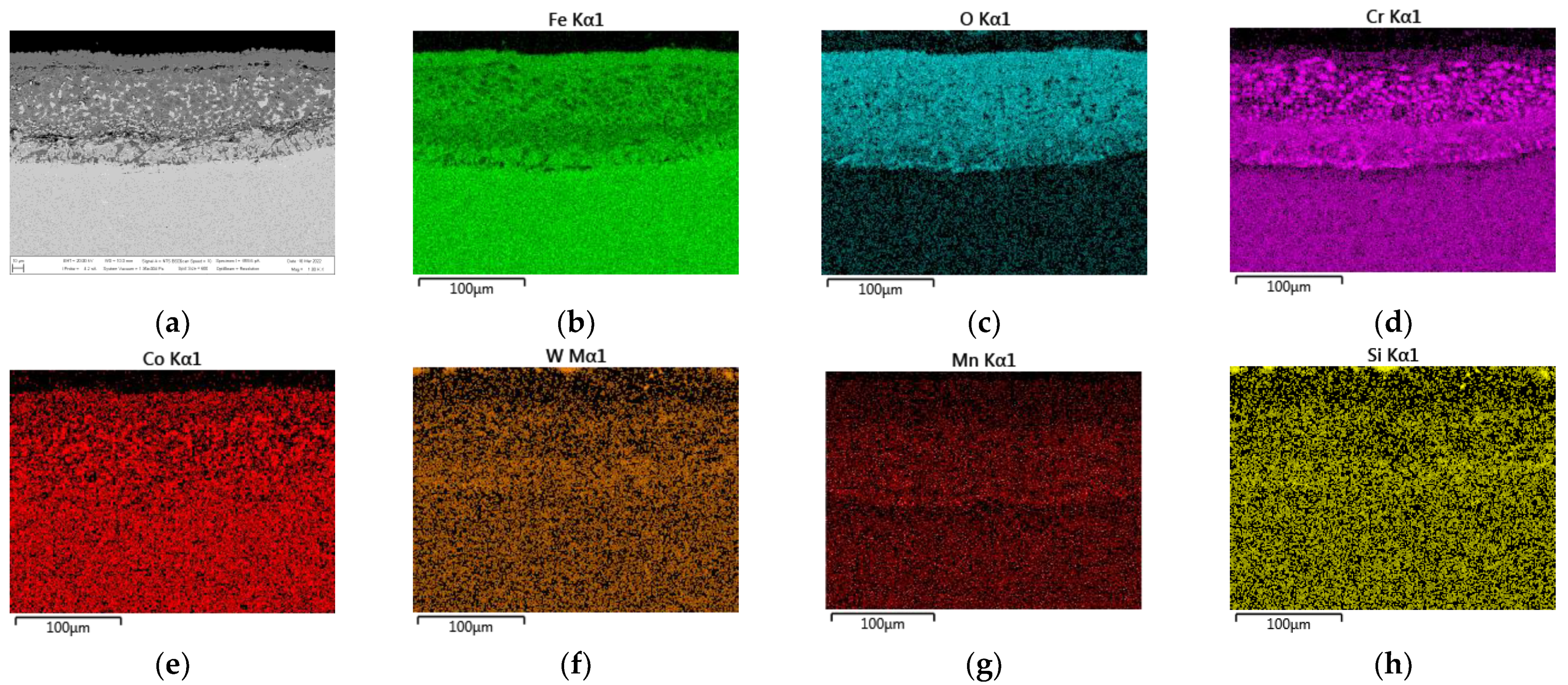
3.2. Oxide Layer Morphology and Identification of MarBN Steel after Oxidation at 600 °C
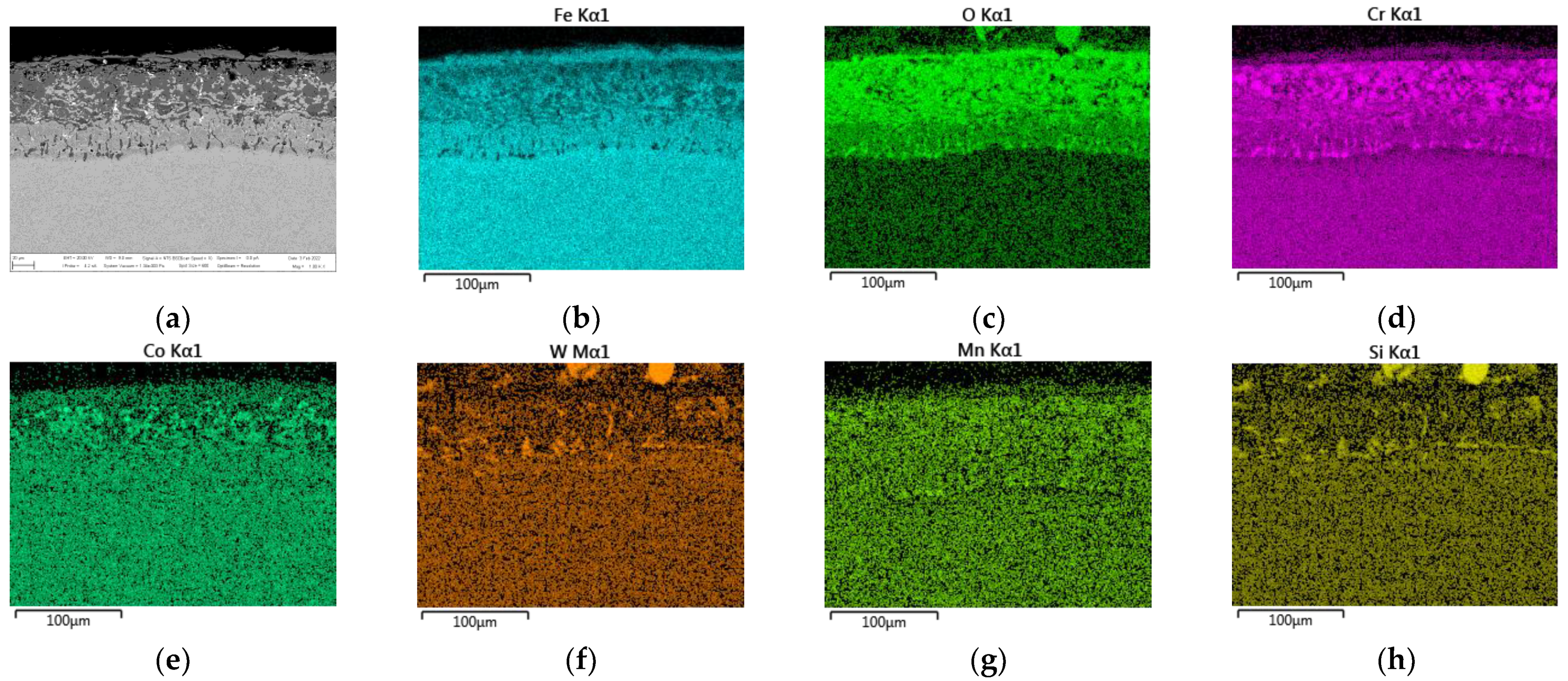
3.2.1. EDS SEM Maps of MarBN Steel Oxide Layer after Oxidation at 600 °C
3.2.2. XRD Phase Analysis of the Oxide Layer at 600 °C
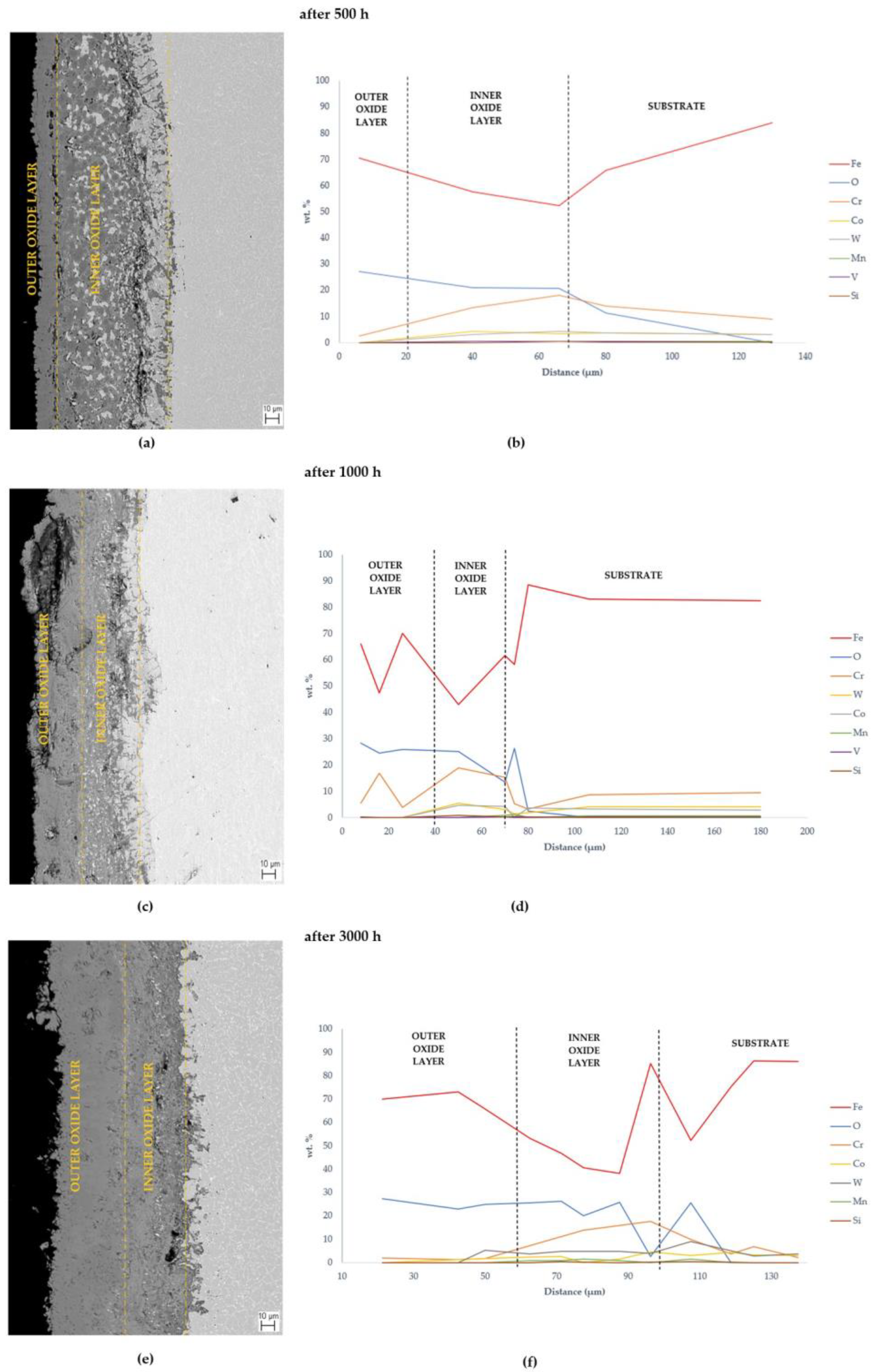
3.3. Morphology and Identification of the Oxide Layer on MarBN Steel after Oxidation at 650 °C
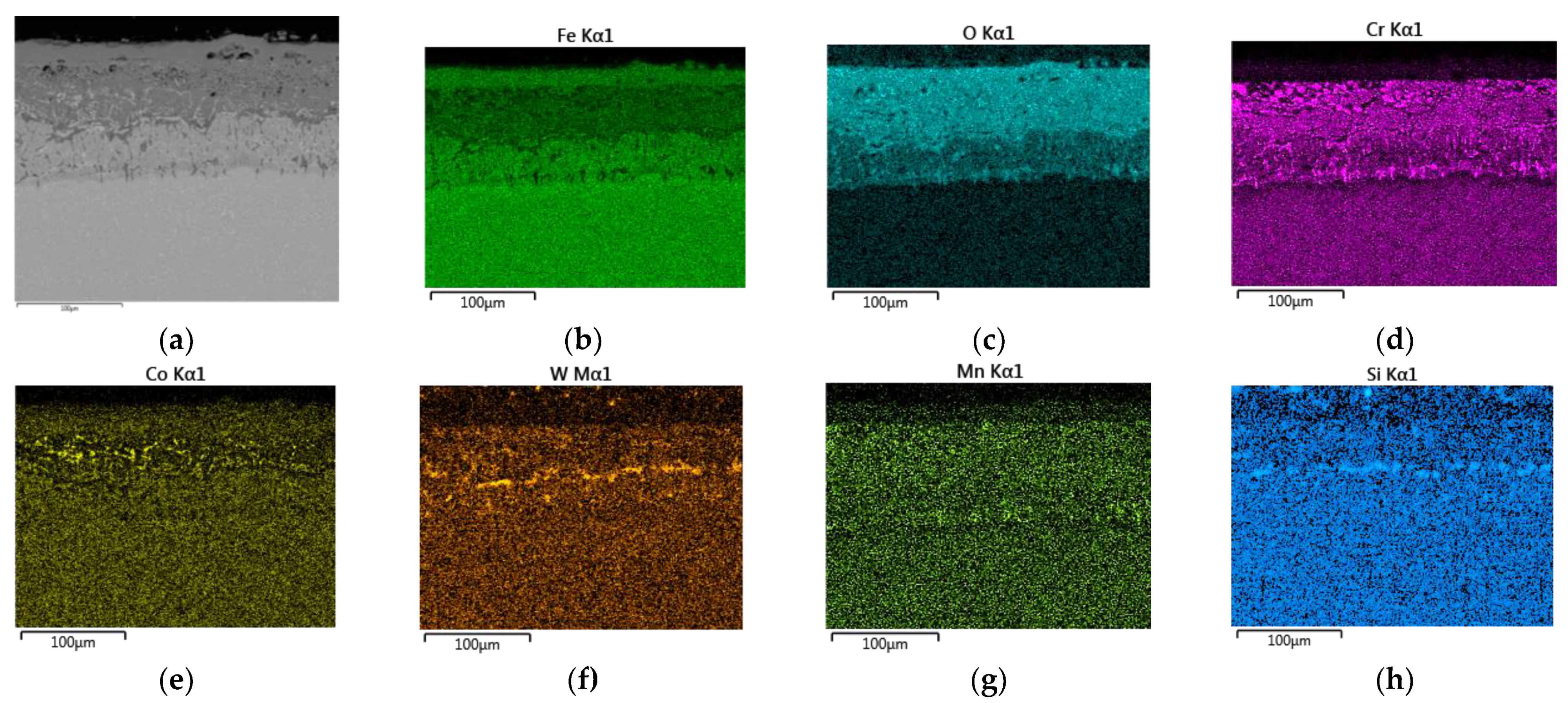
3.3.1. EDS SEM Maps at 650 °C
3.3.2. XRD Phase Analysis of the Oxide Layer at 650 °C
3.3.3. TEM Analysis of MarBN Steel after Oxidation at 650 °C
4. Conclusions
- The microstructure of MarBN steel after normalizing annealing and tempering consisted of tempered martensite and tempered bainite with particles of carbide-based precipitates.
- An oxide layer was formed on the surface of the MarBN martensitic–bainitic steel, which consisted of the outer layer formed by Fe2O3 with Cr2O3 presence and the inner oxide layer formed by Fe3O4 and spinel Fe-Cr.
- EDS SEM analysis showed the importance of the alloys’ transport into the forming oxide layer during long-term exposure. Chromium, as the main alloying element, was essential in the inner oxide layer during the Fe-Cr spinel formation. Mn and Si were important in the passive layer of oxide formation at the oxide/matrix interface already in the earlier stages of oxidation, and during long-term oxidation, their effect only increased during long-term oxidation.
- By XRD analysis, Fe2O3, Cr2O3, and Fe3O4 phases were identified in the oxide layer after 3000 h of oxidation at a temperature of 600 °C, whereas Fe2O3, Cr2O3, CrO2, Fe3O4, and CoO3 were identified at a temperature of 650 °C. By combining the data from the XRD record with EDS SEM analysis, it was possible to identify Fe-Cr spinel, which together with Fe3O4 created an effective barrier against further oxidation.
- TEM analysis from the zone under the oxide layer showed coarser particles of complex carbides of the M23C6 type (M = Fe, W) significantly depleted of Cr due to its diffusion into the oxide layer on the steel surface during the oxidation process.
- In the zone under the oxide layer, very fine particles with a high number of particles per unit area were also observed, which, according to the results of the EDS analysis, contained a high proportion of C with a majority content of V and Nb and a minor Cr content. Diffusion of Cr was directed from the coarser carbide particles from the surface of the steel through the subsurface zone by the formation of fine particles.
- Under the indicated zone, the steel structure’s nature was very similar to the initial state of the analyzed steel.
- The entire described process leads to the result that 9Cr heat-resistant steels have a high resistance to high-temperature oxidation due to the formation of spinel enriched with alloys in the inner oxide layer, supported by passivation by Si oxides at the oxide/matrix interface. The strength properties of the steel remain preserved due to the presence of fine stable carbides V and Nb and due to the structure at a distance from the oxide layer, which preserves the character of the original structure.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ardy, H.; Bangun, D.A. Failure Analysis of Superheater Boiler Tube SA 213 T12. IOP Conf. Ser. Mater. Sci. Eng. 2019, 547, 012035. [Google Scholar] [CrossRef]
- Zhang, T.; Zhang, X.; Bayar, N.; Zhao, Z. Review of oxide scale on high temperature heating surface in boiler. IOP Conf. Ser. Earth Environ. Sci. 2021, 692, 022049. [Google Scholar] [CrossRef]
- Sun, K.; Wang, J.; Yan, Y.; Jiang, J.; Deng, L. Failure Analysis of Tube-Burst on T91 Platen Superheater of 600 MW Supercritical Boiler. In Proceedings of the International Conference on Artificial Intelligence and Electromechanical Automation (AIEA), Tianjin, China, 26–28 June 2020; pp. 453–456. [Google Scholar] [CrossRef]
- Yu, C.; Zhang, J.; Young, D.J. High temperature corrosion of Fe-Cr-(Mn/Si) alloys in CO2-H2O-SO2 gases. Corros. Sci. 2016, 112, 214–225. [Google Scholar] [CrossRef]
- Wang, S.; Zheng, Z.; Zheng, K.; Long, J.; Wang, J.; Ren, Y.; Li, Y. High temperature oxidation behavior of heat resistant steel with rare earth element Ce. Mater. Res. Express. 2020, 7, 016571. [Google Scholar] [CrossRef]
- Dudziak, T.; Łukaszewicz, M.; Simms, N.; Nicholls, J. Analysis of High Temperature Steam Oxidation of Superheater Steels Used in Coal Fired Boilers. Oxid. Met. 2015, 85, 171–187. [Google Scholar] [CrossRef] [Green Version]
- Gupta, G.K.; Chattopadhyaya, S. Critical Failure Analysis of Superheater Tubes of Coal-Based Boiler. J. Mech. Eng. 2017, 63, 287–299. [Google Scholar] [CrossRef]
- Meier, G.H.; Jung, K.; Mu, N.; Yanar, N.M.; Pettit, F.S.; Abella´n, J.P.; Olszewski, T.; Hierro, L.N.; Quadakkers, W.J.; Holcomb, G.R. Effect of Alloy Composition and Exposure Conditions on the Selective Oxidation Behavior of Ferritic Fe-Cr and Fe-Cr-X Alloys. Oxid. Met. 2010, 74, 319–340. [Google Scholar] [CrossRef]
- Zhong, X.; Wu, X.; Han, E. The characteristic of oxide scales on T91 tube after long-term service in an ultra-supercritical coal power plant. J. Supercrit. Fluids 2012, 72, 68–77. [Google Scholar] [CrossRef]
- Abe, F. Research and Development of Heat-Resistant Materials for Advanced USC Power Plants with Steam Temperatures of 700 °C and Above. Engineering 2015, 1, 211–224. [Google Scholar] [CrossRef] [Green Version]
- Dudova, N.; Mishnev, R.; Kaibyshev, R. Effect of Tempering on Microstructure and Mechanical Properties of Boron Containing 10%Cr Steel. ISIJ Int. 2011, 51, 1912–1918. [Google Scholar] [CrossRef] [Green Version]
- Wu, Q.; Liu, Y.; Zhang, Z.; Qi, Y.; Zhang, C.; Zheng, H.; Xu, Y. Oxidation behavior and high-temperature tensile properties of Fe-9Cr-(Mo, Mo/Ni) alloys. Corros. Sci. 2021, 181, 109243. [Google Scholar] [CrossRef]
- Ma, H.; He, Y.; Liu, Y.; Shin, K. Effects of precipitation on the scale and grain growth in 9% Cr tempered martensite steel upon steam oxidation. Mater. Charact. 2020, 167, 110497. [Google Scholar] [CrossRef]
- Hagarová, M.; Vaško, M.; Pástor, M.; Baranová, G.; Matvija, M. Effect of Flue Gases’ Corrosive Components on the Degradation Process of Evaporator Tubes. Materials 2021, 14, 3860. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Jin, X.; Rong, L. Improvement in High Temperature Oxidation Resistance of 9%Cr Ferritic–Martensitic Steel by Enhanced Diffusion of Mn. Oxid. Met. 2015, 85, 189–203. [Google Scholar] [CrossRef]
- Ishitsuka, T.; Inoue, Y.; Ogawa, H. Effect of Silicon on the Steam Oxidation Resistance of a 9%Cr Heat Resistant Steel. Oxid. Met. 2004, 61, 125–142. [Google Scholar] [CrossRef]
- Ennis, P.; Quadakkers, W.J. The steam oxidation resistance of 9-12% chromium steels, in materials for advanced power generation. Energy Technol. 2002, 21, 1131–1142. [Google Scholar]
- Lepingle, V.; Louis, G.; Allué, D.; Lefebvre, B.; Vandenberghe, B. Steam oxidation resistance of new 12%Cr steels: Comparison with some other ferritic steels. Corros. Sci. 2008, 50, 1011–1019. [Google Scholar] [CrossRef]
- Schütze, M.; Schorr, M.; Renusch, D.P.; Donchev, A.; Vossen, J.P.T. The role of alloy composition, environment and stresses for the oxidation resistance of modern 9% Cr steels for fossil power stations. Mater. Res. 2004, 7, 111–123. [Google Scholar] [CrossRef]
- Kang, J.-Y.; Ha, H.-Y.; Kim, S.-D.; Park, J.Y.; Jang, M.-H.; Lee, T.-H. Effect of tungsten on the oxidation of alumina-forming austenitic stainless steel. Appl. Microsc. 2019, 49, 13. [Google Scholar] [CrossRef] [Green Version]
- Dudova, N. 9–12% Cr Heat-Resistant Martensitic Steels with Increased Boron and Decreased Nitrogen Contents. Metals 2022, 12, 1119. [Google Scholar] [CrossRef]
- Yan, P.; Liu, Z.; Bao, H.; Weng, Y.; Liu, W. Effect of normalizing temperature on the strength of 9Cr–3W–3Co martensitic heat resistant steel. Mater. Sci. Eng. A 2014, 597, 148–156. [Google Scholar] [CrossRef]
- Cai, Y.; Wang, Q.; Liu, M.; Jiang, Y.; Zou, T.; Wang, Y.; Li, Q.; Pei, Y.; Zhang, H.; Liu, Y.; et al. Tensile Behavior, Constitutive Model, and Deformation Mechanisms of MarBN Steel at Various Temperatures and Strain Rates. Materials 2022, 15, 8745. [Google Scholar] [CrossRef] [PubMed]
- Chandra-ambhorn, S.; Nilsonthi, T.; Wexternals, Y.; Galerie, A. Oxidation of simulated recycled steels with 0.23 and 1.03 wt.% Si in Ar-20%H2O at 900 °C. Corros. Sci. 2014, 87, 101–110. [Google Scholar] [CrossRef]
- Hagarová, M.; Baranová, G.; Fujda, M.; Matvija, M.; Horňak, P.; Bednarčík, J.; Yudina, D. High Temperature Oxidation Behavior of Creep Resistant Steels in Water Vapour Containing Environments. Materials 2022, 15, 616. [Google Scholar] [CrossRef]
- Naraparaju, R. High Temperature Oxidation Behaviour of Boiler Steels with Emphasis on Shot-Peening Effects—Experimental Results and Simulation. Ph.D. Thesis, Universität Siegen, Siegen, Germany, 2013. [Google Scholar]
- Li, Y.; Macdonald, D.D.; Yang, J.; Qiu, J.; Wang, S. Point defect model for the corrosion of steels in supercritical water: Part I, film growth kinetics. Corros. Sci. 2019, 163, 108280. [Google Scholar] [CrossRef]
- Li, Y.; Xu, T.; Wang, S.; Fekete, B.; Yang, J.; Yang, J.; Qiu, J.; Xu, A.; Wang, J.; Xu, Y.; et al. Modelling and Analysis of the Corrosion Characteristics of Ferritic-Martensitic Steels in Supercritical Water. Materials 2019, 12, 409. [Google Scholar] [CrossRef] [Green Version]
- Shen, Z.; Tweddle, D.; Yu, H.; He, G.; Varambhia, A.; Karamched, P.; Hofmann, F.; Wilkinson, A.J.; Moody, M.P.; Zhang, L.; et al. Microstructural understanding of the oxidation of an austenitic stainless steel in high-temperature steam through advanced characterization. Acta Mater. 2020, 194, 321–336. [Google Scholar] [CrossRef]
- Saunders, S.R.J.; Monteiro, M.; Rizzo, F. The oxidation behaviour of metals and alloys at high temperatures in atmospheres containing water vapour: A review. Prog. Mater. Sci. 2008, 53, 775–837. [Google Scholar] [CrossRef]
- Quadakkers, W.J.; Ennis, P.J.; Zurek, J.; Michalik, M. Steam oxidation of ferritic steels—Laboratory test kinetic data. Mater. High Temp. 2005, 22, 47–60. [Google Scholar] [CrossRef]
- Yan, J.-J.; Huang, X.-F.; Huang, W.-G. High-temperature oxidation behavior of 9Cr-5Si-3Al ferritic heat-resistant steel. Int. J. Miner. Metall. Mater. 2020, 27, 1244–1250. [Google Scholar] [CrossRef]
- Chen, Y.; Sridharan, K.; Ukai, S.; Allen, T.R. Oxidation of 9Cr oxide dispersion strengthened steel exposed in supercritical water. J. Nucl. Mater. 2007, 371, 118–128. [Google Scholar] [CrossRef]
- Gheno, T.; Monceau, D.; Young, D.J. Mechanism of breakaway oxidation of Fe-Cr and Fe-Cr-Ni alloys in dry and wet carbon dioxide. Corros. Sci. 2012, 64, 222–233. [Google Scholar] [CrossRef] [Green Version]
- Bischoff, J.; Motta, A.T.; Comstock, R.J. Evolution of the oxide structure of 9CrODS steel exposed to supercritical water. J. Nucl. Mater. 2009, 392, 272–279. [Google Scholar] [CrossRef]
- Huntz, A.M.; Bague, V.; Beauplé, G.; Haut, C.; Sévérac, C.; Lecour, P.; Longaygue, X.; Ropital, F. Effect of silicon on the oxidation resistance of 9% Cr steels. Appl. Surf. Sci. 2003, 207, 255–275. [Google Scholar] [CrossRef]
- Zhang, Y.; Zou, D.; Li, Y.; Wang, Y.; Zhang, W.; Zhang, X. Effect of Al content on the high-temperature oxidation behavior of 18Cr–Al–Si ferritic heat-resistant stainless steel. J. Mater. Res. Technol. 2021, 11, 1730–1740. [Google Scholar] [CrossRef]
- Jung, I.-H. Critical evaluation and thermodynamic modeling of the Mn-Cr-O system for the oxidation of SOFC interconnect. Solid State Ion. 2006, 177, 765–777. [Google Scholar] [CrossRef]
- Srinivasan, S.; Mallika, C.; Krishna, N.G.; Thinaharan, C.; Jayakumar, T.; Kamachi Mudali, U. Evolution of surface chemistry and morphology of oxide scale formed during initial stage oxidation of modified 9Cr–1Mo steel. Corros. Sci. 2014, 79, 59–68. [Google Scholar] [CrossRef]
- England, D.M.; Virkar, A.V. Oxidation Kinetics of Some Nickel-Based Superalloy Foils and Electronic Resistance of the Oxide Scale Formed in Air Part, I. J. Electrochem. Soc. 1999, 146, 3196. [Google Scholar] [CrossRef]
- Laverde, D.; Gómez-Acebo, T.; Castro, F. Continuous and cyclic oxidation of T91 ferritic steel under steam. Corros. Sci. 2004, 46, 613–631. [Google Scholar] [CrossRef]
- Mathiazhagan, P.; Khanna, A.S. High Temperature Oxidation Behavior of P91, P92 and E911 Alloy Steels in Dry and Wet Atmospheres. High Temp. Mater. Process. 2011, 30, 43–50. [Google Scholar] [CrossRef]
- Yan, J.; Qiu, Y.; Da, B.; Li, Z.; Li, Y.; Ding, Z.; Ma, L.; Zhang, P.; Yuan, Y.; Gu, Y. Impact of the voids on the cracking behavior of the duplex oxide scale on the 18%Cr austenite alloy surface. Corros. Sci. 2020, 163, 108298. [Google Scholar] [CrossRef]
- Chen, K.; Zhang, L.; Shen, Z. Understanding the surface oxide evolution of T91 ferritic-martensitic steel in supercritical water through advanced characterization. Acta Mater. 2020, 194, 156–167. [Google Scholar] [CrossRef]
- Abellán, J.P.; Olszewski, T.; Meier, G.H.; Singheisera, L.; Quadakkers, W.J. The oxidation behaviour of the 9% Cr steel P92 in CO2 and H2O-rich gases relevant to oxyfuel environments. Int. J. Mater. Res. 2010, 101, 287–299. [Google Scholar] [CrossRef]
- Demizieux, M.-C.; Desgranges, C.; Martinelli, L.; Favergeon, J.; Ginestar, K. Morphology and Buckling of the Oxide Scale after Fe-9Cr Steel Oxidation in Water Vapor Environment. Oxid. Met. 2019, 91, 191–212. [Google Scholar] [CrossRef]
- Bao, Z.; Han, R.; Zhu, Y.; Li, H.; Li, N.; Tang, M.; Zhang, H.; Zhao, C. High Temperature Oxidation Behavior of New Martensitic Heat-Resistant Steel. Mater. Sci. 2022, 28, 164–170. [Google Scholar] [CrossRef]
- Oleksak, R.P.; Addou, R.; Gwalani, B.; Baltrus, J.P.; Liu, T.; Trey Diulus, J.; Devaraj, A.; Herman, G.S.; Doğan, Ö.N. Molecular-scale investigation of the oxidation behavior of chromia-forming alloys in high-temperature CO2. NPJ Mater. Degrad. 2021, 5, 46. [Google Scholar] [CrossRef]






| Element | C | Mn | Si | P | S | Cr | Ni | Cu | Mo | V |
|---|---|---|---|---|---|---|---|---|---|---|
| wt. % | 0.08 | 0.47 | 0.29 | 0.009 | 0.002 | 8.80 | 0.15 | 0.07 | 0.04 | 0.20 |
| Element | W | Co | Al | Ti | Nb | B | As | Sn | N | B + N |
| wt. % | 2.96 | 2.95 | 0.011 | 0.002 | 0.06 | 0.014 | 0.009 | <0.01 | 0.0079 | 0.0219 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baranová, G.; Hagarová, M.; Matvija, M.; Csík, D.; Girman, V.; Bednarčík, J.; Bekeč, P. Experimental Study of the Evolution of Creep-Resistant Steel’s High-Temperature Oxidation Behavior. Crystals 2023, 13, 982. https://doi.org/10.3390/cryst13060982
Baranová G, Hagarová M, Matvija M, Csík D, Girman V, Bednarčík J, Bekeč P. Experimental Study of the Evolution of Creep-Resistant Steel’s High-Temperature Oxidation Behavior. Crystals. 2023; 13(6):982. https://doi.org/10.3390/cryst13060982
Chicago/Turabian StyleBaranová, Gabriela, Mária Hagarová, Miloš Matvija, Dávid Csík, Vladimír Girman, Jozef Bednarčík, and Pavel Bekeč. 2023. "Experimental Study of the Evolution of Creep-Resistant Steel’s High-Temperature Oxidation Behavior" Crystals 13, no. 6: 982. https://doi.org/10.3390/cryst13060982
APA StyleBaranová, G., Hagarová, M., Matvija, M., Csík, D., Girman, V., Bednarčík, J., & Bekeč, P. (2023). Experimental Study of the Evolution of Creep-Resistant Steel’s High-Temperature Oxidation Behavior. Crystals, 13(6), 982. https://doi.org/10.3390/cryst13060982







