A Study of the Hot Salt Corrosion Behavior of Three Nickel-Based Single-Crystal Superalloys at 900 °C
Abstract
:1. Introduction
2. Materials and Methods
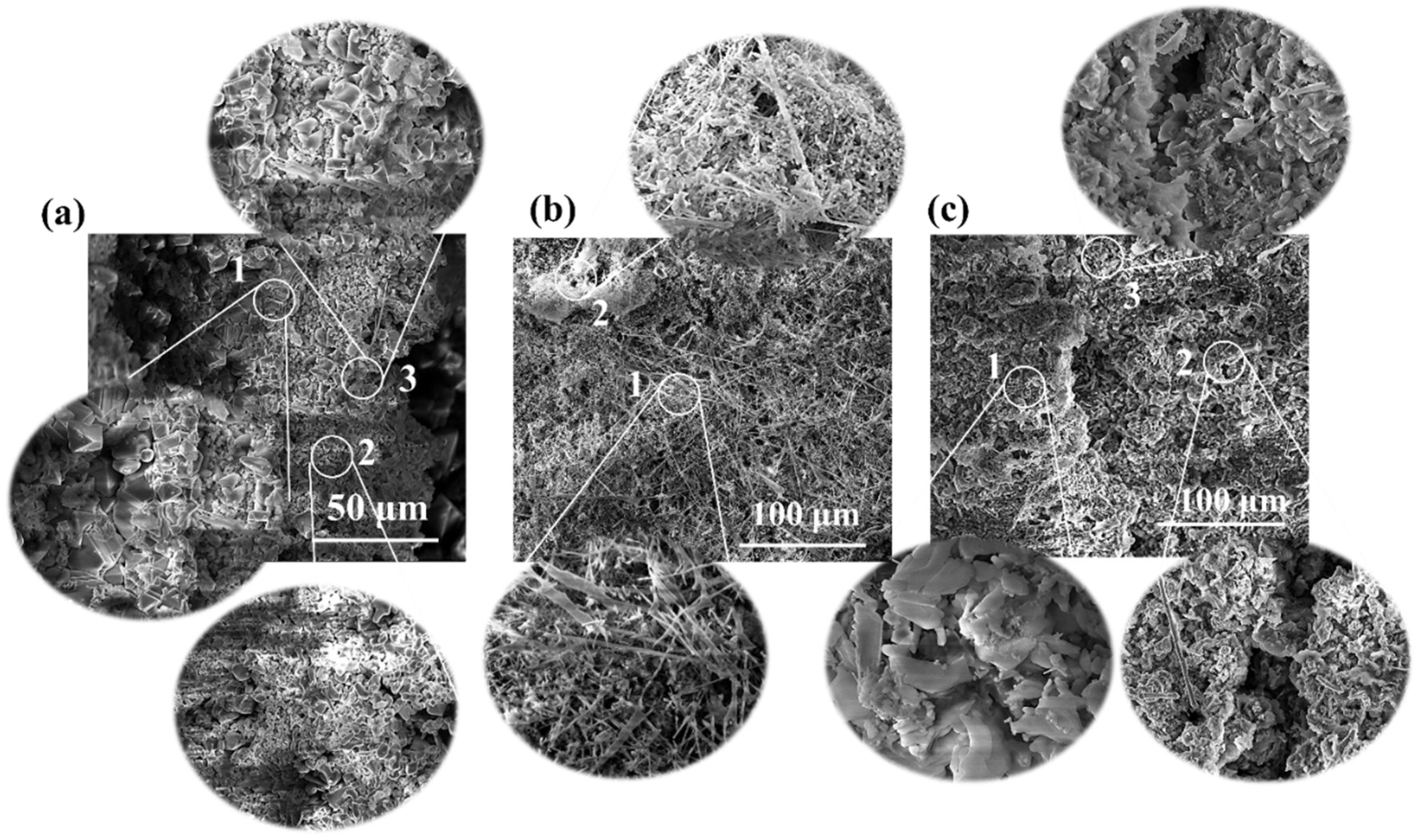
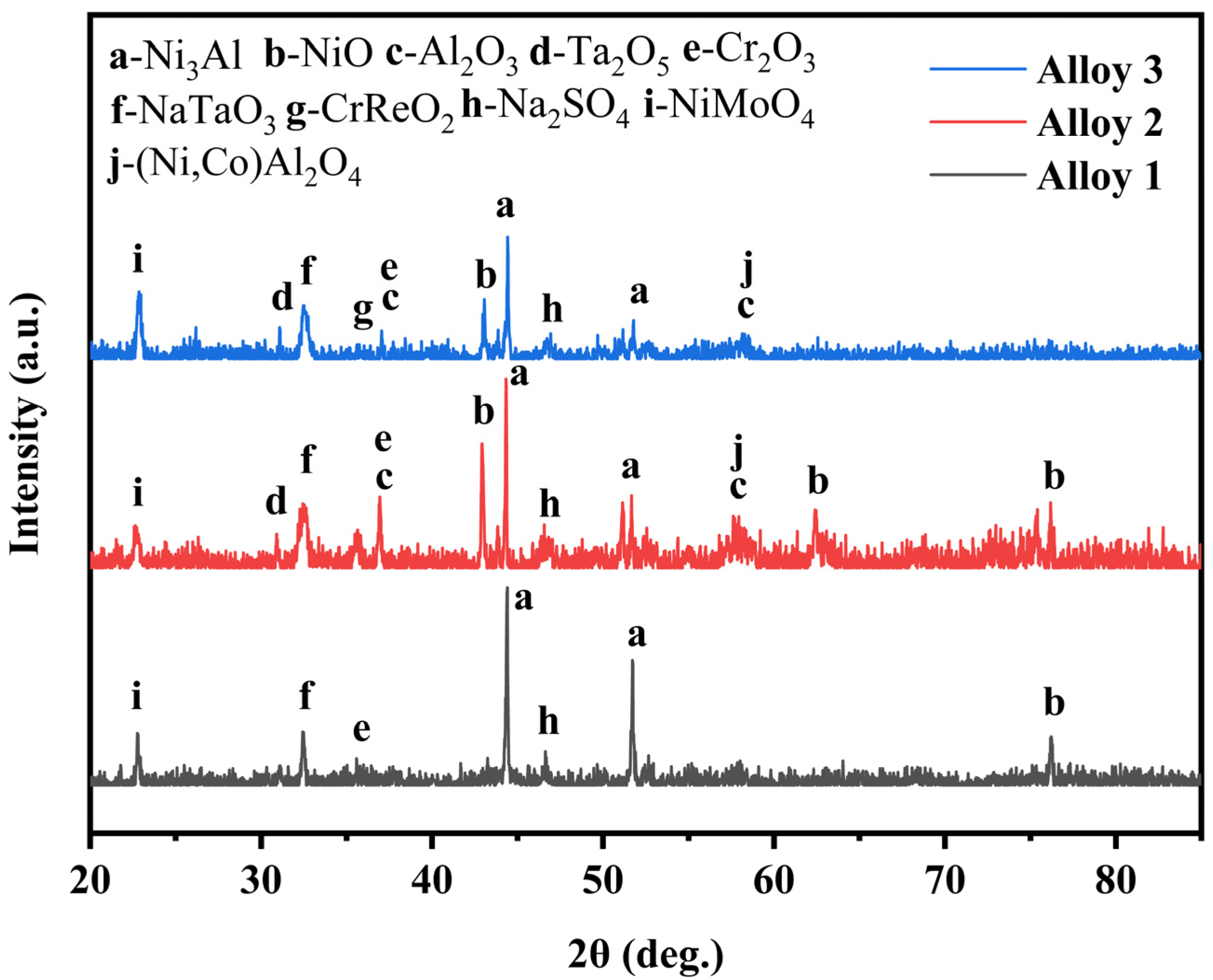
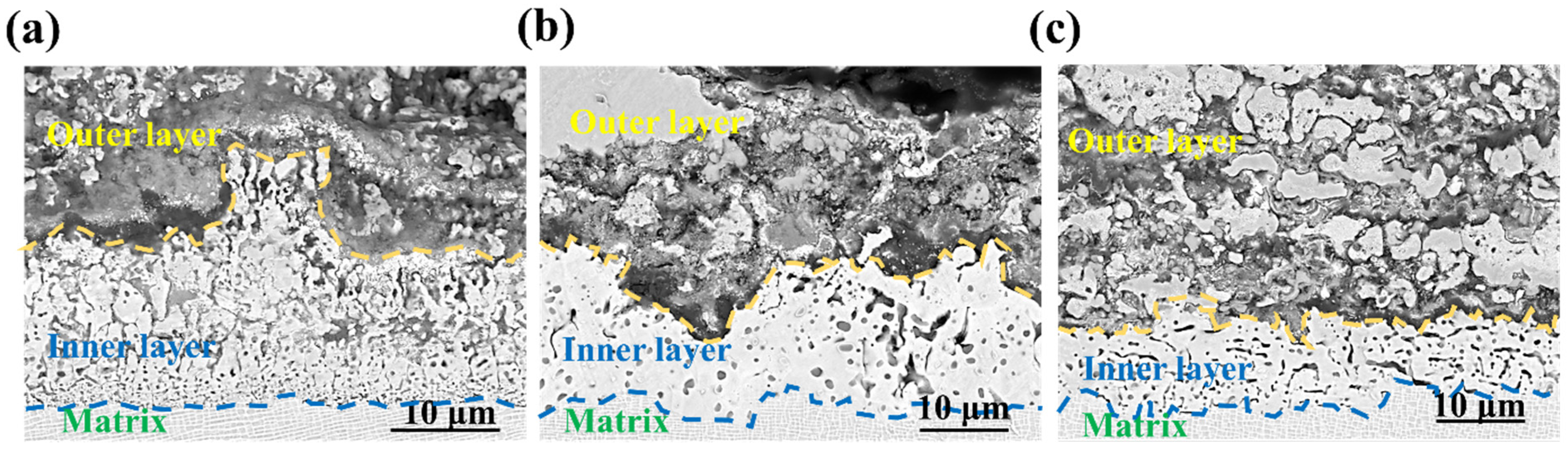
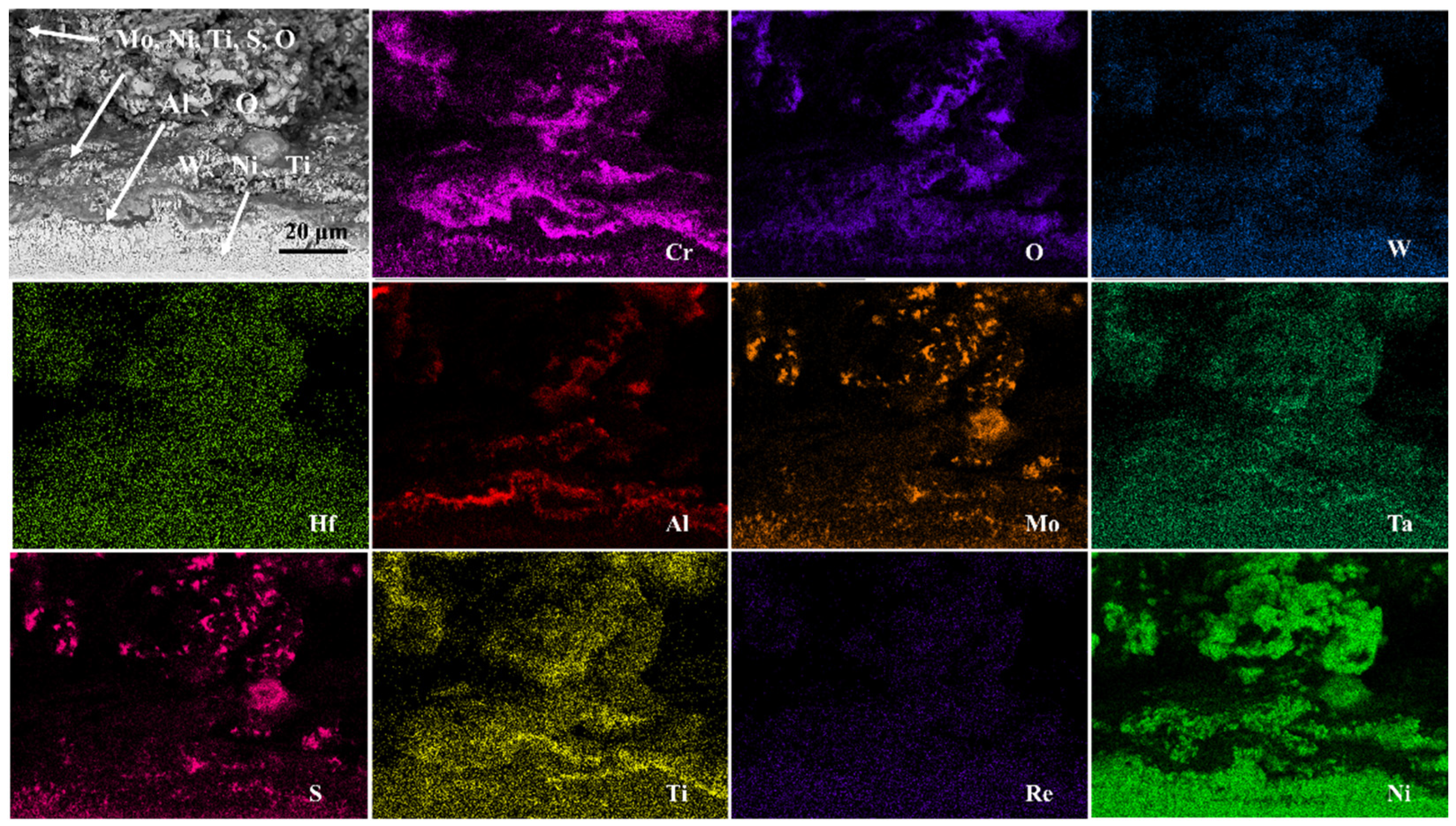
3. Results and Discussion
4. Conclusions
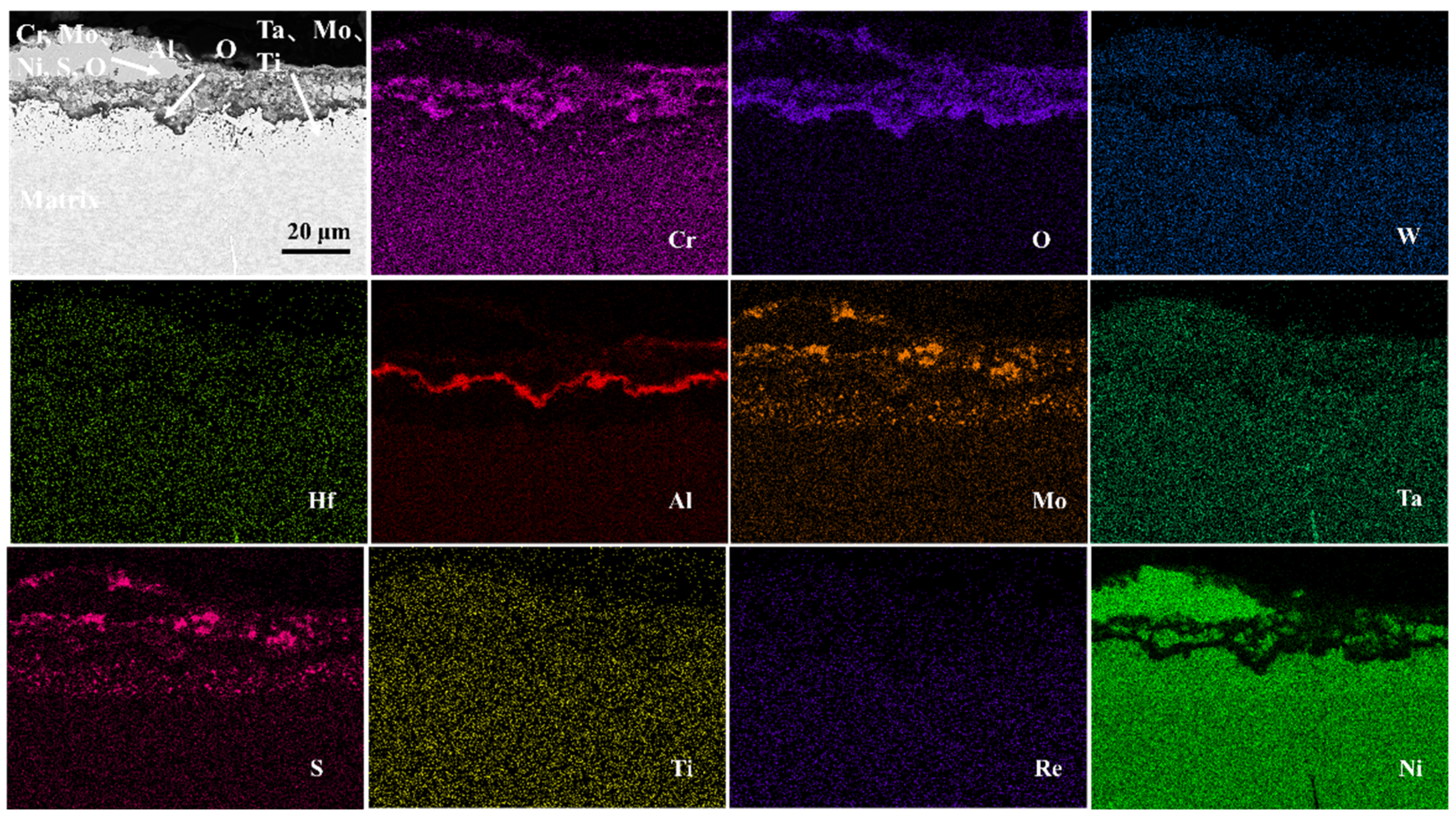
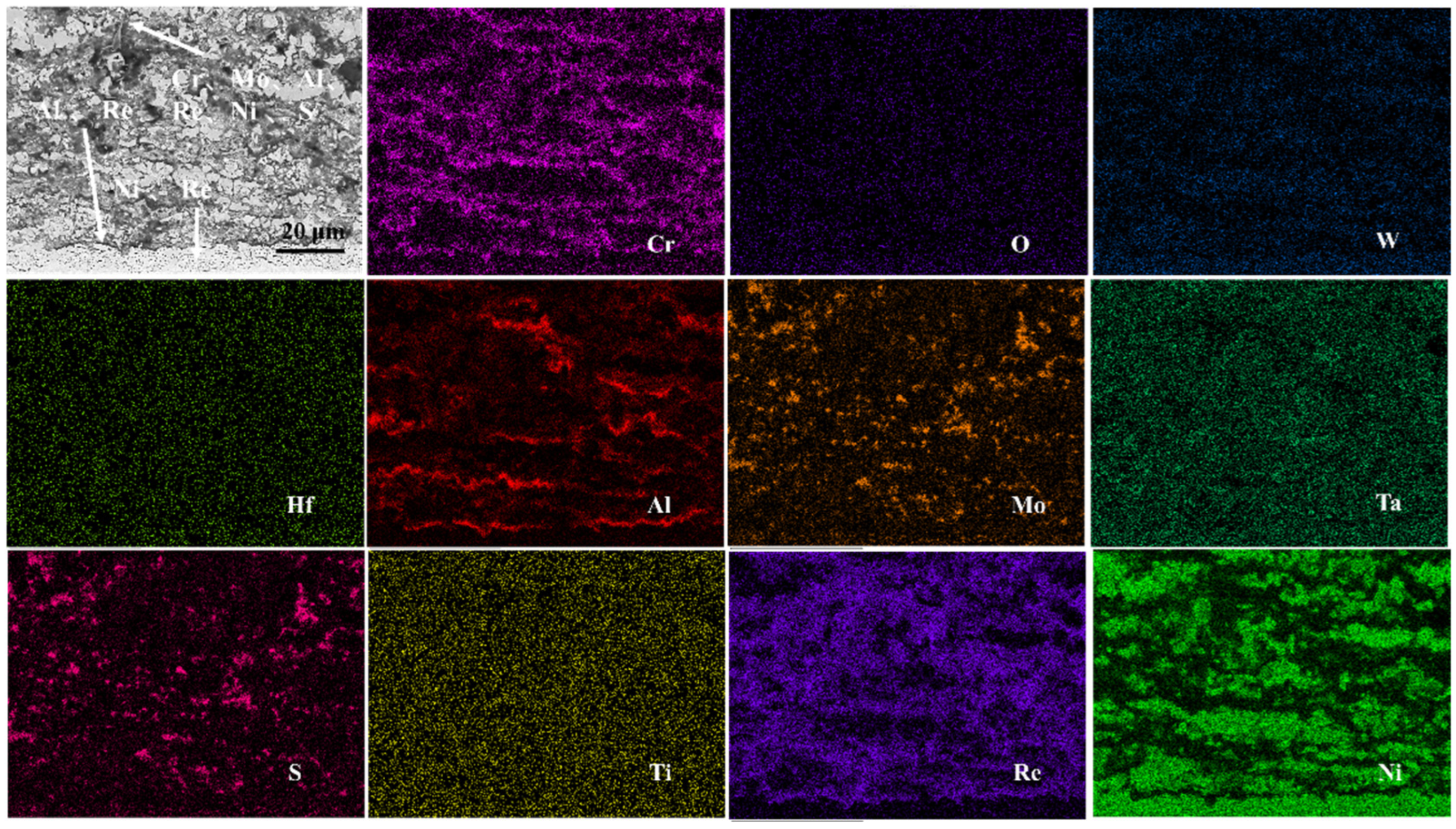
- The outer corrosion layers of all three alloys were enriched in Mo, Ni, S, and O. However, Alloy 1 showed O enrichment in all corrosion processes, whereas Alloy 2 showed O enrichment only in the outer layer. On the other hand, Alloy 3 showed very little O enrichment in the corrosion products.
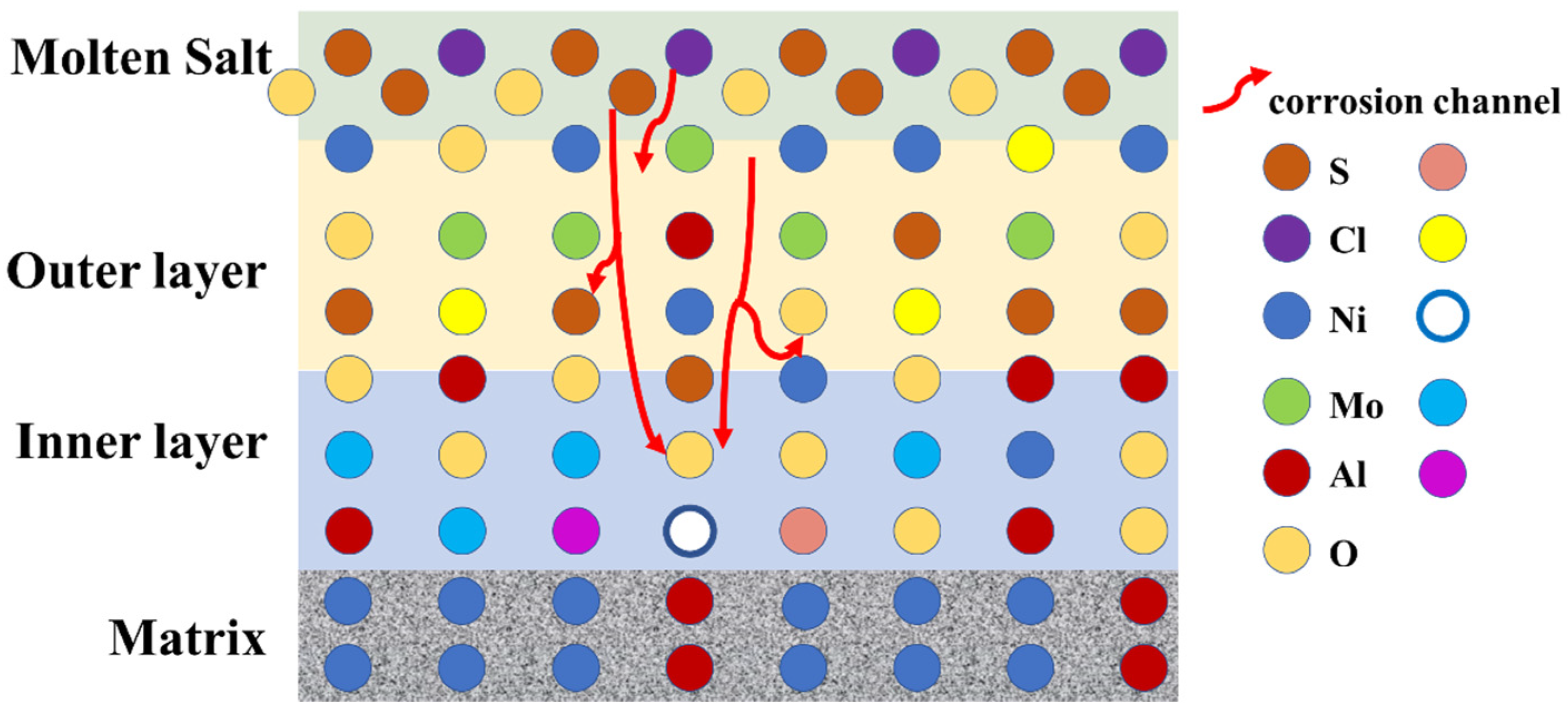
- The corrosion mechanism of nickel-based single-crystal superalloys at high temperatures was briefly analyzed. In the early stage of corrosion, S and O first reacted with Al, Mo, and Cr to form oxides, and then with Ni and Ti. With the oxidation reaction and the presence of corrosive substances, the substrate from the void channel underwent further corrosion, during which some oxides were dissolved, and thus the protective oxide film was reduced, and the substrate was further corroded.
- It was also found that the presence of the elements Re, Cr, and Co enhanced the corrosion resistance of the alloy. By contrast, the presence of elements Mo and W exerted the opposite effect.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gäumann, M.; Bezençon, C.; Canalis, P.; Kurz, W. Single-crystal laser deposition of superalloys: Processing–microstructure maps. Acta Mater. 2001, 49, 1051–1062. [Google Scholar] [CrossRef]
- Pradhan, D.; Shankar Mahobia, G.; Chattopadhyay, K.; Singh, V. Salt induced corrosion behaviour of superalloy IN718. Mater. Today Proc. 2018, 5 Pt 2, 7047–7054. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, Q.; Zhuang, Y.; Kovalenko, V.; Yao, J. Microstructures and cyclic hot corrosion behavior of laser deposited Inconel 718 alloy under different heat treatment conditions. Opt. Laser Technol. 2021, 135, 106659. [Google Scholar] [CrossRef]
- Yang, Y.Q.; Zhao, Y.C.; Wen, Z.X.; Lu, G.; Pei, H.; Yue, Z. Synergistic effect of multiple molten salts on hot corrosion behaviour of Ni-based single crystal superalloy. Corros. Sci. 2022, 204, 110381. [Google Scholar] [CrossRef]
- Pollock, T.M.; Tin, S. Nickel-Based Superalloys for Advanced Turbine Engines: Chemistry, Microstructure and Properties. J. Propuls. Power 2006, 22, 361–374. [Google Scholar] [CrossRef]
- Kawagishi, K.; Sato, A.; Sato, A.; Kobayashi, T.; Harada, H. Oxidation Behavior of Ru-Containing Ni-Base Single-Crystal Superalloys. Mater. Sci. Forum 2006, 522–523, 317–322. [Google Scholar] [CrossRef]
- Akhtar, A.; Hegde, S.; Reed, R.C. The oxidation of single-crystal nickel-based superalloys. JOM 2006, 58, 37–42. [Google Scholar] [CrossRef]
- Kawagishi, K.; Harada, H.; Sato, A.; Sato, A.; Kobayashi, T. The oxidation properties of fourth generation single-crystal nickel-based superalloys. JOM J. Miner. Met. 2006, 58, 43–46. [Google Scholar] [CrossRef]
- Song, P.; Liu, M.; Jiang, X.; Feng, Y.; Wu, J.; Zhang, G.; Wang, D.; Dong, J.; Chen, X.-Q.; Lou, L. Influence of alloying elements on hot corrosion resistance of nickel-based single crystal superalloys coated with Na2SO4 salt at 900 °C. Mater. Des. 2021, 197, 109197. [Google Scholar] [CrossRef]
- Gong, N.; Meng, T.L.; Teo, S.L.; Cao, J.; Lee, C.J.; Tan, C.K.I.; Tan, D.C.; Suwardi, A.; Lin, M.; Misra, R.; et al. High-temperature oxidation and hot corrosion of Ni-based single crystal superalloy in the incubation stage. Corros. Sci. 2023, 214, 111026. [Google Scholar] [CrossRef]
- Jokisaari, A.M.; Naghavi, S.S.; Wolverton, C.; Voorhees, P.W.; Heinonen, O.G. Predicting the morphologies of γ precipitates in cobalt-based superalloys. Acta Mater. 2017, 141, 273–284. [Google Scholar] [CrossRef]
- Chen, Z.; Qu, W.; Zhang, Z.; Wang, J.; Chen, M.; Li, S.; Wang, F. Hot corrosion mechanism of nickel-based single crystal superalloy IC21 under flowing atmosphere. Corros. Sci. 2023, 218, 111170. [Google Scholar] [CrossRef]
- Chen, Z.; Wu, M.; Pei, Y.; Li, S.; Gong, S. Study on abnormal hot corrosion behavior of nickel-based single-crystal superalloy at 900 C after drilling. NPJ Mater. Degrad. 2021, 5, 21. [Google Scholar] [CrossRef]
- Zhang, L.N.; Ojo, O.A. Corrosion behavior of wire arc additive manufactured Inconel 718 superalloy. J. Alloys Compd. 2020, 829, 154455. [Google Scholar] [CrossRef]
- Nychka, J.A.; Clarke, D.R.; Meier, G.H. Spallation and transient oxide growth on PWA 1484 superalloy. Mater. Sci. Eng. A 2008, 490, 359–368. [Google Scholar] [CrossRef]
- Koizumi, Y.; Kawagishi, K.; Yokokawa, T.; Yuyama, M.; Takata, Y.; Harada, H. Hot Corrosion and Creep Properties of Ni-Base Single-Crystal Superalloys. Superalloys 2020, 2020, 747–752. [Google Scholar]


| Cr | Re | Co | Mo | Ta | Ti | W | Al | Hf | Ni | |
|---|---|---|---|---|---|---|---|---|---|---|
| Alloy 1 | 6.25 | 2.82 | 9.51 | 0.58 | 6.51 | 0.94 | 6.37 | 5.72 | 0.09 | Bal. |
| Alloy 2 | 7 | 3.08 | 7.83 | 1.52 | 6.50 | - | 5.01 | 6.01 | 0.11 | Bal. |
| Alloy 3 | 3.5 | 5 | 6 | 0.4 | 8 | 0.15 | 6.5 | 5.8 | 0.03 | Bal. |
| Alloy 1 | Alloy 2 | Alloy 3 | |
|---|---|---|---|
| Modelling | ExpDec1 | ExpDec1 | ExpDec1 |
| Equation | y = A1*exp(−x/t1) + y0 | y = A1*exp(−x/t1) + y0 | y = A1*exp(−x/t1) + y0 |
| y0 | 2.46 ± 0.03 | 2.57 ± 0.13 | 2.59 ± 1.44 |
| A1 | 0.11 ± 0.022 | 0.02 ± 0.02 | 0.02 ± 0.25 |
| t1 | −20.97 ± 1.26 | −14.88 ± 2.56 | −15.87 ± 36.43 |
| Reduced Chi-Sqr | 0.58 | 8.64 | 919.05 |
| R2 (COD) | 0.99 | 0.95 | −0.69 |
| Adjust R2 | 0.99 | 0.91 | −1.81 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Q.; Liu, F.; Li, Y.; Yao, J.; Yang, J.; Tan, L.; Wang, Z.; Huang, L.; Liu, Y. A Study of the Hot Salt Corrosion Behavior of Three Nickel-Based Single-Crystal Superalloys at 900 °C. Crystals 2024, 14, 307. https://doi.org/10.3390/cryst14040307
Li Q, Liu F, Li Y, Yao J, Yang J, Tan L, Wang Z, Huang L, Liu Y. A Study of the Hot Salt Corrosion Behavior of Three Nickel-Based Single-Crystal Superalloys at 900 °C. Crystals. 2024; 14(4):307. https://doi.org/10.3390/cryst14040307
Chicago/Turabian StyleLi, Qianyi, Feng Liu, Yixin Li, Jian Yao, Jingyu Yang, Liming Tan, Zi Wang, Lan Huang, and Yong Liu. 2024. "A Study of the Hot Salt Corrosion Behavior of Three Nickel-Based Single-Crystal Superalloys at 900 °C" Crystals 14, no. 4: 307. https://doi.org/10.3390/cryst14040307




