Preparation of N, Cl Co-Doped Lignin Carbon Quantum Dots and Detection of Microplastics in Water
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Fabrication of CQDs
2.3. Synthesis of PS Microspheres
2.4. CQDs for PS Detection
2.5. Characterization
3. Result and Discussion
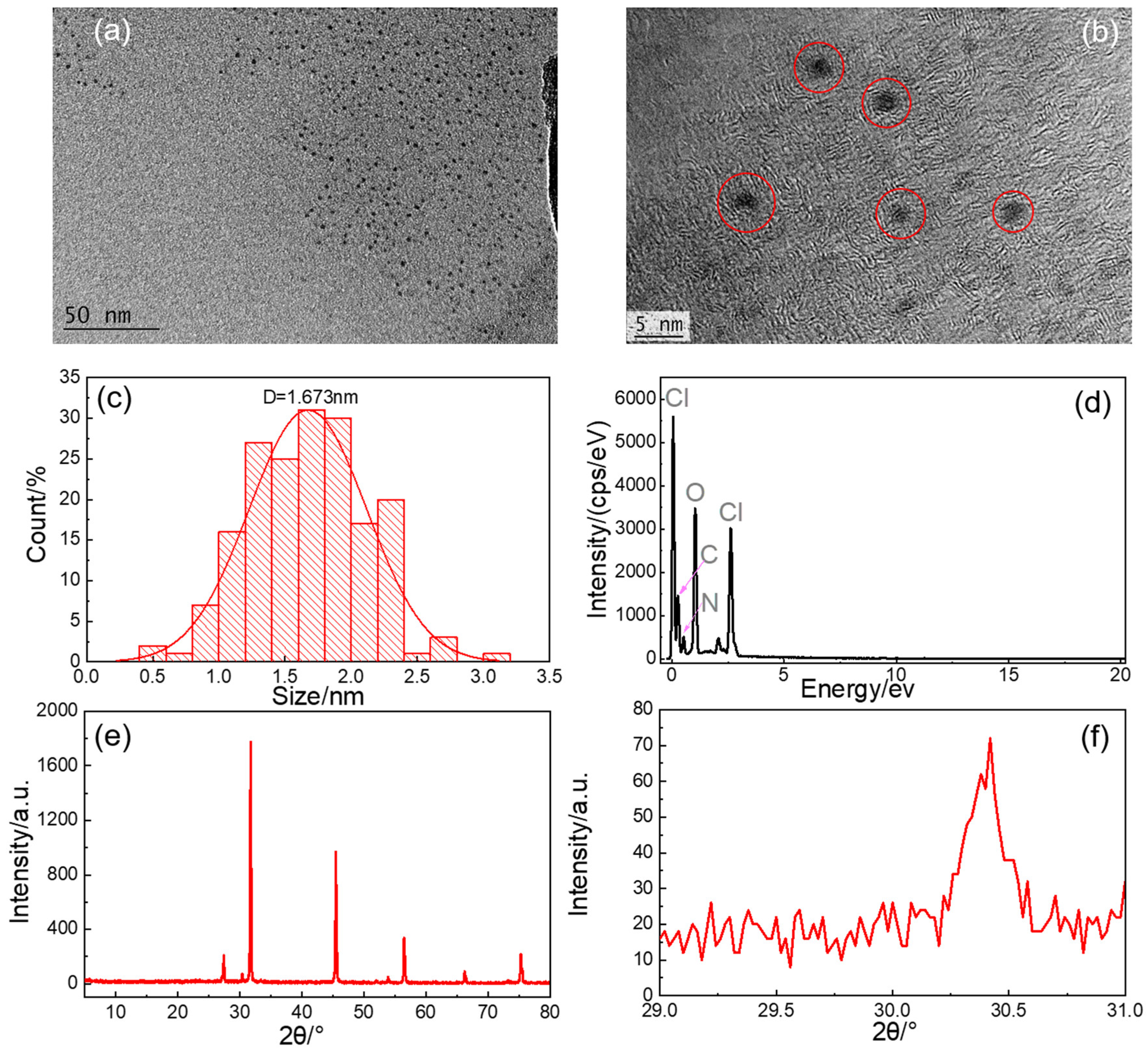
3.1. Characterization of CQDs
3.2. Detection of PS Microplastics by CQDs
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Geyer, R.; Jambeck, J.R.; Law, K.L. Production, Use, and Fate of all Plastics Ever Made. Sci. Adv. 2017, 3, e1700782. [Google Scholar] [CrossRef] [Green Version]
- Thompson, R.C.; Olsen, Y.; Mitchell, R.P.; Davis, A.; Rowland, S.J.; John, A.W.G.; McGonigle, D.; Russell, A.E. Lost at Sea: Where is All the Plastic? Science 2004, 304, 838. [Google Scholar] [CrossRef] [PubMed]
- Reed, S.; Clark, M.; Thompson, R.; Hughes, K.A. Microplastics in Marine Sediments near Rothera Research Station, Antarctica. Mar. Pollut. Bull. 2018, 133, 460–463. [Google Scholar] [CrossRef] [PubMed]
- Ross, P.S.; Chastain, S.; Vassilenko, E.; Etemadifar, A.; Zimmermann, S.; Quesnel, S.-A.; Eert, J.; Solomon, E.; Patankar, S.; Posacka, A.M.; et al. Pervasive Distribution of Polyester Fibres in the Arctic Ocean is Driven by Atlantic Inputs. Nat. Commun. 2021, 12, 106. [Google Scholar] [CrossRef]
- Silvestrova, K.; Stepanova, N. The Distribution of Microplastics in the Surface Layer of the Atlantic Ocean from the Subtropics to the Equator According to Visual Analysis. Mar. Pollut. Bull. 2021, 162, 111836. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.; Wang, X.; Yin, L.; Chen, S.; Tao, J.; Zhou, W.; Chen, H.; Zhang, G.; Xiao, R. Research Progress of Microplastics in Soil-Plant System: Ecological Effects and Potential Risks. Sci. Total Environ. 2022, 812, 151487. [Google Scholar] [CrossRef] [PubMed]
- Karbalaei, S.; Hanachi, P.; Walker, T.R.; Cole, M. Occurrence, Sources, Human Health Impacts and Mitigation of Microplastic Pollution. Environ. Sci. Pollut. Res. 2018, 25, 36046–36063. [Google Scholar] [CrossRef]
- Yong, C.Q.Y.; Valiyaveettil, S.; Tang, B.L. Toxicity of Microplastics and Nanoplastics in Mammalian Systems. Int. J. Environ. Res. Public Health 2020, 17, 1509. [Google Scholar] [CrossRef] [Green Version]
- Brennecke, D.; Duarte, B.; Paiva, F.; Cacador, I.; Canning-Clode, J. Microplastics as Vector for Heavy Metal Contamination from the Marine Environment. Estuar. Coast. Shelf Sci. 2016, 178, 189–195. [Google Scholar] [CrossRef]
- Wang, F.; Zhang, M.; Sha, W.; Wang, Y.; Hao, H.; Dou, Y.; Li, Y. Sorption Behavior and Mechanisms of Organic Contaminants to Nano and Microplastics. Molecules 2020, 25, 1827. [Google Scholar] [CrossRef] [Green Version]
- Kaeppler, A.; Fischer, D.; Oberbeckmann, S.; Schernewski, G.; Labrenz, M.; Eichhorn, K.-J.; Voit, B. Analysis of Environmental Microplastics by Vibrational Microspectroscopy: FTIR, Raman or Both? Anal. Bioanal. Chem. 2016, 408, 8377–8391. [Google Scholar] [CrossRef]
- Fischer, M.; Scholz-Boettcher, B.M. Simultaneous Trace Identification and Quantification of Common Types of Microplastics in Environmental Samples by Pyrolysis-Gas Chromatography-Mass Spectrometry. Environ. Sci. Technol. 2017, 51, 5052–5060. [Google Scholar] [CrossRef]
- Duemichen, E.; Eisentraut, P.; Bannick, C.G.; Barthel, A.-K.; Senz, R.; Braun, U. Fast Identification of Microplastics in Complex Environmental Samples by a Thermal Degradation Method. Chemosphere 2017, 174, 572–584. [Google Scholar] [CrossRef]
- Shim, W.J.; Song, Y.K.; Hong, S.H.; Jang, M. Identification and Quantification of Microplastics Using Nile Red Staining. Mar. Pollut. Bull. 2016, 113, 469–476. [Google Scholar] [CrossRef] [PubMed]
- Gbogbo, F.; Takyi, J.B.; Billah, M.K.; Ewool, J. Analysis of Microplastics in Wetland Samples from Coastal Ghana Using the Rose Bengal Stain. Environ. Monit. Assess. 2020, 192, 208. [Google Scholar] [CrossRef] [PubMed]
- Parker, B.; Andreou, D.; Green, I.D.; Britton, J.R. Microplastics in Freshwater Fishes: Occurrence, Impacts and Future Perspectives. Fish Fish. 2021, 22, 467–488. [Google Scholar] [CrossRef]
- Lim, S.Y.; Shen, W.; Gao, Z. Carbon Quantum Dots and their Applications. Chem. Soc. Rev. 2015, 44, 362–381. [Google Scholar] [CrossRef]
- Magesh, V.; Sundramoorthy, A.K.; Ganapathy, D. Recent Advances on Synthesis and Potential Applications of Carbon Quantum Dots. Front. Mater. 2022, 9, 906838. [Google Scholar] [CrossRef]
- Nazri, N.A.A.; Azeman, N.H.; Luo, Y.; Bakar, A.A.A. Carbon quantum dots for optical sensor applications: A review. Opt. Laser Technol. 2021, 139, 106928. [Google Scholar] [CrossRef]
- Atchudan, R.; Edison, T.N.J.I.; Shanmugam, M.; Perumal, S.; Somanathan, T.; Lee, Y.R. Sustainable Synthesis of Carbon Quantum Dots from Banana Peel Waste using Hydrothermal Process for in Vivo Bioimaging. Phys. E-Low-Dimens. Syst. Nanostruct. 2021, 126, 114417. [Google Scholar] [CrossRef]
- Choppadandi, M.; Guduru, A.T.; Gondaliya, P.; Arya, N.; Kalia, K.; Kumar, H.; Kapusetti, G. Structural Features Regulated Photoluminescence Intensity and Cell Internalization of Carbon and Graphene Quantum Dots for Bioimaging. Mater. Sci. Eng. C-Mater. Biol. Appl. 2021, 129, 112366. [Google Scholar] [CrossRef] [PubMed]
- Molaei, M.J. Carbon Quantum Dots and their Biomedical and Therapeutic Applications: A Review. R. Soc Adv. 2019, 9, 6460–6481. [Google Scholar] [CrossRef] [PubMed]
- Shi, Z.-J.; Ma, M.-G. Synthesis, Structure, and Applications of Lignin-Based Carbon Materials: A Review. Sci. Adv. Mater. 2019, 11, 18–32. [Google Scholar] [CrossRef]
- Bressi, V.; Chiarotto, I.; Ferlazzo, A.; Celesti, C.; Michenzi, C.; Len, T.; Iannazzo, D.; Neri, G.; Espro, C. Voltammetric Sensor Based on Waste-Derived Carbon Nanodots for Enhanced Detection of Nitrobenzene. ChemElectroChem 2023, e202300004. [Google Scholar] [CrossRef]
- Iravani, S.; Varma, R.S. Greener Synthesis of Lignin Nanoparticles and their Applications. Green Chem. 2020, 22, 612–636. [Google Scholar] [CrossRef]
- Hao, L.; Yu, Y.; Liang, Z.; Hou, H.; Liu, X.; Chen, C.; Min, D. Deciphering Photocatalytic Degradation of Methylene Blue by Surface-Tailored Nitrogen-Doped Carbon Quantum Dots Derived from Kraft lignin. Int. J. Biol. Macromol. 2023, 242 Pt 2, 124958. [Google Scholar] [CrossRef]
- Ferlazzo, A.; Bressi, V.; Espro, C.; Iannazzo, D.; Piperopoulos, E.; Neri, G. Electrochemical Determination of Nitrites and Sulfites by Using Waste-Derived Nanobiochar. J. Electroanal. Chem. 2023, 928, 117071. [Google Scholar] [CrossRef]
- Zhang, B.; Liu, Y.; Ren, M.; Li, W.; Zhang, X.; Vajtai, R.; Ajayan, P.M.; Tour, J.M.; Wang, L. Sustainable Synthesis of Bright Green Fluorescent Nitrogen-Doped Carbon Quantum Dots from Alkali Lignin. Chemsuschem 2019, 12, 4202–4210. [Google Scholar] [CrossRef]
- Yang, X.; Hou, S.; Chu, T.; Han, J.; Li, R.; Guo, Y.; Gong, Y.; Li, H.; Wan, Z. Preparation of Magnesium, Nitrogen-Codoped Carbon Quantum Dots from Lignin with Bright Green Fluorescence and Sensitive pH Response. Ind. Crops Prod. 2021, 167, 113507. [Google Scholar] [CrossRef]
- Devi, P.; Rajput, P.; Thakur, A.; Kim, K.-H.; Kumar, P. Recent Advances in Carbon Quantum Dot-Based Sensing of Heavy Metals in Water. Trac-Trends Anal. Chem. 2019, 114, 171–195. [Google Scholar] [CrossRef]
- Wareing, T.C.; Gentile, P.; Phan, A.N. Biomass-Based Carbon Dots: Current Development and Future Perspectives. ACS Nano 2021, 15, 15471–15501. [Google Scholar] [CrossRef] [PubMed]
- Long, C.; Jiang, Z.; Shangguan, J.; Qing, T.; Zhang, P.; Feng, B. Applications of Carbon Dots in Environmental Pollution Control: A Review. Chem. Eng. J. 2021, 406, 126848. [Google Scholar] [CrossRef]
- Zhu, L.; Shen, D.; Luo, K.H. Triple-Emission Nitrogen and Boron Co-Doped Carbon Quantum Dots from Lignin: Highly Fluorescent Sensing Platform for Detection of Hexavalent Chromium Ions. J. Colloid Interface Sci. 2022, 617, 557–567. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Tang, L.; Xiang, J.; Ji, R.; Yuan, J.; Zhao, J.; Yu, R.; Tai, Y.; Song, L. Chlorine Doped Graphene Quantum Dots: Preparation, Properties, and Photovoltaic Detectors. Appl. Phys. Lett. 2014, 105, 111–116. [Google Scholar] [CrossRef]
- Janus, L.; Radwan-Praglowska, J.; Piatkowski, M.; Bogdal, D. Coumarin-Modified CQDs for Biomedical Applications-Two-Step Synthesis and Characterization. Int. J. Mol. Sci. 2020, 21, 8073. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, H.; Ma, C.; Luo, S.; Xu, M.; Wu, Z.; Li, W.; Liu, S. Luminescent Transparent Wood Based on Lignin-Derived Carbon Dots as a Building Material for Dual-Channel, Real-Time, and Visual Detection of Formaldehyde Gas. ACS Appl. Mater. Interfaces 2020, 12, 36628–36638. [Google Scholar] [CrossRef]
- Yang, X.; Guo, Y.; Liang, S.; Hou, S.; Chu, T.; Ma, J.; Chen, X.; Zhou, J.; Sun, R. Preparation of Sulfur-Doped Carbon Quantum Dots from Lignin as a Sensor to Detect Sudan I in an Acidic Environment. J. Mater. Chem. B 2020, 8, 10788–10796. [Google Scholar] [CrossRef]
- Yang, Y.; Xing, X.; Zou, T.; Wang, Z.; Zhao, R.; Hong, P.; Peng, S.; Zhang, X.; Wang, Y. A Novel and Sensitive Ratiometric Fluorescence Assay for Carbendazim Based on N-Doped Carbon Quantum Dots and Gold Nanocluster Nanohybrid. J. Hazard. Mater. 2020, 386, 121958. [Google Scholar] [CrossRef]
- Adsetts, J.R.; Hoesterey, S.; Gao, C.; Love, D.A.; Ding, Z. Electrochemiluminescence and Photoluminescence of Carbon Quantum Dots Controlled by Aggregation-Induced Emission, Aggregation-Caused Quenching, and Interfacial Reactions. Langmuir 2020, 36, 14432–14442. [Google Scholar] [CrossRef]
- Erni-Cassola, G.; Gibson, M.I.; Thompson, R.C.; Christie-Oleza, J.A. Lost, but Found with Nile Red: A Novel Method for Detecting and Quantifying Small Microplastics (1 mm to 20 μm) in Environmental Samples. Environ. Sci. Technol. 2017, 51, 13641–13648. [Google Scholar] [CrossRef] [Green Version]
- Zhu, T.; Cao, L.; Kou, X.; Liu, Y.; Dong, W.-F.; Ge, M.; Li, L. Nitrogen-Doped Cyan-Emissive Carbon Quantum Dots for Fluorescence Tetracycline Detection and Lysosome Imaging. R. Soc. Adv. 2022, 12, 33761–33771. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.L.; Chen, B.B.; Yang, T.; Wang, J.; Liu, X.D.; Huang, C.Z. One-Pot Carbonization Synthesis of Europium-Doped Carbon Quantum Dots for Highly Selective Detection of Tetracycline. Methods Appl. Fluoresc. 2017, 5, 015003. [Google Scholar] [CrossRef] [PubMed]
- Chai, S.Q.; He, J.H.; Zhan, L.; Li, Y.F.; Li, C.M.; Huang, C.Z. Dy(III)-Induced Aggregation Emission Quenching Effect of Single-Layered Graphene Quantum Dots for Selective Detection of Phosphate in the Artificial Wetlands. Talanta 2019, 196, 100–108. [Google Scholar] [CrossRef]
- Lv, L.L.; Qu, J.H.; Yu, Z.H.; Chen, D.H.; Zhou, C.X.; Hong, P.Z.; Sun, S.L.; Li, C.Y. A simple method for detecting and quantifying microplastics utilizing fluorescent dyes—Safranine T, fluorescein isophosphate, Nile red based on thermal expansion and contraction property. Environ. Pollut. 2019, 255, 113283. [Google Scholar] [CrossRef] [PubMed]
- Cai, H.; Xu, E.G.; Du, F.; Li, R.; Liu, J.; Shi, H. Analysis of Environmental Nanoplastics: Progress and Challenges. Chem. Eng. J. 2021, 410, 128208. [Google Scholar] [CrossRef]
- Khan, H.M.; Khan, A.A. Characterization of Aqueous Rose Bengal Dye Solution for the Measurement of Low Doses of Gamma Radiation. J. Radioanal. Nucl. Chem. 2010, 284, 37–42. [Google Scholar] [CrossRef]





| Nano PS | Micro PS | Mm PS | ||||
|---|---|---|---|---|---|---|
| 426 nm | 659 nm | 426 nm | 659 nm | 426 nm | 659 nm | |
| y0 | 0.00209 | 0.0517 | 0.00205 | 0.00222 | 0.00201 | 0.0231 |
| A1 | 7.374 × 10−6 | −0.0313 | 1.342 × 10−5 | 0.0273 | 7.862 × 10−5 | 0.0695 |
| t | −0.00388 | −0.0239 | −0.00499 | 0.00299 | −0.00468 | 0.00269 |
| R2 | 0.923 | 0.980 | 0.792 | 0.994 | 0.579 | 0.985 |
| Nano PS | Micro PS | Mm PS | |
|---|---|---|---|
| a | 52,745.613 | 206,260.436 | 15,256.532 |
| b | 36.640 | −74.569 | 27.695 |
| R2 | 0.992 | 0.939 | 0.963 |
| Nile Red [14,39] | Rose Bengal [15,46] | CQDs | |
|---|---|---|---|
| applicable environment | organic solvent | water | water |
| light stability | good | poor | good |
| sensitivity | poor | ordinary | good |
| pre-processing | need | need | need |
| Detecting of particle size | mm, μm | mm, μm | mm, μm, nm |
| Dyeing ability to PS | good | poor | ordinary |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, H.; Jiang, Z.; Wang, C.; Li, Y. Preparation of N, Cl Co-Doped Lignin Carbon Quantum Dots and Detection of Microplastics in Water. Crystals 2023, 13, 983. https://doi.org/10.3390/cryst13060983
Zhao H, Jiang Z, Wang C, Li Y. Preparation of N, Cl Co-Doped Lignin Carbon Quantum Dots and Detection of Microplastics in Water. Crystals. 2023; 13(6):983. https://doi.org/10.3390/cryst13060983
Chicago/Turabian StyleZhao, Hao, Zishuai Jiang, Chengyu Wang, and Yudong Li. 2023. "Preparation of N, Cl Co-Doped Lignin Carbon Quantum Dots and Detection of Microplastics in Water" Crystals 13, no. 6: 983. https://doi.org/10.3390/cryst13060983
APA StyleZhao, H., Jiang, Z., Wang, C., & Li, Y. (2023). Preparation of N, Cl Co-Doped Lignin Carbon Quantum Dots and Detection of Microplastics in Water. Crystals, 13(6), 983. https://doi.org/10.3390/cryst13060983







