Cobalt Ferrite Nanoparticles Capped with Perchloric Acid for Life-Science Application
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Suspensions of CoxFe3−xO4 Nanoparticles Stabilized with Perchloric Acid
2.3. Nanoparticle Characterization Methods
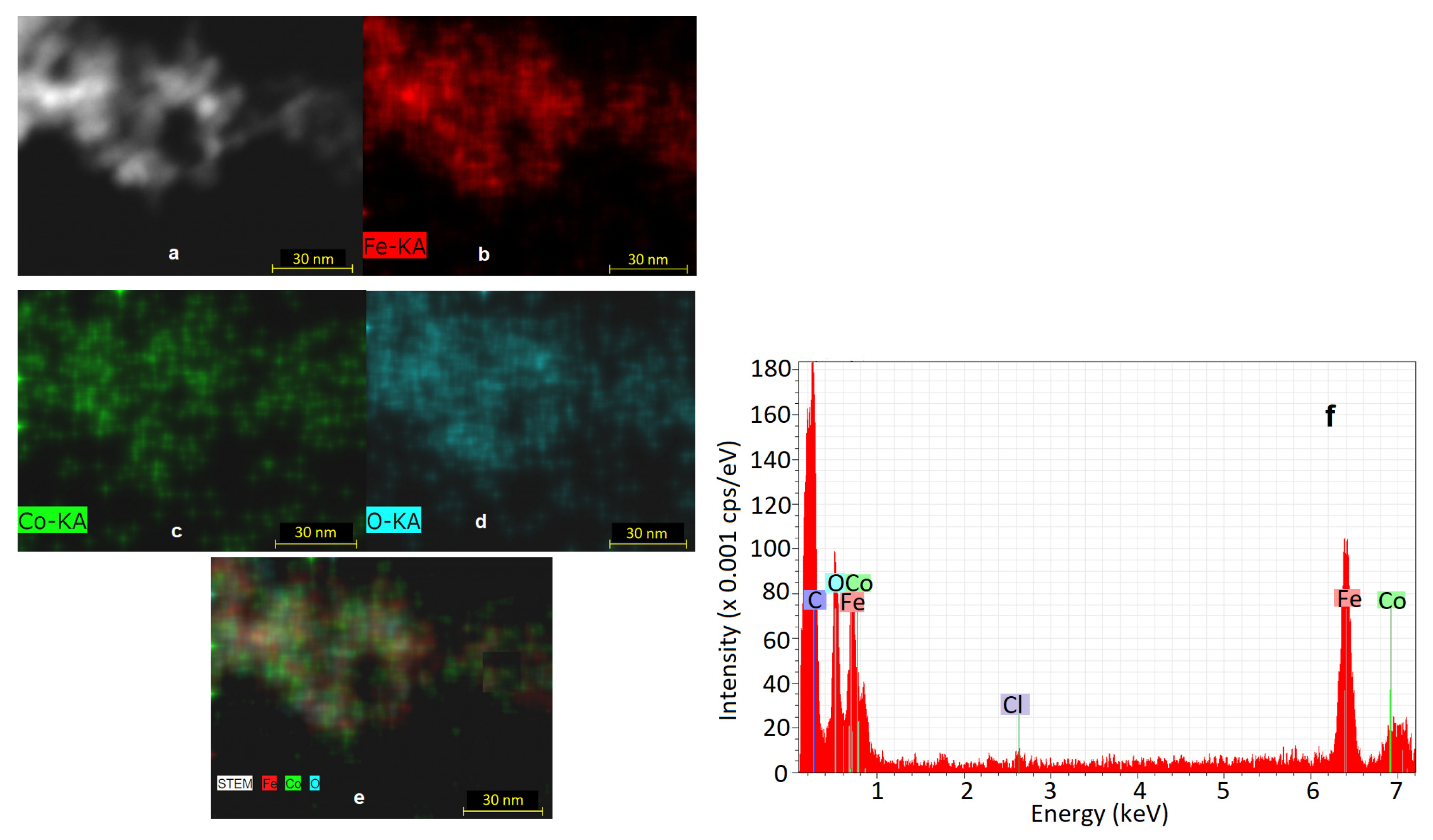
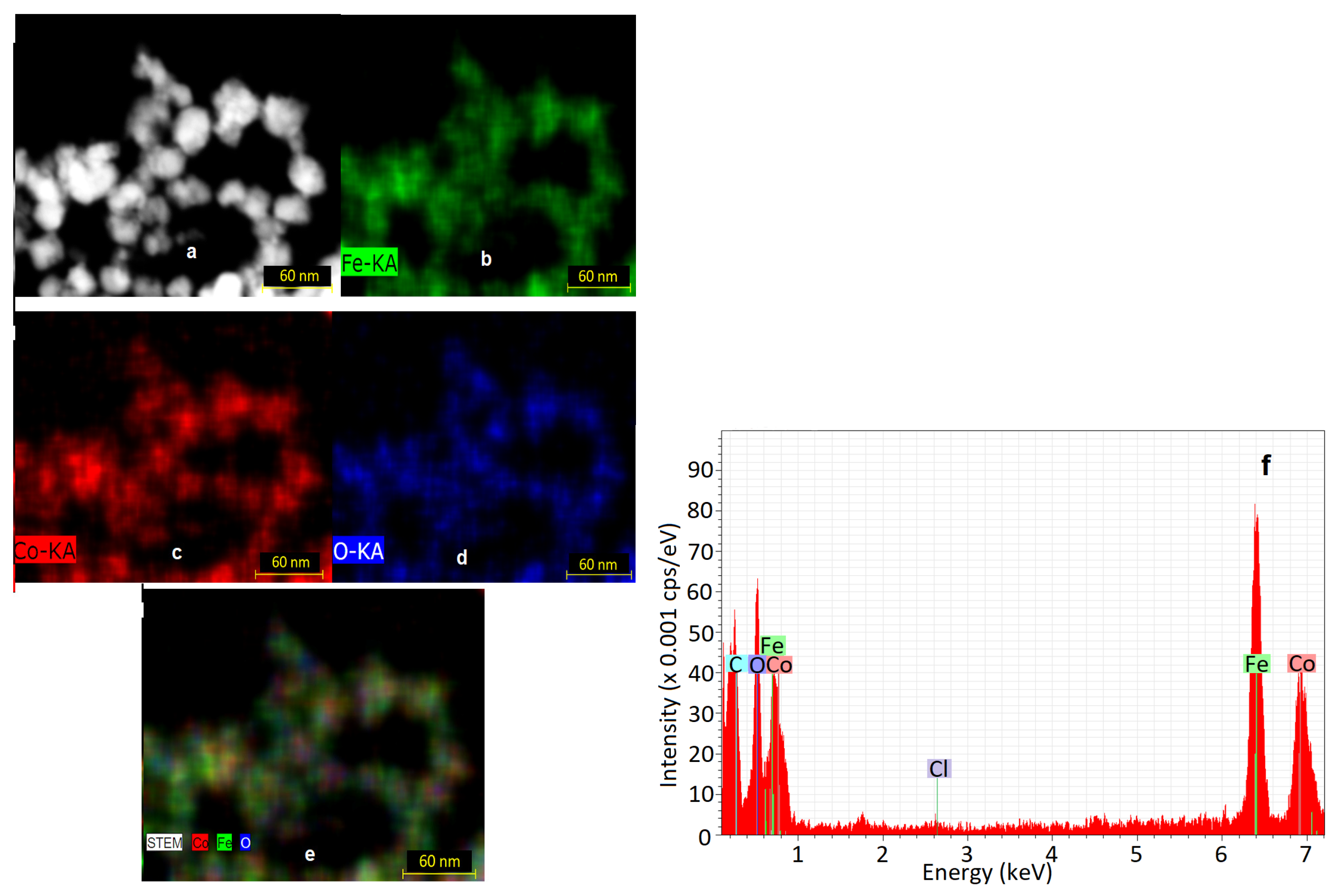
2.3.1. Microstructural and Elemental Investigation
2.3.2. Investigations of Crystallinity Properties
2.3.3. Magnetic Properties Investigation by Vibrating Sample Magnetometry (VSM)
2.3.4. FTIR Investigation
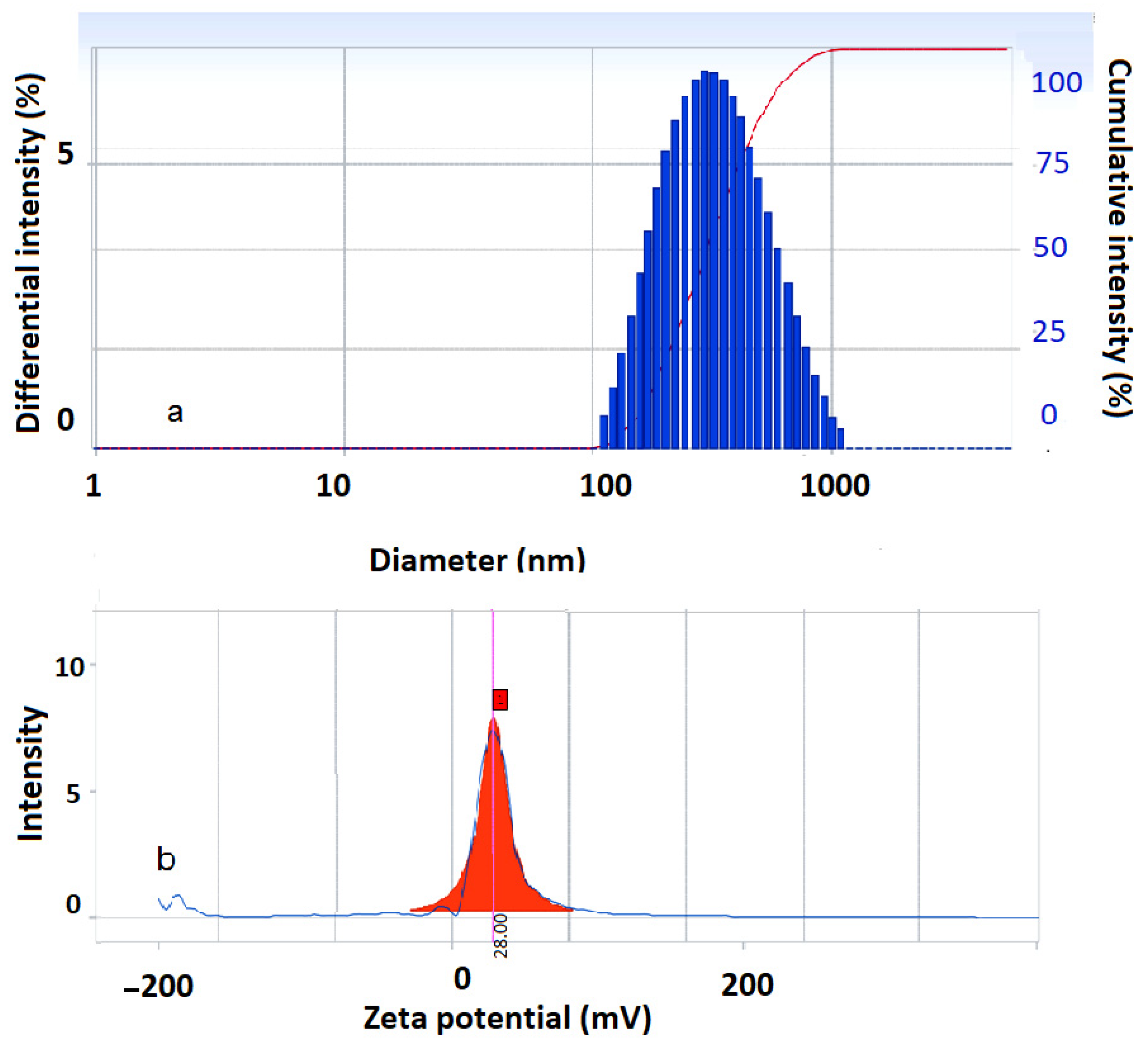
2.3.5. Dynamic Light Scattering Investigation
3. Results and Discussion
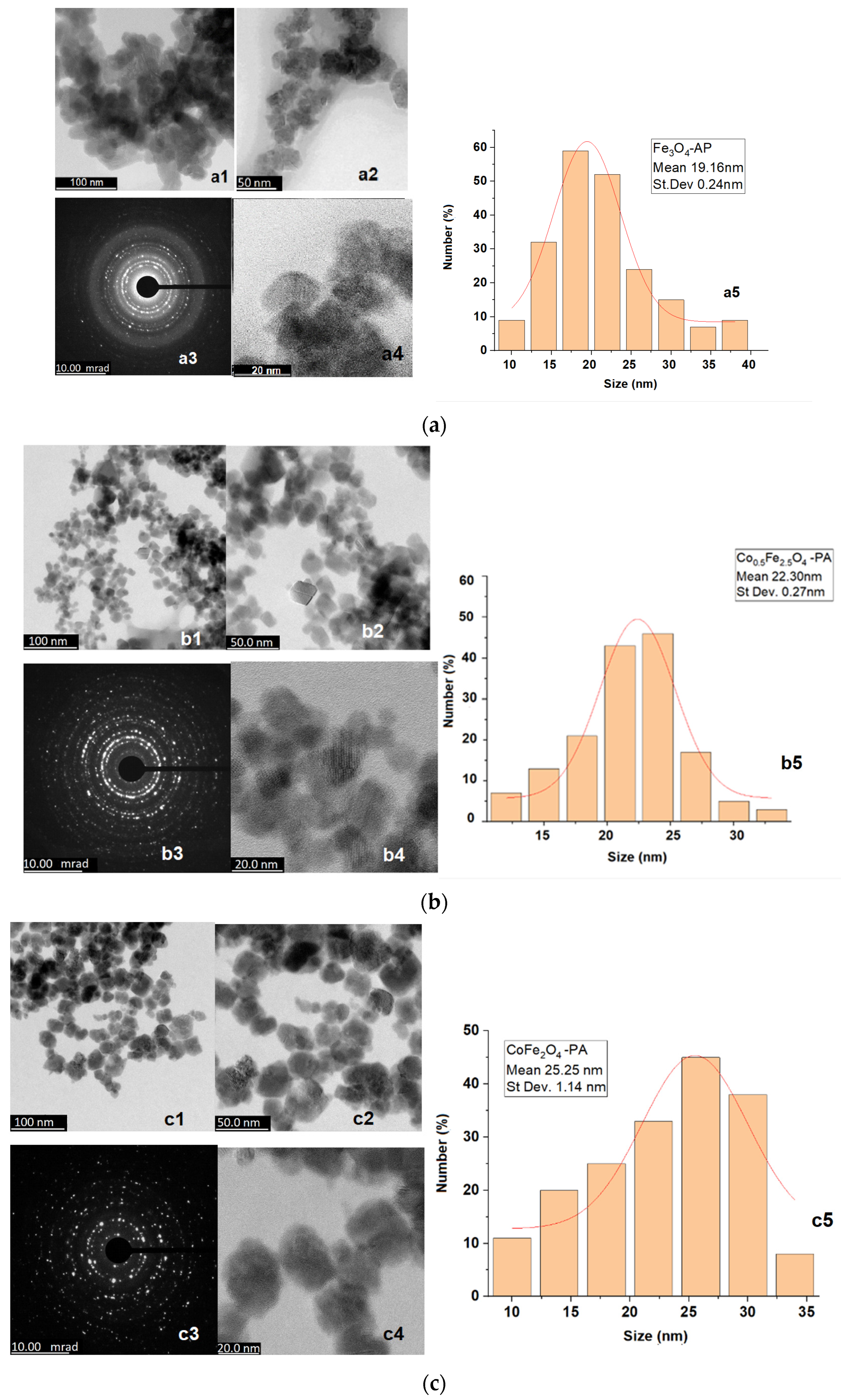
3.1. Microstructural Characterization
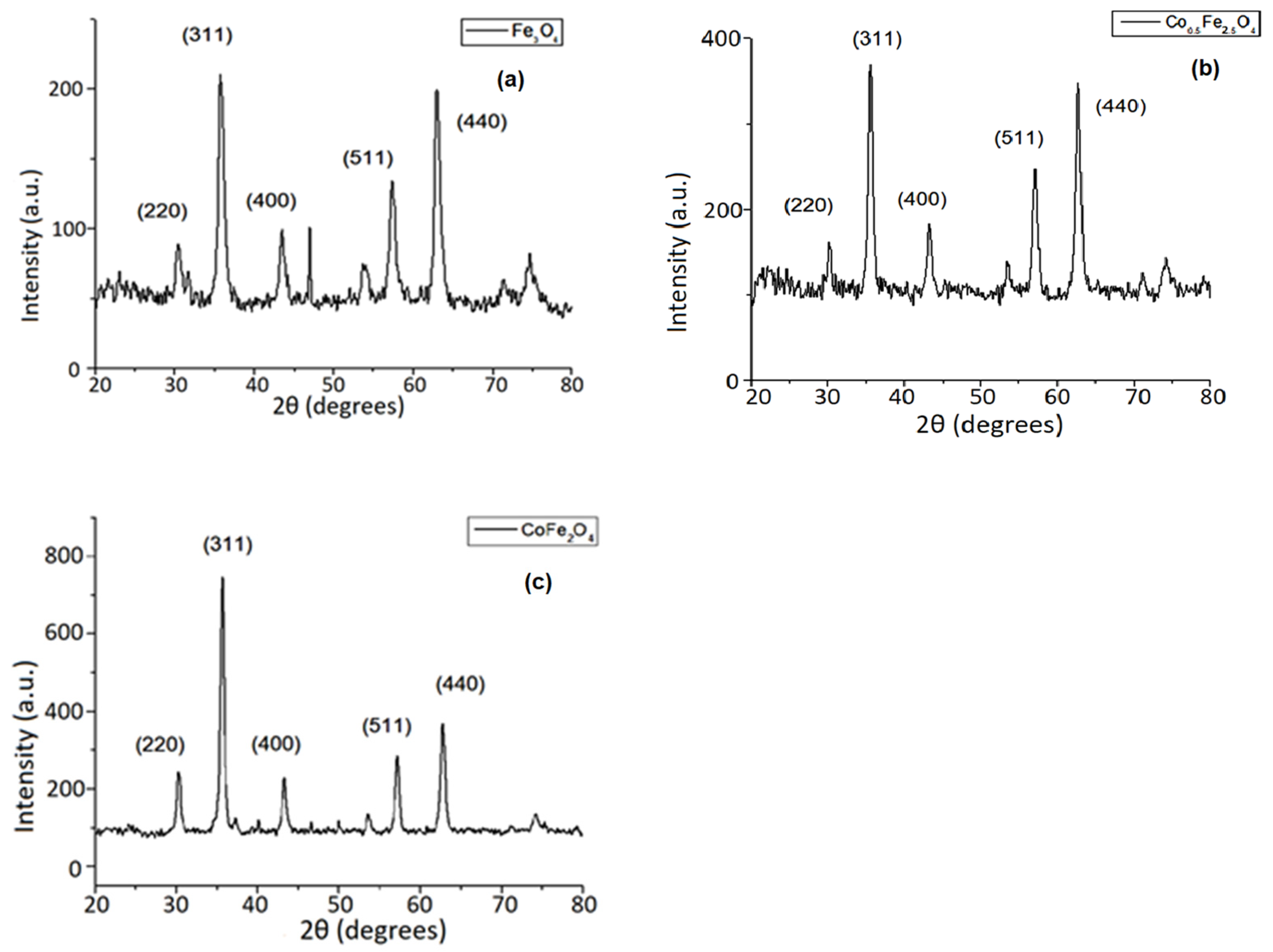
3.2. XRD Investigation Results
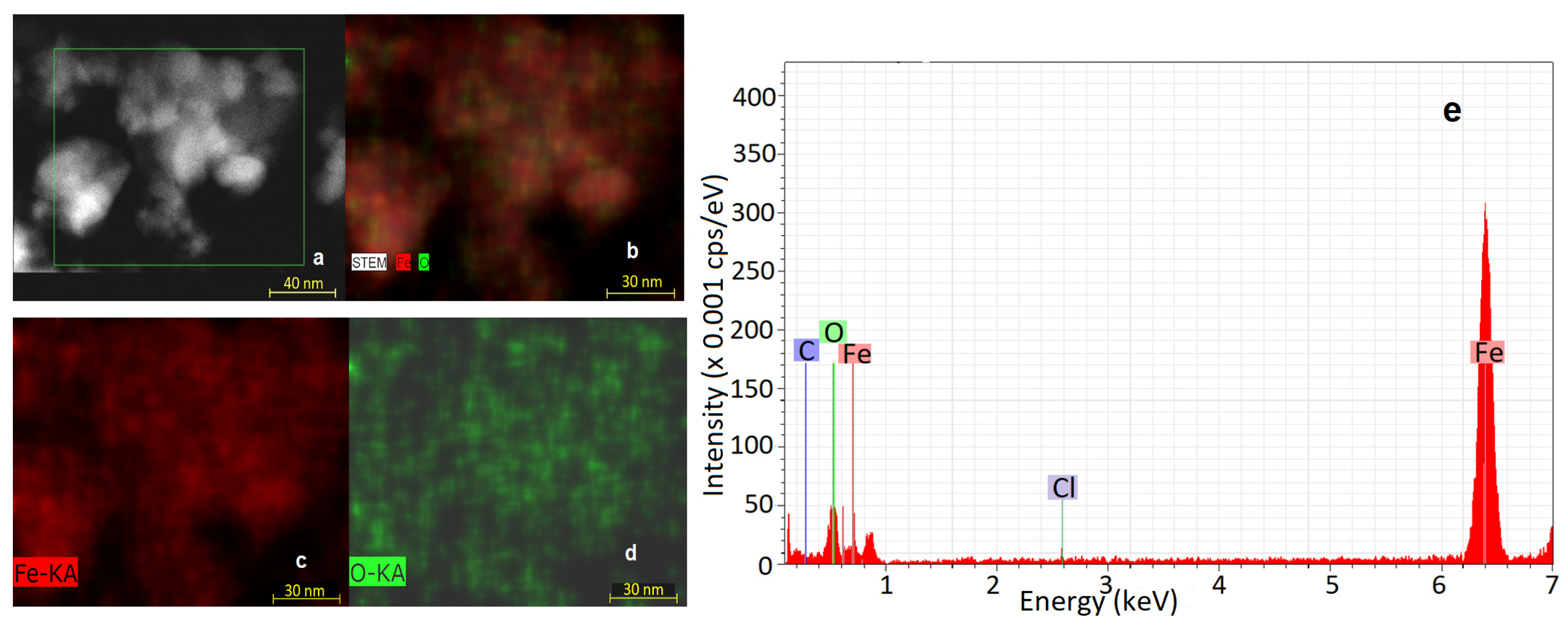
3.3. EDS Analysis Results
3.4. DLS Investigation Results
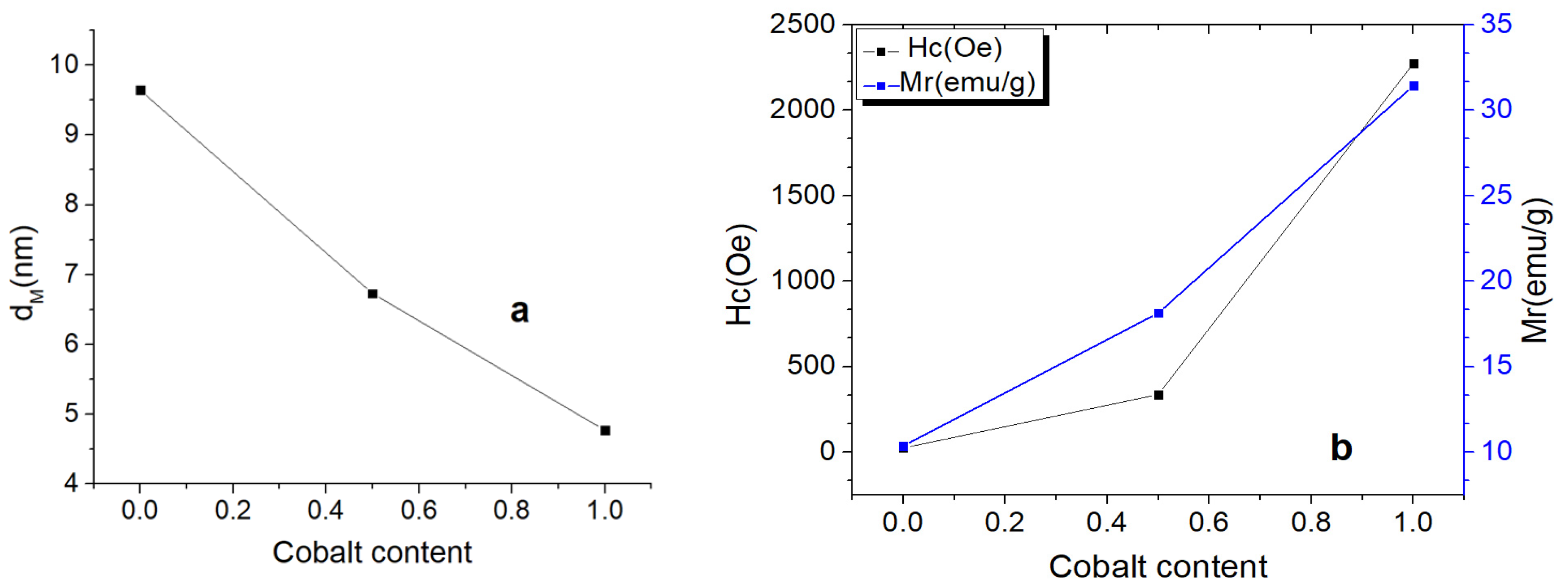
3.5. VSM Investigation Results
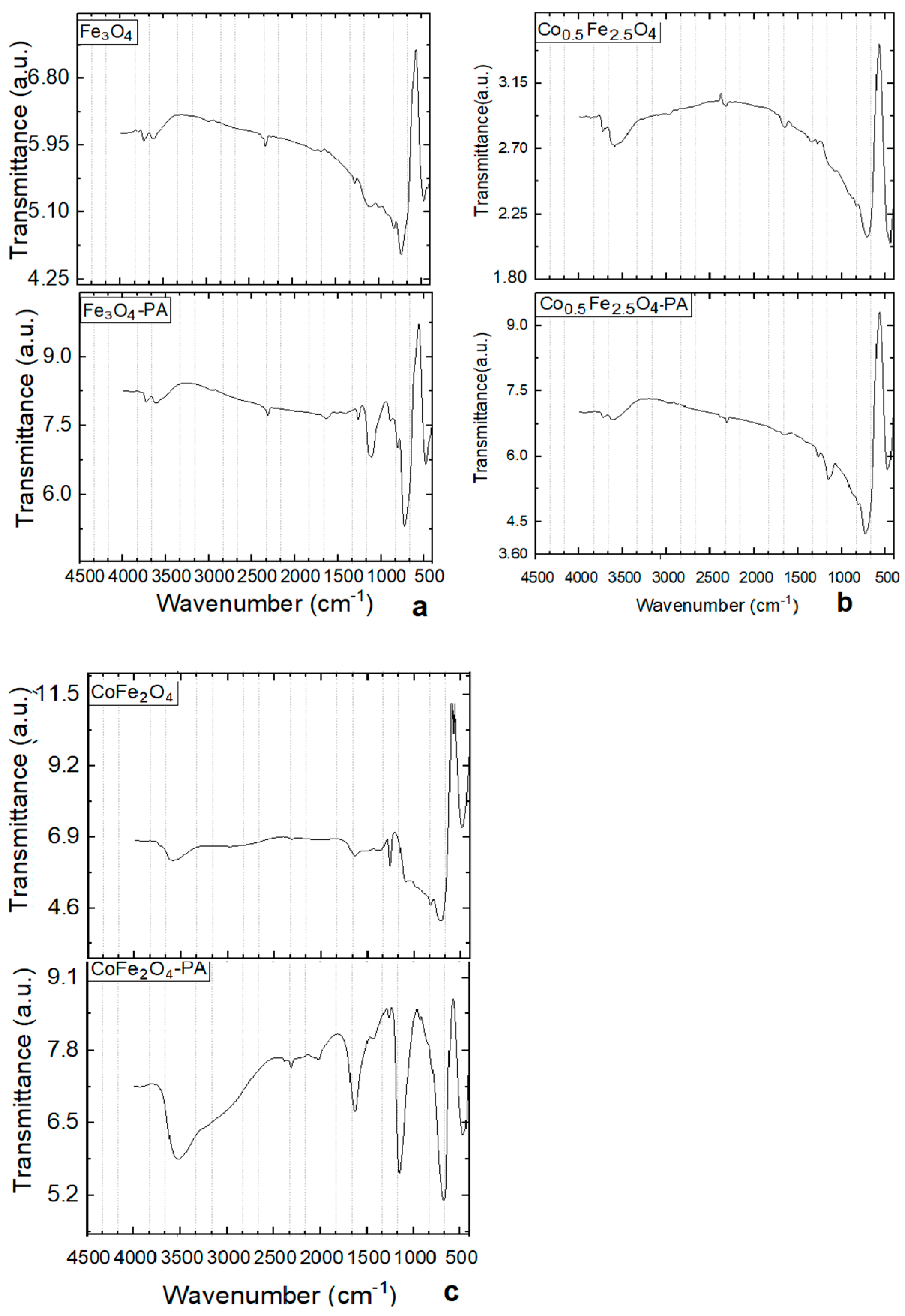
3.6. FTIR Investigatig Results
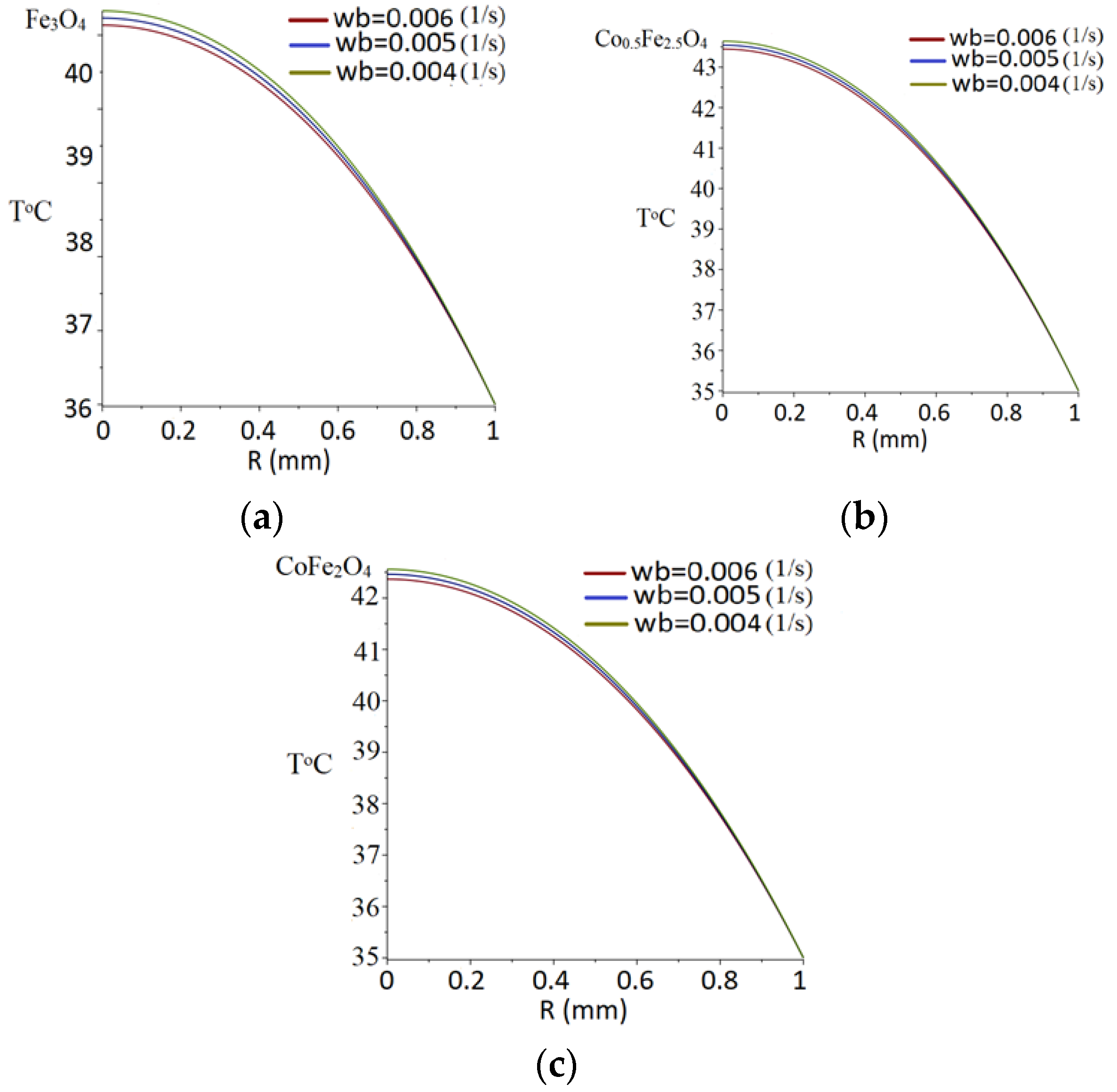
3.7. Temperature Field Modeling
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Liu, F.; Laurent, S.; Roch, A.; Elst, L.V.; Muller, R.N. Size-controlled synthesis of CoFe2O4 nanoparticles potential contrast agent for MRI and investigation on their size-dependent magnetic properties. J. Nanomater. 2013, 2013, 462540. [Google Scholar] [CrossRef] [Green Version]
- Garanina, A.S.; Nikitin, A.A.; Abakumova, T.O.; Semkina, A.S.; Prelovskaya, A.O.; Naumenko, V.A.; Erofeev, A.S.; Gorelkin, P.V.; Majouga, A.G.; Abakumov, M.A.; et al. Cobalt ferrite nanoparticles for tumor therapy: Effective heating versus possible toxicity. Nanomaterials 2021, 12, 38. [Google Scholar] [CrossRef] [PubMed]
- Dey, C.; Ghosh, A.; Ahir, M.; Ghosh, A.; Goswami, M.M. Improvement of anticancer drug release by cobalt ferrite magnetic nanoparticles through combined pH and temperature responsive technique. ChemPhysChem 2018, 9, 2872–2878. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.Q.; Moon, S.J.; Park, K.H.; Paek, S.H.; Chung, K.-W.; Bae, S. Feasibility of TEOS coated CoFe2O4 nanoparticles to a GMR biosensor agent for single molecular detection. J. Nanosci. Nanotechnol. 2011, 11, 82–89. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Uzunoğlu, D.; Ergüt, M.; Karacabey, P.; Özer, A. Synthesis of cobalt ferrite nanoparticles via chemical precipitation as an effective photocatalyst for photo Fenton-like degradation of methylene blue. Desalin. Water Treat. 2019, 172, 96–105. [Google Scholar] [CrossRef]
- Krishna, R.; Titus, E.; Chandra, S.; Bardhan, N.K.; Krishna, R.; Bahadur, D.; Gracio, J. Fabrication of a glucose biosensor based on citric acid assisted cobalt ferrite magnetic nanoparticles. J. Nanosci. Nanotechnol. 2012, 12, 6631–6638. [Google Scholar] [CrossRef]
- Lu, L.T.; Dung, N.T.; Tung, L.D.; Thanh, C.T.; Quy, O.K.; Chuc, N.V.; Maenosonoe, S.; Thanh, N.T.K. Synthesis of magnetic cobalt ferrite nanoparticles with controlled morphology, monodispersity and composition: The influence of solvent, surfactant, reductant and synthetic conditions. Nanoscale 2015, 7, 19596–19610. [Google Scholar] [CrossRef] [Green Version]
- Mohamed, R.M.; Rashad, M.M.; Haraz, F.A.; Sigmund, W. Structure and magnetic properties of nanocrystalline cobalt ferrite powders synthesized using organic acid precursor method. J. Magn. Magn. Mater. 2010, 322, 2058–2064. [Google Scholar] [CrossRef]
- Kurian, S.; Thankachan, D.S.; Nair, A.E.K.; Thomas, A.B.A.; Binu Krishna, K.T. Structural, magnetic, and acidic properties of cobalt ferrite nanoparticles synthesised by wet chemical methods. J. Adv. Ceram. 2015, 4, 199–205. [Google Scholar] [CrossRef] [Green Version]
- Tamboli, Q.Y.; Patange, S.M.; Mohanta, Y.K.; Sharma, R.; Zakde, K.R. Green Synthesis of cobalt ferrite nanoparticles: An emerging material for environmental and biomedical applications. J. Nanomater. 2023, 2023, 9770212. [Google Scholar] [CrossRef]
- Jeevanantham, B.; Song, Y.; Choe, H.; Shobana, M.K. Structural and optical characteristics of cobalt ferrite nanoparticles. Mater. Lett. X 2021, 12, 100105. [Google Scholar] [CrossRef]
- Stein, C.R.; Bezerra, M.T.S.; Holanda, G.H.A.; André-Filho, J.; Morais, P.C. Structural and magnetic properties of cobalt ferrite nanoparticles synthesized by co-precipitation at increasing temperatures. AIP Adv. 2018, 8, 056303. [Google Scholar] [CrossRef]
- Sangsuriyonk, K.; Paradee, N.; Rotjanasuworapong, K.; Sirivat, A. Synthesis and characterization of CoxFe1−xFe2O4 nanoparticles by anionic, cationic, and non-ionic surfactant templates via co-precipitation. Sci. Rep. 2022, 12, 4611. [Google Scholar] [CrossRef] [PubMed]
- Fantechi, E.; Innocenti, C.; Albino, M.; Lottini, E.; Sangregorio, C. Influence of cobalt doping on the hyperthermic efficiency of magnetite nanoparticles. J. Magn. Magn. Mater. 2015, 380, 365–371. [Google Scholar] [CrossRef]
- Sathya, A.; Guardia, P.; Brescia, R.; Silvestri, N.; Pugliese, G.; Nitti, S.; Manna, L.; Pellegrino, T. CoxFe3−xO4 nanocubes for theranostic applications: Effect of cobalt content and particle size. Chem. Mater. 2016, 28, 1769–1780. [Google Scholar] [CrossRef]
- Mazario, E.; Menéndez, N.; Herrasti, P.; Cañete, M.; Connord, V.; Carrey, J. Magnetic hyperthermia properties of electrosynthesized cobalt ferrite nanoparticles. J. Phys. Chem. C 2013, 117, 11405–11411. [Google Scholar] [CrossRef]
- Pennes, H.H. Analysis of tissue and arterial blood temperatures in the resting human forearm. J. Appl. Physiol. 1948, 1, 93–122. [Google Scholar] [CrossRef] [PubMed]
- Shih, T.-C.; Yuan, P.; Lin, W.-L.; Kou, H.-S. Analytical analysis of the Pennes bioheat transfer equation with sinusoidal heat flux condition on skin surface. Med. Eng. Phys. 2007, 29, 946–953. [Google Scholar] [CrossRef]
- Massart, R. Preparation of aqueous magnetic liquids in alkaline and acidic media. IEEE Trans. Magn. 1981, 17, 2–5. [Google Scholar] [CrossRef]
- Blanco-Andujar, C.; Ortega, D.; Pankhursta, Q.A.; Thanh, N.T.K. Elucidating the morphological and structural evolution of iron oxide nanoparticles formed by sodium carbonate in aqueous medium. J. Mater. Chem. 2012, 22, 12498. [Google Scholar] [CrossRef] [Green Version]
- Trivedi, M.K.; Sethi, K.K.; Panda, P.; Jana, S. Physicochemical, Thermal and Spectroscopic Characterization of Sodium Selenate Using XRD, PSD, DSC, TGA/DTG, UV-vis, and FT-IR. Marmara Pharm. J. 2017, 21, 311–318. [Google Scholar] [CrossRef] [Green Version]
- Sanpo, N.; Wang, J.; Berndt, C.C. Sol-Gel Synthesized Copper-Substituted Cobalt Ferrite Nanoparticles for Biomedical Applications. J. Nano Res. 2013, 22, 95–106. [Google Scholar] [CrossRef]
- Allaedini, G.; Tasirin, S.M.; Aminayi, P. Magnetic properties of cobalt ferrite synthesized by hydrothermal method. Int. Nano Lett. 2015, 5, 183–186. [Google Scholar] [CrossRef] [Green Version]
- Culita, D.C.; Patron, L.; Oprea, O.; Bartha, C.; Palade, P.; Teodorescu, V.; Filoti, G. Detailed characterization of functionalized magnetite and ascertained effects. J. Nanopart. Res. 2013, 15, 1916. [Google Scholar] [CrossRef]
- Ghorbani, H.; Eshraghi, M.; Sabouri Dodaran, A.A. Structural and magnetic properties of cobalt ferrite nanoparticles doped with cadmium. Phys. B Condens. Matter 2022, 634, 413816. [Google Scholar] [CrossRef]
- Olsson, R.T.; Salazar-Alvarez, G.; Hedenqvist, M.S.; Gedde, U.W.; Lindberg, F.; Savage, S.J. Controlled synthesis of near-stoichiometric cobalt ferrite nanoparticles. Chem. Mater. 2005, 17, 5109–5118. [Google Scholar] [CrossRef]
- El-Hiti, G.A.; Alotaibi, M.H.; Ahmed, A.A.; Hamad, B.A.; Ahmed, D.S.; Ahmed, A.; Hashim, H.; Yousif, E. The morphology and performance of poly(vinyl chloride) containing melamine schiff bases against ultraviolet light. Molecules 2019, 24, 803. [Google Scholar] [CrossRef] [Green Version]
- Vinosha, P.A.; Manikandan, A.; Preetha, A.C.; Dinesh, A.; Slimani, Y.; Almessiere, M.A.; Baykal, A.; Xavier, B.; Nirmala, G.F. Review on recent advances of synthesis, magnetic properties, and water treatment applications of cobalt ferrite nanoparticles and nanocomposites. J. Supercond. Nov. Magn. 2021, 34, 995–1018. [Google Scholar] [CrossRef]
- Wetterskog, E.; Tai, C.W.; Bergström, L.; Salazar-Alvarez, G. Anomalous magnetic properties of nanoparticles arising from defect structures: Topotaxial oxidation of Fe1−xO/Fe3−δO4 core /shell nanocubes to single-phase particles. ACS Nano 2013, 7, 7132–7144. [Google Scholar] [CrossRef]
- Shendruk, T.N.; Desautels, R.D.; Southern, B.W.; van Lierop, J. The effect of surface spin disorder on the magnetism of γ-Fe2O3 nanoparticle dispersions. Nanotechnology 2007, 18, 455704. [Google Scholar] [CrossRef]
- George, T.; Sunnya, A.T.; Varghese, T. Magnetic properties of cobalt ferrite nanoparticles synthesized by sol-gel method. IOP Conf. Ser. Mater. Sci. Eng. 2015, 73, 012050. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, Z.; Yin, D.; Liu, Y.; Fei, C.L.; Xiong, R.; Shi, J.; Yan, G.L. Composition and magnetic properties of cobalt ferrite nano-particles prepared by the co-precipitation method. J. Magn. Magn. Mater. 2010, 322, 3470–3475. [Google Scholar] [CrossRef]
- D’yachenko, S.V.; Zhernovoi, A.I. The Langevin formula for describing the magnetization curve of a magnetic liquid. Tech. Phys. 2016, 61, 1835–1837. [Google Scholar] [CrossRef]
- Herzer, G. Grain size dependence of coercivity and permeability in nanocrystalline ferromagnets. IEEE Trans. Magn. 1990, 26, 1397–1402. [Google Scholar] [CrossRef]
- Tajiri, T.; Terashita, N.; Hamamoto, K.; Deguchi, H.; Mito, M.; Morimoto, Y.; Konishi, K.; Kohno, A. Size dependences of crystal structure and magnetic properties of DyMnO3 nanoparticles. J. Magn. Magn. Mater. 2013, 345, 288–293. [Google Scholar] [CrossRef]
- Saafan, S.A.; Assar, S.T.; Mansour, S.F. Magnetic and electrical properties of Co1−xCax Fe2O4 nanoparticles synthesized by the auto combustion method. J. Alloys Compd. 2012, 542, 32–36. [Google Scholar] [CrossRef]
- Dehsari, H.S.; Asadi, K. Impact of stoichiometry and size on the magnetic properties of cobalt ferrite nanoparticles. J. Phys. Chem. C 2018, 122, 29106–29121. [Google Scholar] [CrossRef]
- Muthuselvi, C.; Rathika, N.; Selvalakshmi, K. Vibrational, Optical and antimicrobial activity studies on diglycine perchlorate single crystal. J. Appl. Sci. 2019, 19, 848–856. [Google Scholar] [CrossRef] [Green Version]
- Chaki, S.H.; Malek, T.J.; Chaudhary, M.D.; Tailor, J.P.; Deshpande, M.P. Magnetite Fe3O4 nanoparticles synthesis by wet chemical reduction and their characterization. Adv. Nat. Sci. Nanosci. Nanotechnol. 2015, 6, 035009. [Google Scholar] [CrossRef]
- Music, S. Mössbauer, FT-IR and FE SEM investigation of iron oxides precipitated from FeSO4 solutions. J. Mol. Struct. 2007, 834–836, 445–453. [Google Scholar]
- Prasad, P.S.R.; Prasad, K.S.; Chaitanya, V.K.; Babu, E.V.S.S.K.; Sreedhar, B.; Murthy, S.R.J. In situ FTIR study on the dehydration of natural goethite. J. Asian Earth Sci. 2006, 27, 503–511. [Google Scholar] [CrossRef]
- Cui, H.; Ren, W.; Lin, P.; Liu, Y. Structure control synthesis of iron oxide polymorph nanoparticles through an epoxide precipitation route. J. Exp. Nanosci. 2013, 8, 869–875. [Google Scholar] [CrossRef]
- Ristic, M.; De Grave, E.; Music, S.; Popovic, S.; Orehovec, Z. Transformation of low crystalline ferrihydrite to α-Fe2O3 in the solid state. J. Mol. Struct. 2007, 454, 834–836. [Google Scholar] [CrossRef]
- Ghosh, M.K.; Poinern, G.E.J.; Issa, T.B.; Singh, P. Arsenic adsorption on goethite nanoparticles produced through hydrazine sulfate assisted synthesis method. Korean J. Chem. Eng. 2012, 29, 95–102. [Google Scholar] [CrossRef]
- Safi, R.; Ghasemi, A.; Shoja-Razavi, R.; Tavousi, M. The role of pH on the particle size and magnetic consequence of cobalt ferrite. J. Magn. Magn. Mater. 2015, 396, 288–294. [Google Scholar] [CrossRef]
- Anjum, S.; Tufail, R.; Rashid, K.; Zia, R.; Riaz, S. Effect of cobalt doping on crystallinity, stability, magnetic and optical properties of magnetic iron oxide nano-particles. J. Magn. Magn. Mater. 2017, 432, 198–207. [Google Scholar] [CrossRef]
- Karthickraja, D.; Karthi, S.; Kumar, G.A.; Sardar, D.K.; Dannangoda, G.C.; Martirosyan, K.S.; Girija, E.K. Fabrication of core–shell CoFe2O4@HAp nanoparticles: A novel magnetic platform for biomedical applications. New J. Chem. 2019, 43, 13584. [Google Scholar] [CrossRef]
- Rana, S.; Philip, J.; Raj, B. Micelle based synthesis of cobalt ferrite nanoparticles and its characterization using Fourier transform infrared transmission spectrometry and thermogravimetry. Mater. Chem. Phys. 2010, 124, 264–269. [Google Scholar] [CrossRef]
- Oliveira, P.N.; Silva, D.M.; Dias, G.S.; Santos, I.A.; Cotica, L.F. Synthesis and physical property measurements of CoFe2O4:BaTiO3 core-shell composite nanoparticles. Ferroelectrics 2016, 499, 76–82. [Google Scholar] [CrossRef]
- Vitello, D.J.; Ripper, R.M.; Fettiplace, M.R.; Weinberg, G.L.; Vitello, J.M. Blood density is nearly equal to water density: A validation study of the gravimetric method of measuring intraoperative blood loss. J. Vet. Med. 2015, 2015, 152730. [Google Scholar] [CrossRef] [Green Version]
- Nie, S.; Zhang, C.; Song, J. Thermal management of epidermal electronic devices/skin system considering insensible sweating. Sci. Rep. 2018, 8, 14121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Specific Heat Capacity and Thermal Conduction of Blood. Available online: https://biology.stackexchange.com/questions/111370/specific-heat-capacity-and-thermal-conduction-of-blood (accessed on 30 May 2023).
- Pankhurst, Q.A.; Connolly, J.; Jones, S.K.; Dobson, J. Applications of magnetic nanoparticles in biomedicine. J. Phys. D 2003, 36, R167–R181. [Google Scholar] [CrossRef] [Green Version]
- Rosensweig, R.A. Heating magnetic fluid with alternating magnetic field. J. Magn. Magn. Mater. 2002, 252, 370–374. [Google Scholar] [CrossRef]
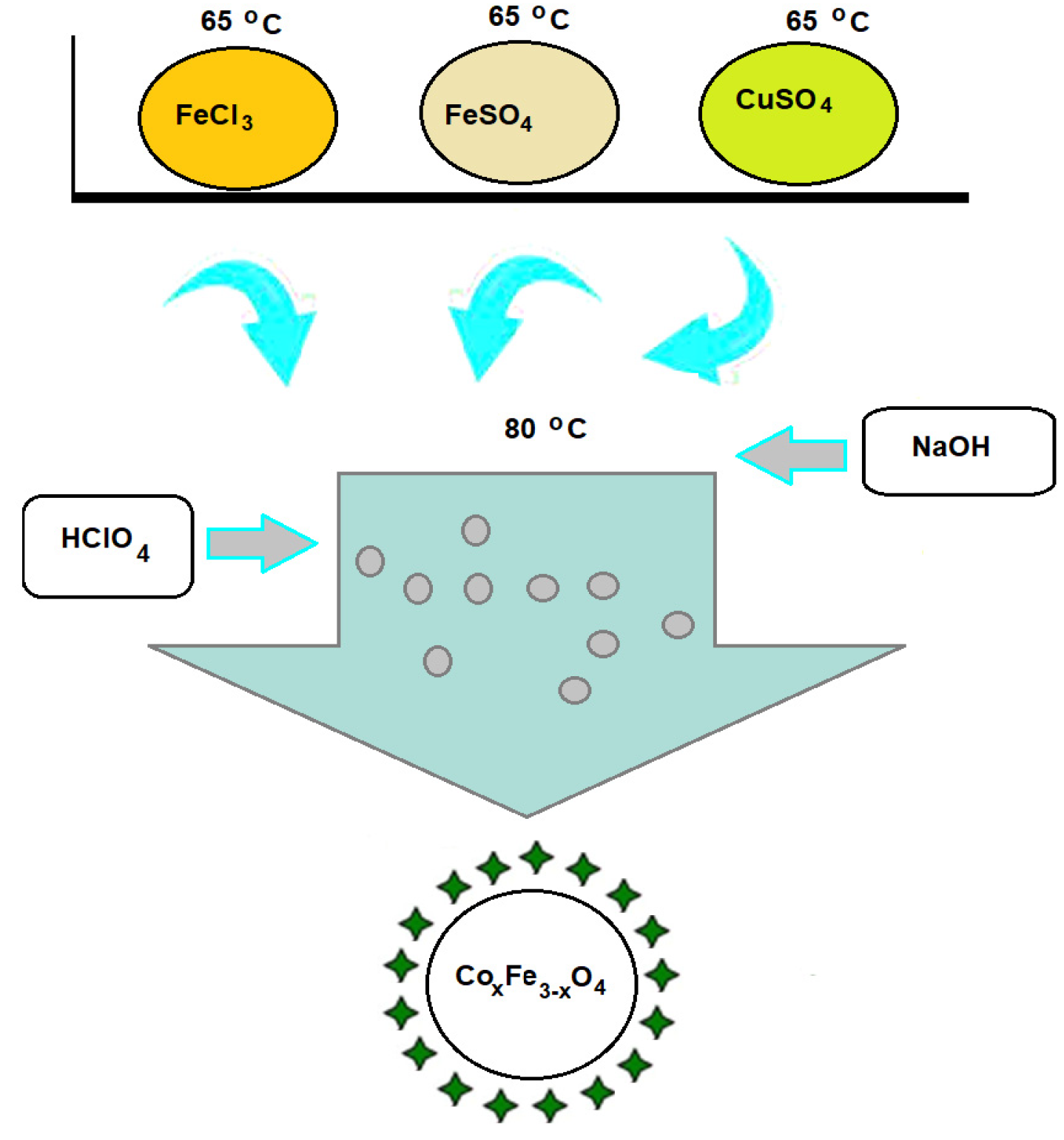




| Sample | x | FeCl3 × 6H2O (134 mM) | FeSO4 × 7H2O (67 mM) | CoSO4 × 7H2O (67 mM) | NaOH (1.7 M) | HClO4 (25%) |
|---|---|---|---|---|---|---|
| Fe3O4 | 0 | 3.62 g (100 mL) | 1.86 g (100 mL) | - | 3.4 g (50 mL) | 3 mL |
| Co0.5Fe2.5O4 | 0.5 | 3.62 g (100 mL) | 0.93 g (50 mL) | 0.94 g (50 mL) | 3.4 g (50 mL) | 3 mL |
| CoFe2O4 | 1 | 3.62 g (100 mL) | - | 1.88 g (100 mL) | 3.4 g (50 mL) | 3 mL |
| Cobalt Content | Ferrophase | D (nm) | St Dev (nm) |
|---|---|---|---|
| 0 | Fe3O4 | 19.16 | 0.24 |
| 0.5 | Co0.5Fe2.5O4 | 22.30 | 0.27 |
| 1 | CoFe2O4 | 25.25 | 1.14 |
| Sample | Dh (nm) | PI | V (mV) |
|---|---|---|---|
| Fe3O4-PA | 311.2 | 0.234 | 28.0 |
| Co0.5Fe2.5O4-PA | 157.4 | 0.235 | 20.47 |
| CoFe2O4-PA | 77.8 | 0.239 | 30.29 |
| Sample | Cobalt Content | dM (nm) | MS (emu/g) | Hc (Oe) | Mr (emu/g) | Mr/Hc (emu/g/Oe) |
|---|---|---|---|---|---|---|
| Fe3O4 | 0 | 9.64 | 64.68 | 24.238 | 10.36 | 0.427 |
| Co0.5Fe2.5O4 | 0.5 | 6.73 | 72.483 | 337.23 | 18.14 | 0.053 |
| CoFe2O4 | 1 | 4.77 | 54.132 | 2274.7 | 31.44 | 0.013 |
| Sample | dM (nm) | D311 (nm) | DTEM (nm) | Dh (nm) |
|---|---|---|---|---|
| Fe3O4 | 9.64 | 9.8 | 19.1 | 311.2 |
| Co0.5Fe2.5O4 | 6.73 | 11.3 | 22.3 | 157.4 |
| CoFe2O4 | 4.77 | 15.2 | 25.2 | 77.8 |
| Thermal and Magnetic Characteristics | Values |
|---|---|
| Te (K) | 308 |
| Ta (K) | 310 |
| ρb (kg/m3) | 1000 |
| cb (J/kg K) | 4200 |
| k (W/m·K) | 0.2 |
| ωb (1/s) | 0.004/0.005/0.006 |
| Qm (W/m3) | 400 |
| Qext (W/m3) Fe3O4 | 2.06 × 105 |
| Qext (W/m3) Co0.5Fe2.5O4 | 3.50 × 105 |
| Qext (W/m3) CoFe2O4 | 3.03 × 105 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ardeleanu, H.; Ababei, G.; Grigoras, M.; Ursu, L.; Melniciuc-Puica, N.; Astefanoaei, I.; Pricop, D.; Lupu, N.; Creanga, D. Cobalt Ferrite Nanoparticles Capped with Perchloric Acid for Life-Science Application. Crystals 2023, 13, 1058. https://doi.org/10.3390/cryst13071058
Ardeleanu H, Ababei G, Grigoras M, Ursu L, Melniciuc-Puica N, Astefanoaei I, Pricop D, Lupu N, Creanga D. Cobalt Ferrite Nanoparticles Capped with Perchloric Acid for Life-Science Application. Crystals. 2023; 13(7):1058. https://doi.org/10.3390/cryst13071058
Chicago/Turabian StyleArdeleanu, Helmina, Gabriel Ababei, Marian Grigoras, Laura Ursu, Nicoleta Melniciuc-Puica, Iordana Astefanoaei, Daniela Pricop, Nicoleta Lupu, and Dorina Creanga. 2023. "Cobalt Ferrite Nanoparticles Capped with Perchloric Acid for Life-Science Application" Crystals 13, no. 7: 1058. https://doi.org/10.3390/cryst13071058






