Large-Scale Production and Optical Properties of a High-Quality SnS2 Single Crystal Grown Using the Chemical Vapor Transportation Method
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Details
2.2. Characterizations
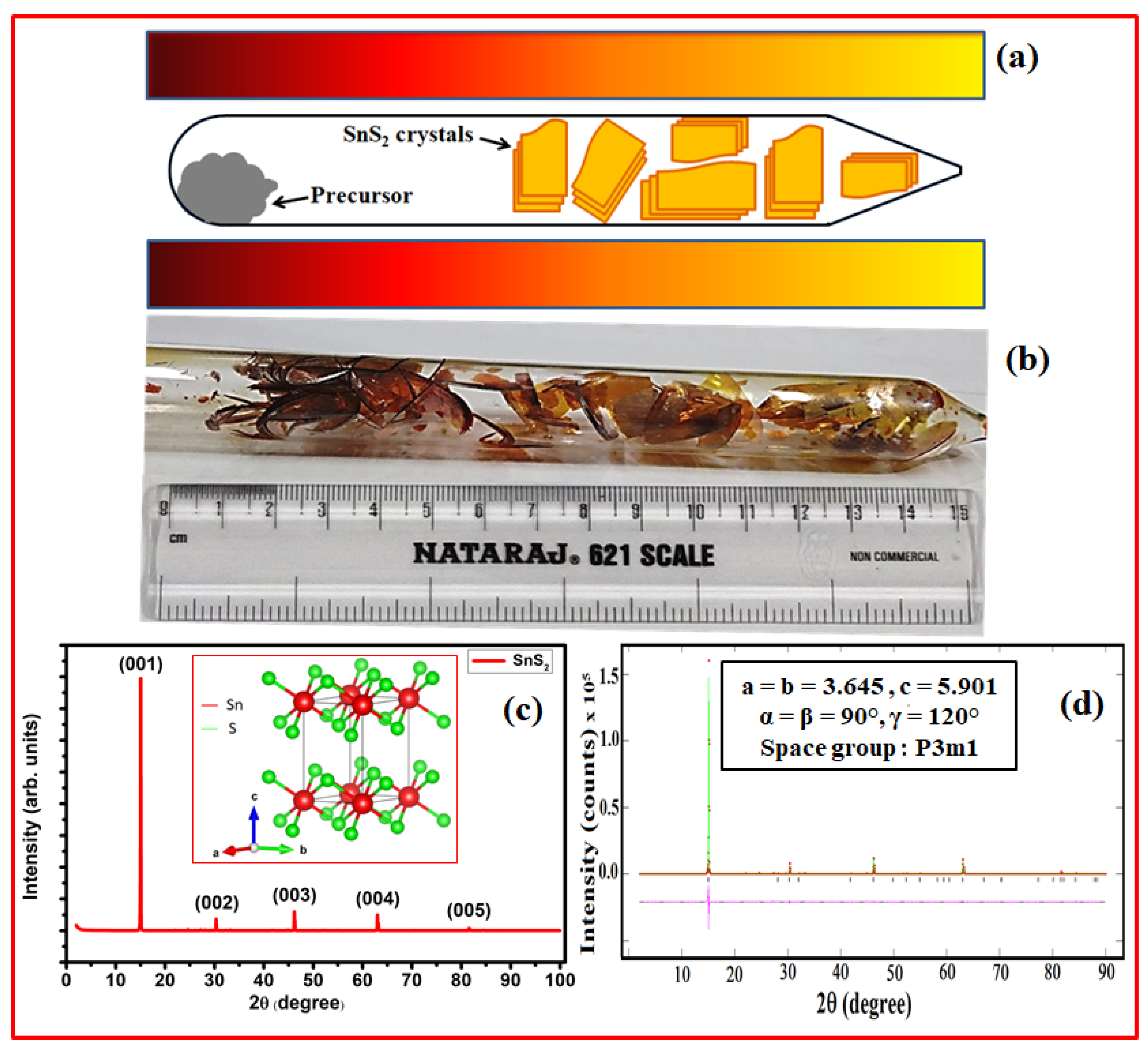
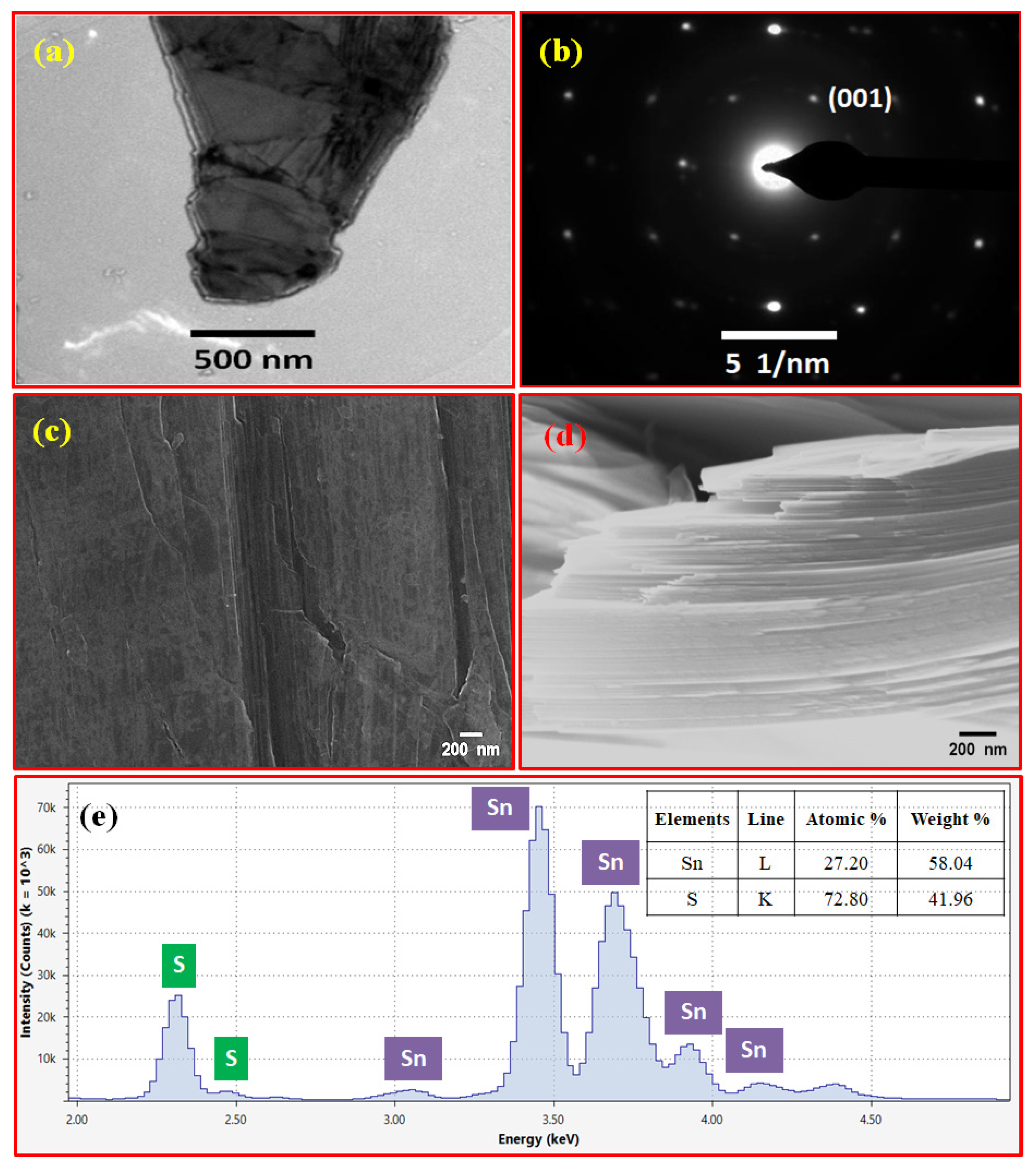
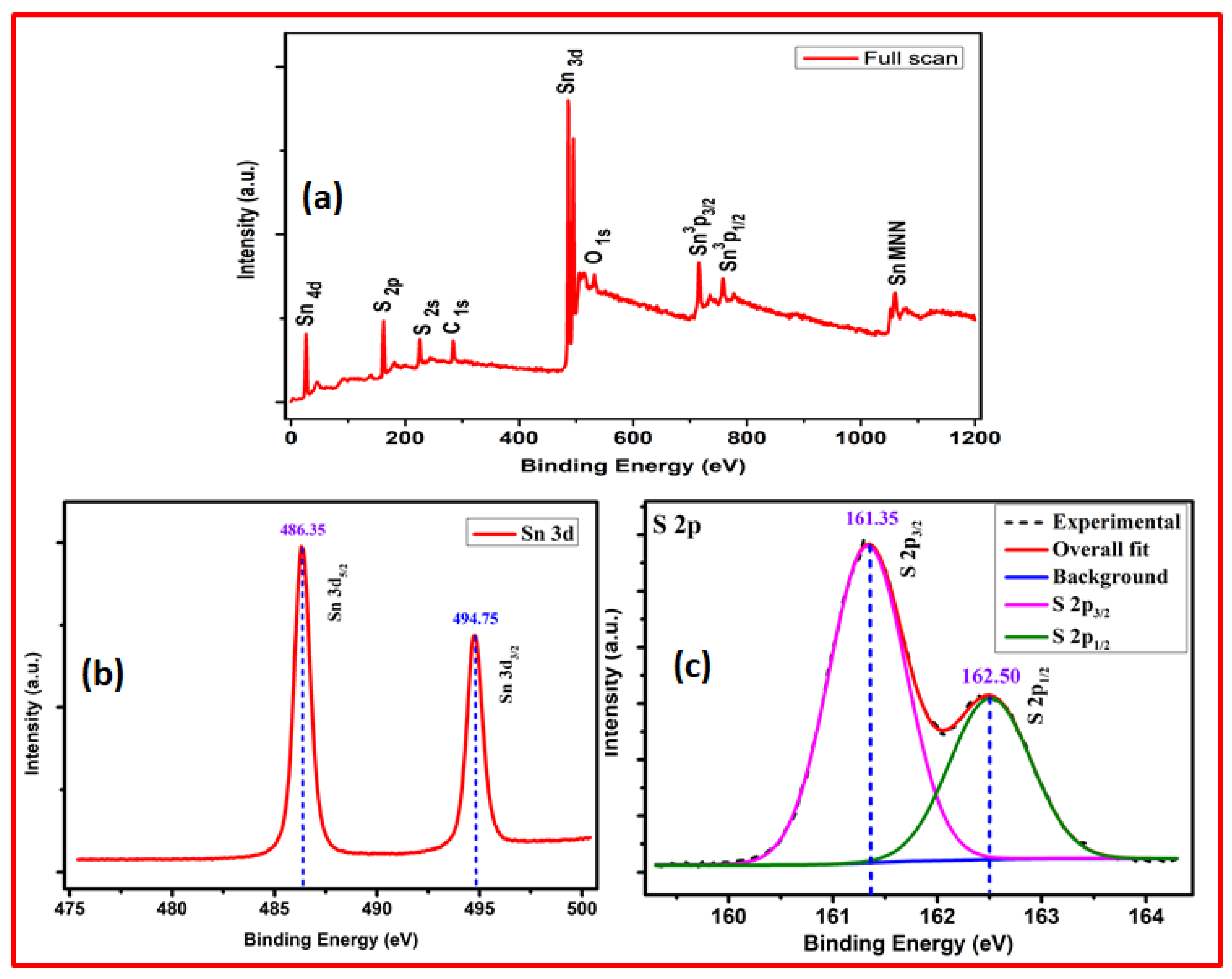
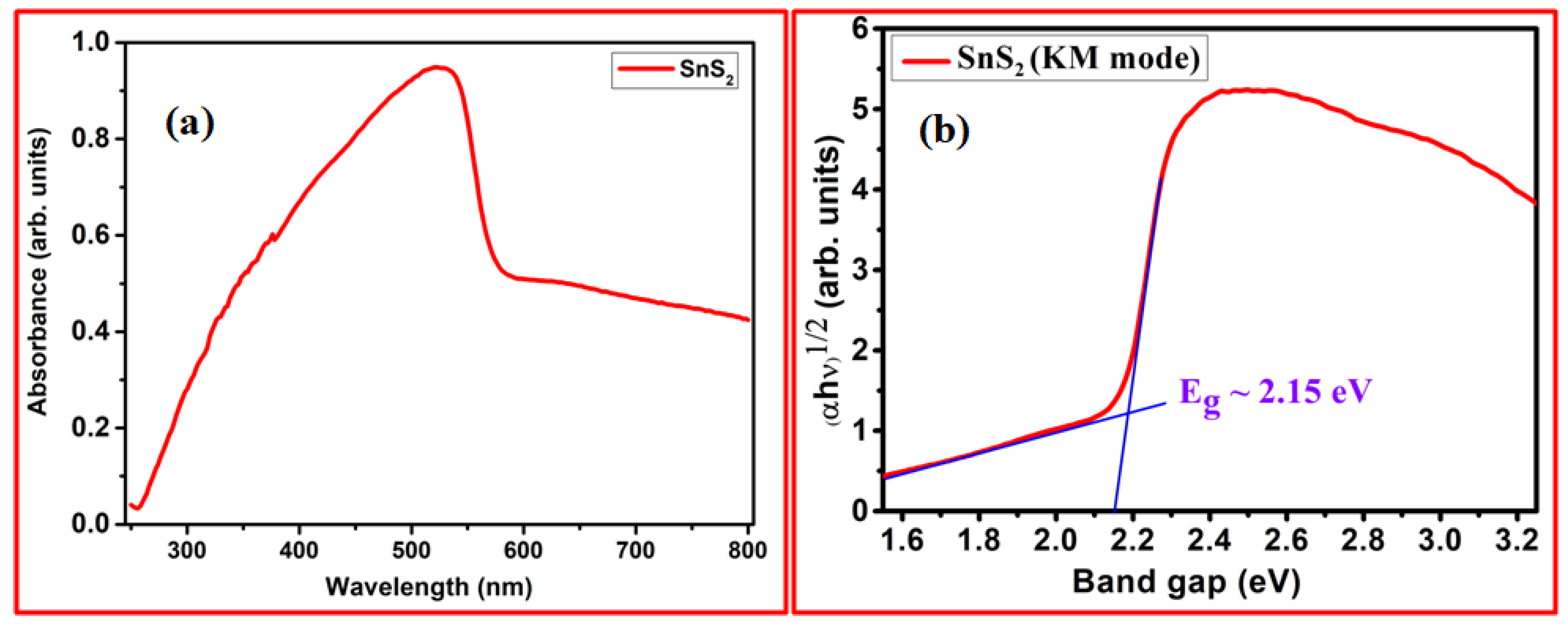
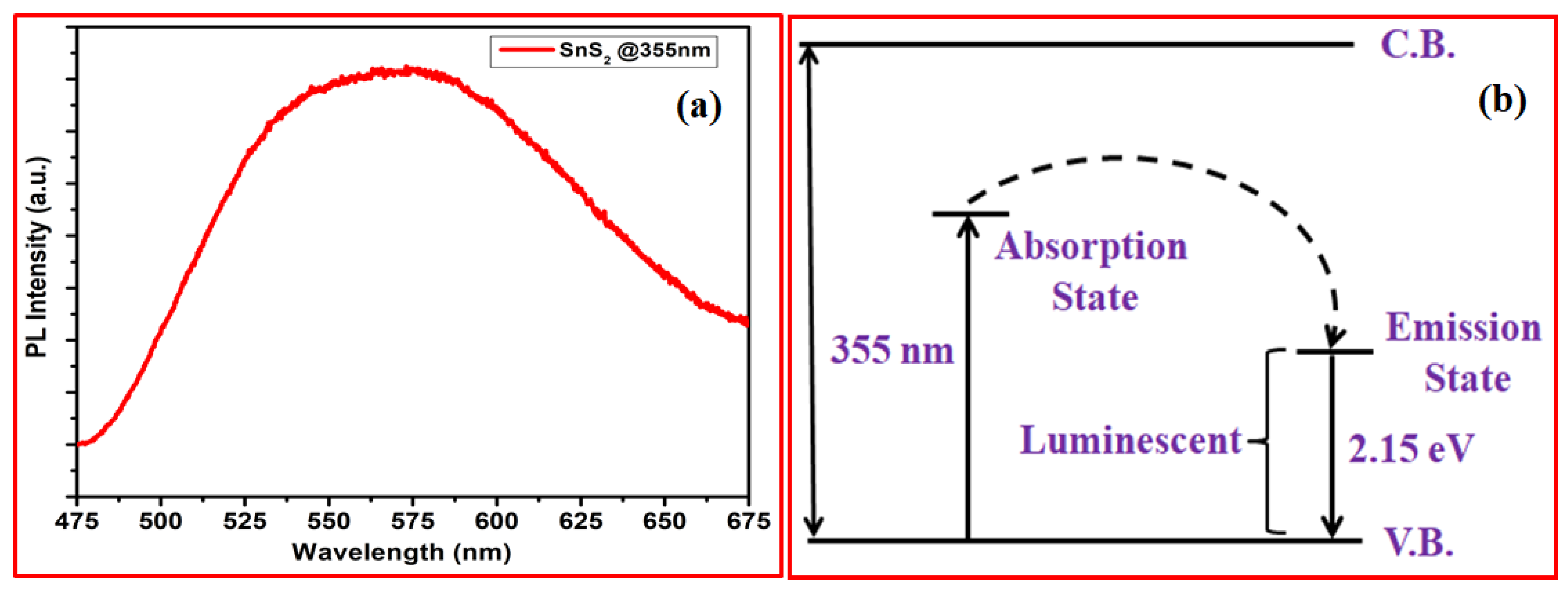
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Abderrahmane, A.; Ko, P.J.; Thu, T.V.; Ishizawa, S.; Takamura, T.; Sandhu, A. High photosensitivity few-layered MoSe2 back-gated field-effect phototransistors. Nanotechnology 2014, 25, 365202. [Google Scholar] [CrossRef] [PubMed]
- Chuang, H.J.; Chamlagain, B.; Koehler, M.; Perera, M.M.; Yan, J.; Mandrus, D.; Tománek, D.; Zhou, Z. Low-resistance 2D/2D ohmic contacts: A universal approach to high-performance WSe2, MoS2, and MoSe2 transistors. Nano Lett. 2016, 16, 1896–1902. [Google Scholar] [CrossRef] [PubMed]
- Di Bartolomeo, A.; Pelella, A.; Urban, F.; Grillo, A.; Iemmo, L.; Passacantando, M.; Liu, X.; Giubileo, F. Field Emission in Ultrathin PdSe 2 Back-Gated Transistors. Adv. Electron. Mater. 2020, 6, 2000094. [Google Scholar] [CrossRef]
- Di Bartolomeo, A.; Grillo, A.; Urban, F.; Iemmo, L.; Giubileo, F.; Luongo, G.; Amato, G.; Croin, L.; Sun, L.; Liang, S.-J.; et al. Asymmetric Schottky Contacts in Bilayer MoS2 Field Effect Transistors. Adv. Funct. Mater. 2018, 28, 1800657. [Google Scholar] [CrossRef]
- Yu, S.H.; Lee, Y.B.; Jang, S.K.; Kang, J.; Jeon, J.; Lee, C.G.; Lee, J.Y.; Kim, H.; Hwang, E.; Lee, S.; et al. Dye-Sensitized MoS2 Photodetector with Enhanced Spectral Photoresponse. ACS Nano 2014, 8, 8285–8291. [Google Scholar] [CrossRef]
- Godde, T.; Schmidt, D.; Schmutzler, J.; Aßmann, M.; Debus, J.; Withers, F.; Alexeev, E.M.; Del Pozo-Zamudio, O.; Skrypka, O.V.; Novoselov, K.S.; et al. Exciton and trion dynamics in atomically thinMoSe2andWSe2: Effect of localization. Phys. Rev. B 2016, 94, 165301. [Google Scholar] [CrossRef]
- Guo, J.; Yang, B.; Zheng, Z.; Jiang, J. Observation of abnormal mobility enhancement in multilayer MoS2 transistor by synergy of ultraviolet illumination and ozone plasma treatment. Phys. E Low-Dimens. Syst. Nanostructures 2017, 87, 150–154. [Google Scholar] [CrossRef]
- Huang, Y.; Deng, H.-X.; Xu, K.; Wang, Z.-X.; Wang, Q.-S.; Wang, F.-M.; Zhan, X.-Y.; Li, S.-S.; Luo, J.-W.; He, J. Highly sensitive and fast phototransistor based on large size CVD-grown SnS2nanosheets. Nanoscale 2015, 7, 14093–14099. [Google Scholar] [CrossRef]
- Pelella, A.; Grillo, A.; Urban, F.; Giubileo, F.; Passacantando, M.; Pollmann, E.; Sleziona, S.; Schleberger, M.; Di Bartolomeo, A. Gate-Controlled Field Emission Current from MoS2 Nanosheets. Adv. Electron. Mater. 2021, 7, 2000838. [Google Scholar] [CrossRef]
- Shimakawa, K. Electrical Transport Properties. Mater. Energy 2021, 177–202. [Google Scholar] [CrossRef]
- Urban, F.; Gity, F.; Hurley, P.K.; McEvoy, N.; Di Bartolomeo, A. Isotropic conduction and negative photoconduction in ultrathin PtSe2 films. Appl. Phys. Lett. 2020, 117, 193102. [Google Scholar] [CrossRef]
- Urban, F.; Passacantando, M.; Giubileo, F.; Iemmo, L.; Di Bartolomeo, A. Transport and Field Emission Properties of MoS2 Bilayers. Nanomaterials 2018, 8, 151. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Shang, J.; Wang, J.; Shen, X.; Cao, B.; Peimyoo, N.; Zou, C.; Chen, Y.; Wang, Y.; Cong, C.; et al. Electrically Tunable Valley-Light Emitting Diode (vLED) Based on CVD-Grown Monolayer WS2. Nano Lett. 2016, 16, 1560–1567. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Zhang, Q.; Gan, L.; Li, H.; Zhai, T. Large-Size Growth of Ultrathin SnS2 Nanosheets and High Performance for Pho-totransistors. Adv. Funct. Mater. 2016, 26, 4405–4413. [Google Scholar] [CrossRef]
- Lee, C.; Yan, H.; Brus, L.E.; Heinz, T.F.; Hone, J.; Ryu, S. Anomalous Lattice Vibrations of Single- and Few-Layer MoS2. ACS Nano 2010, 4, 2695–2700. [Google Scholar] [CrossRef]
- Mak, K.F.; Lee, C.; Hone, J.; Shan, J.; Heinz, T.F. Atomically thin MoS2: A new direct-gap semiconductor. Phys. Rev. Lett. 2010, 105, 2–5. [Google Scholar] [CrossRef]
- Ramasubramaniam, A. Large excitonic effects in monolayers of molybdenum and tungsten dichalcogenides. Phys. Rev. B 2012, 86, 115409. [Google Scholar] [CrossRef]
- Chernikov, A.; Berkelbach, T.C.; Hill, H.M.; Rigosi, A.; Li, Y.; Aslan, O.B.; Reichman, D.R.; Hybertsen, M.S.; Heinz, T.F. Exciton binding energy and nonhydrogenic Rydberg series in monolayer WS2. Phys. Rev. Lett. 2014, 113, 1–5. [Google Scholar] [CrossRef]
- Radisavljevic, B.; Radenovic, A.; Brivio, J.; Giacometti, V.; Kis, A. Single-layer MoS2 transistors. Nat. Nanotechnol. 2011, 6, 147–150. [Google Scholar] [CrossRef]
- Sanne, A.; Ghosh, R.; Rai, A.; Yogeesh, M.N.; Shin, S.H.; Sharma, A.; Jarvis, K.; Mathew, L.; Rao, R.; Akinwande, D.; et al. Radio Frequency Transistors and Circuits Based on CVD MoS2. Nano Lett. 2015, 15, 5039–5045. [Google Scholar] [CrossRef]
- Fan, C.; Li, Y.; Lu, F.; Deng, H.-X.; Wei, Z.; Li, J. Wavelength dependent UV-Vis photodetectors from SnS2 flakes. RSC Adv. 2016, 6, 422–427. [Google Scholar] [CrossRef]
- Furchi, M.M.; Pospischil, A.; Libisch, F.; Burgdörfer, J.; Mueller, T. Photovoltaic Effect in an Electrically Tunable van der Waals Heterojunction. Nano Lett. 2014, 14, 4785–4791. [Google Scholar] [CrossRef] [PubMed]
- Deng, L.; Zhu, Z.; Liu, L.; Liu, H. Synthesis of Ag2O and Ag co-modified flower-like SnS2 composites with enhanced photocatalytic activity under solar light irradiation. Solid State Sci. 2017, 63, 76–83. [Google Scholar] [CrossRef]
- Hu, Y.; Chen, T.; Wang, X.; Ma, L.; Chen, R.; Zhu, H.; Yuan, X.; Yan, C.; Zhu, G.; Lv, H.; et al. Controlled growth and photo-conductive properties of hexagonal SnS2 nanoflakes with mesa-shaped atomic steps. Nano Res. 2017, 10, 1434–1447. [Google Scholar] [CrossRef]
- Jia, X.; Tang, C.; Pan, R.; Long, Y.-Z.; Gu, C.; Li, J. Thickness-Dependently Enhanced Photodetection Performance of Vertically Grown SnS2 Nanoflakes with Large Size and High Production. ACS Appl. Mater. Interfaces 2018, 10, 18073–18081. [Google Scholar] [CrossRef]
- Yang, Y.B.; Dash, J.K.; Xiang, Y.; Wang, Y.; Shi, J.; Dinolfo, P.H.; Lu, T.-M.; Wang, G.-C. Tuning the Phase and Optical Properties of Ultrathin SnSx Films. J. Phys. Chem. C 2016, 120, 13199–13214. [Google Scholar] [CrossRef]
- Gong, Y.; Yuan, H.; Wu, C.-L.; Tang, P.; Yang, S.-Z.; Yang, A.; Li, G.; Liu, B.; van de Groep, J.; Brongersma, M.L.; et al. Spatially controlled doping of two-dimensional SnS2 through intercalation for electronics. Nat. Nanotechnol. 2018, 13, 294–299. [Google Scholar] [CrossRef]
- Manzeli, S.; Ovchinnikov, D.; Pasquier, D.; Yazyev, O.V.; Kis, A. 2D transition metal dichalcogenides. Nat. Rev. Mater. 2017, 2, 17033. [Google Scholar] [CrossRef]
- Zhou, X.; Hu, X.; Zhou, S.; Song, H.; Zhang, Q.; Pi, L.; Li, L.; Li, H.; Lü, J.; Zhai, T. Tunneling Diode Based on WSe2 /SnS2 Het-erostructure Incorporating High Detectivity and Responsivity. Adv. Mater. 2018, 30, 1703286. [Google Scholar] [CrossRef]
- Fu, X.; Ilanchezhiyan, P.; Kumar, G.M.; Cho, H.D.; Zhang, L.; Chan, A.S.; Lee, D.J.; Panin, G.N.; Kang, T.W. Tunable UV-visible absorption of SnS2layered quantum dots produced by liquid phase exfoliation. Nanoscale 2017, 9, 1820–1826. [Google Scholar] [CrossRef]
- Xia, J.; Zhu, D.; Wang, L.; Huang, B.; Huang, X.; Meng, X.-M. Large-Scale Growth of Two-Dimensional SnS2Crystals Driven by Screw Dislocations and Application to Photodetectors. Adv. Funct. Mater. 2015, 25, 4255–4261. [Google Scholar] [CrossRef]
- Yang, T.; Zheng, B.; Wang, Z.; Xu, T.; Pan, C.; Zou, J.; Zhang, X.; Qi, Z.; Liu, H.; Feng, Y.; et al. Van der Waals epitaxial growth and optoelectronics of large-scale WSe2/SnS2 vertical bilayer p–n junctions. Nat. Commun. 2017, 8, 1906. [Google Scholar] [CrossRef] [PubMed]
- Ying, H.; Li, X.; Wu, Y.; Yao, Y.; Xi, J.; Su, W.; Jin, C.; Xu, M.; He, Z.; Zhang, Q. High-performance ultra-violet phototransistors based on CVT-grown high quality SnS2flakes. Nanoscale Adv. 2019, 1, 3973–3979. [Google Scholar] [CrossRef] [PubMed]
- Giri, B.; Masroor, M.; Yan, T.; Kushnir, K.; Carl, A.D.; Doiron, C.; Zhang, H.; Zhao, Y.; McClelland, A.; Tompsett, G.A.; et al. Balancing Light Absorption and Charge Transport in Vertical SnS 2 Nanoflake Photoanodes with Stepped Layers and Large Intrinsic Mobility. Adv. Energy Mater. 2019, 9, 1901236. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhu, P.; Huang, L.; Xie, J.; Zhang, S.; Cao, G.; Zhao, X. Few-Layered SnS2on Few-Layered Reduced Graphene Oxide as Na-Ion Battery Anode with Ultralong Cycle Life and Superior Rate Capability. Adv. Funct. Mater. 2014, 25, 481–489. [Google Scholar] [CrossRef]
- Zhang, Z.; Huang, J.; Zhang, M.; Yuan, Q.; Dong, B. Ultrathin hexagonal SnS2 nanosheets coupled with g-C3N4 nanosheets as 2D/2D heterojunction photocatalysts toward high photocatalytic activity. Appl. Catal. B: Environ. 2015, 163, 298–305. [Google Scholar] [CrossRef]
- Wang, B.; Zhong, S.P.; Bin Zhang, Z.; Zheng, Z.Q.; Zhang, Y.P.; Zhang, H. Broadband photodetectors based on 2D group IVA metal chalcogenides semiconductors. Appl. Mater. Today 2019, 15, 115–138. [Google Scholar] [CrossRef]
- Zhu, H.L.; Cheng, J.; Zhang, D.; Liang, C.; Reckmeier, C.J.; Huang, H.; Rogach, A.L.; Choy, W.C.H. Room-Temperature Solu-tion-Processed NiOx:PbI2 Nanocomposite Structures for Realizing High-Performance Perovskite Photodetectors. ACS Nano 2016, 10, 6808–6815. [Google Scholar] [CrossRef]
- Song, H.S.; Li, S.L.; Gao, L.; Xu, Y.; Ueno, K.; Tang, J.; Cheng, Y.B.; Tsukagoshi, K. High-performance top-gated monolayer SnS2 field-effect transistors and their integrated logic circuits. Nanoscale 2013, 5, 9666–9670. [Google Scholar] [CrossRef]
- Su, G.; Hadjiev, V.G.; Loya, P.E.; Zhang, J.; Lei, S.; Maharjan, S.; Dong, P.; Ajayan, P.M.; Lou, J.; Peng, H. Chemical Vapor Deposition of Thin Crystals of Layered Semiconductor SnS2 for Fast Photodetection Application. Nano Lett. 2015, 15, 506–513. [Google Scholar] [CrossRef]
- Wen, S.; Pan, H.; Zheng, Y. Electronic properties of tin dichalcogenide monolayers and effects of hydrogenation and tension. J. Mater. Chem. C 2015, 3, 3714–3721. [Google Scholar] [CrossRef]
- Zschieschang, U.; Holzmann, T.; Kuhn, A.; Aghamohammadi, M.; Lotsch, B.V.; Klauk, H. Threshold-voltage control and enhancement-mode characteristics in multilayer tin disulfide field-effect transistors by gate-oxide passivation with an al-kylphosphonic acid self-assembled monolayer. J. Appl. Phys. 2015, 117, 104509. [Google Scholar] [CrossRef]
- Fu, Y.; Gou, G.; Wang, X.; Chen, Y.; Wan, Q.; Sun, J.; Xiao, S.; Huang, H.; Yang, J.; Dai, G. High-performance photodetectors based on CVD-grown high-quality SnS2 nanosheets. Appl. Phys. A 2017, 123, 1–8. [Google Scholar] [CrossRef]
- Wang, S.; Yu, H.; Zhang, H.; Wang, A.; Zhao, M.; Chen, Y.; Mei, L.; Wang, J. Broadband Few-Layer MoS2 Saturable Absorbers. Adv. Mater. 2014, 26, 3538–3544. [Google Scholar] [CrossRef]
- Liu, W.; Liu, M.; Wang, X.; Shen, T.; Chang, G.; Lei, M.; Deng, H.-X.; Wei, Z.; Wei, Z.-Y. Thickness-Dependent Ultrafast Photonics of SnS2 Nanolayers for Optimizing Fiber Lasers. ACS Appl. Nano Mater. 2019, 2, 2697–2705. [Google Scholar] [CrossRef]
- Vyas, S.M.; Desai, C.F.; Desai, C.F.; Shah, R.C.; Pandya, G.R. Microhardness Creep in Single Crystals of Tin-chalcogenides. Turk. J. Phys. 2000, 24, 21–27. [Google Scholar]
- Kana, A.; Hibbert, T.; Mahon, M.; Molloy, K.; Parkin, I.; Price, L. Organotin unsymmetric dithiocarbamates: Synthesis, formation and characterisation of tin(II) sulfide films by atmospheric pressure chemical vapour deposition. Polyhedron 2001, 20, 2989–2995. [Google Scholar] [CrossRef]
- Nair, P.; Nair, M.; García, V.; Arenas, O.; Peña, A.C.Y.; Ayala, I.; Gomezdaza, O.; Sánchez, A.; Campos, J.; Hu, H.; et al. Semiconductor thin films by chemical bath deposition for solar energy related applications. Sol. Energy Mater. Sol. Cells 1998, 52, 313–344. [Google Scholar] [CrossRef]
- Kim, J.Y.; George, S.M. Tin Monosulfide Thin Films Grown by Atomic Layer Deposition Using Tin 2,4-Pentanedionate and Hydrogen Sulfide. J. Phys. Chem. C 2010, 114, 17597–17603. [Google Scholar] [CrossRef]
- Ghazali, A.; Zainal, Z.; Hussein, M.Z.; Kassim, A. Cathodic electrodeposition of SnS in the presence of EDTA in aqueous media. Sol. Energy Mater. Sol. Cells 1998, 55, 237–249. [Google Scholar] [CrossRef]
- Sugiyama, M.; Miyauchi, K.; Minemura, T.; Ohtsuka, K.; Noguchi, K.; Nakanishi, H. Preparation of SnS Films by Sulfurization of Sn Sheet. Jpn. J. Appl. Phys. 2008, 47, 4494–4495. [Google Scholar] [CrossRef]
- Xu, Z.; Chen, Y. Fabrication of SnS thin films by a novel multilayer-based solid-state reaction method. Semicond. Sci. Technol. 2012, 27, 35007. [Google Scholar] [CrossRef]
- Ghosh, B.; Das, M.; Banerjee, P.; Das, S. Fabrication and optical properties of SnS thin films by SILAR method. Appl. Surf. Sci. 2008, 254, 6436–6440. [Google Scholar] [CrossRef]
- Schäfer, H.; Jacob, H.; Etzel, K.; Chemische Transportreaktionen. II. Die Verwendung der Zerfallsgleichgewichte der Eisen(II)- und Nickel(II)-halogenide zum Metalltransport im Temperaturgefälle, Z. Anorg. Allg. Chem. 1956, 286, 42–55. [Google Scholar] [CrossRef]
- Nitsche, R.; Bölsterli, H.; Lichtensteiger, M. Crystal growth by chemical transport reactions—I. J. Phys. Chem. Solids 1961, 21, 199–205. [Google Scholar] [CrossRef]
- Colombara, D.; Delsante, S.; Borzone, G.; Mitchels, J.; Molloy, K.; Thomas, L.; Mendis, B.; Cummings, C.; Marken, F.; Peter, L. Crystal growth of Cu2ZnSnS4 solar cell absorber by chemical vapor transport with I2. J. Cryst. Growth 2012, 364, 101–110. [Google Scholar] [CrossRef]
- Wang, Y.; Huang, L.; Wei, Z. Photoresponsive field-effect transistors based on multilayer SnS2 nanosheets. J. Semicond. 2017, 38, 034001. [Google Scholar] [CrossRef]
- Voznyi, A.; Kosyak, V.; Opanasyuk, A.; Tirkusova, N.; Grase, L.; Medvids, A.; Mezinskis, G. Structural and electrical properties of SnS2 thin films. Mater. Chem. Phys. 2016, 173, 52–61. [Google Scholar] [CrossRef]
- Sriv, T.; Kim, K.; Cheong, H. Low-Frequency Raman Spectroscopy of Few-Layer 2H-SnS2. Sci. Rep. 2018, 8, 10194. [Google Scholar] [CrossRef]
- Gedi, S.; Alhammadi, S.; Noh, J.; Reddy, V.R.M.; Park, H.; Rabie, A.M.; Shim, J.-J.; Kang, D.; Kim, W.K. SnS2 Nanoparticles and Thin Film for Application as an Adsorbent and Photovoltaic Buffer. Nanomaterials 2022, 12, 282. [Google Scholar] [CrossRef]
- Crist, B.V. Handbook of Monochromatic XPS Spectra; Wiley: Chichester, UK; New York, NY, USA, 2000. [Google Scholar]
- Burton, L.A.; Colombara, D.; Abellon, R.D.; Grozema, F.C.; Peter, L.M.; Savenije, T.J.; Dennler, G.; Walsh, A. Synthesis, Characterization, and Electronic Structure of Single-Crystal SnS, Sn2S3, and SnS2. Chem. Mater. 2013, 25, 4908–4916. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tripathi, P.; Kumar, A.; Bankar, P.K.; Singh, K.; Gupta, B.K. Large-Scale Production and Optical Properties of a High-Quality SnS2 Single Crystal Grown Using the Chemical Vapor Transportation Method. Crystals 2023, 13, 1131. https://doi.org/10.3390/cryst13071131
Tripathi P, Kumar A, Bankar PK, Singh K, Gupta BK. Large-Scale Production and Optical Properties of a High-Quality SnS2 Single Crystal Grown Using the Chemical Vapor Transportation Method. Crystals. 2023; 13(7):1131. https://doi.org/10.3390/cryst13071131
Chicago/Turabian StyleTripathi, Prashant, Arun Kumar, Prashant K. Bankar, Kedar Singh, and Bipin Kumar Gupta. 2023. "Large-Scale Production and Optical Properties of a High-Quality SnS2 Single Crystal Grown Using the Chemical Vapor Transportation Method" Crystals 13, no. 7: 1131. https://doi.org/10.3390/cryst13071131
APA StyleTripathi, P., Kumar, A., Bankar, P. K., Singh, K., & Gupta, B. K. (2023). Large-Scale Production and Optical Properties of a High-Quality SnS2 Single Crystal Grown Using the Chemical Vapor Transportation Method. Crystals, 13(7), 1131. https://doi.org/10.3390/cryst13071131






