Alkali-Induced Phase Transition to β-Spodumene along the LiAlSi2O6-LiAlSi4O10 Join
Abstract
:1. Introduction
2. Materials and Methods
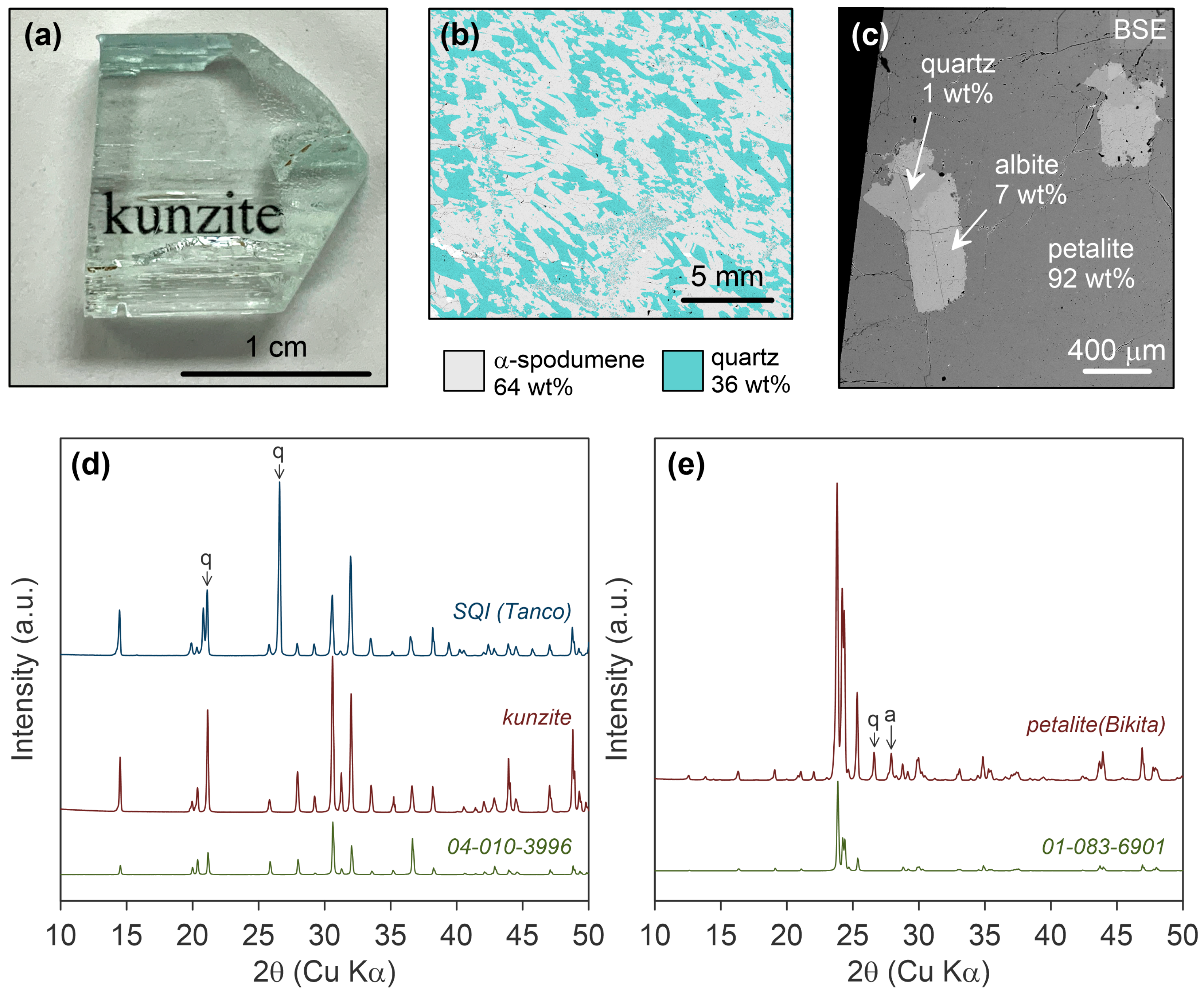
2.1. Materials: Natural Lithium Aluminosilicates
2.2. Analytical Techniques
2.2.1. X-ray Diffraction
2.2.2. Electron Backscatter Diffraction
2.2.3. Automated Mineralogy
2.2.4. Electron Probe X-ray Microanalysis
2.2.5. Laser-Ablation—Inductively Coupled Plasma—Mass Spectrometry (LA-ICP-MS)
2.2.6. Raman Spectroscopy
2.3. Experimental Methods
2.3.1. Heat Treatment
2.3.2. Synthetic Growth of Li-Stuffed Silica Derivatives
2.3.3. Alkali Exchange Experiment
3. Results and Discussion
3.1. Phase Transitions during Heat-Treatment of Natural Lithium Aluminosilicates
3.1.1. α-Spodumene
3.1.2. Petalite
3.2. Synthetic β-Phases along the LiAlSi2O6-LiAlSi4O10 Join
3.2.1. Synthetic Growth
3.2.2. Alkali Exchange
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, C.-T.; Peacor, D.R. The crystal structure of LiAlSi2O6 II (“β-spodumene”). Z. Kristallogr. 1968, 126, 46–65. [Google Scholar] [CrossRef]
- Beall, G.H. Chapter 14: Industrial Applications of Silica. In Reviews in Mineralogy, Silica: Physical Behaviour, Geochemistry, and Materials Applications; Heaney, P.J., Prewitt, C.T., Gibbs, G.V., Eds.; Mineralogical Society of America; De Gruyter: Boston, MA, USA; Berlin, Germany, 1994; Volume 29, pp. 469–505. [Google Scholar] [CrossRef]
- Müller, G.; Hoffmann, M.; Neeff, R. Hydrogen substitution in lithium aluminosilicates. J. Mater. Sci. 1988, 23, 1779–1785. [Google Scholar] [CrossRef]
- Yelantontsev, D.; Mukhachev, A. Processing of lithium ores: Industrial technologies and case studies—A review. Hydrometallurgy 2021, 201, 105578. [Google Scholar] [CrossRef]
- Salakjani, N.K.; Singh, P.; Nikoloski, N. Production of lithium—A literature review part 2: Extraction from spodumene. Min. Proc. Ext. Met. Rev. 2021, 42, 268–283. [Google Scholar] [CrossRef]
- Alhadad, M.F.; Oskierski, H.S.; Ghischi, J.; Senanayake, G.; Dlugogorski, B.Z. Lithium extraction from β-spodumene: A comparison of keatite and analcime process. Hydrometallurgy 2023, 215, 105985. [Google Scholar] [CrossRef]
- Chen, Y.; Tian, Q.; Chen, B.; Shi, X.; Liao, T. Preparation of lithium carbonate from spodumene by a sodium carbonate autoclave process. Hydrometallurgy 2011, 109, 43–46. [Google Scholar] [CrossRef]
- Buerger, M.J. The stuffed derivatives of the silica structures. Am. Mineral. 1954, 39, 600–614. [Google Scholar]
- Strnad, Z. Glass-Ceramic Materials, Glass Science and Technology, Vol. 8; Elsevier: Amsterdam, The Netherlands, 1986; 268p, ISBN 9780444415776. [Google Scholar]
- Nordmann, A.; Cheng, Y.-B. Crystallization behaviour and microstructural evolution of a Li2O-Al2O3-SiO2 glass derived from spodumene mineral. J. Mater. Sci. 1997, 32, 83–89. [Google Scholar] [CrossRef]
- Xu, H.; Heaney, P.J.; Navrotsky, A. Thermal expansion and structural transformations of stuffed derivatives of quartz along the LiAlSiO4-SiO2 join: A variable-temperature powder synchrotron XRD study. Phys. Chem. Miner. 2001, 28, 302–312. [Google Scholar] [CrossRef]
- Roy, R.; Roy, D.M.; Osborn, E.F. Compositional and stability relationships among the lithium aluminosilicates: Eucryptite, spodumene, and petalite. J. Am. Ceram. Soc. 1950, 33, 152–159. [Google Scholar] [CrossRef]
- Munoz, J.L. Stability Relations of LiAlSi2O6 at High Pressure. In Mineralogical Society of America Special Paper 2, Pyroxenes and Amphiboles: Crystal Chemistry and Phase Petrology; Papike, J.J., Ed.; Mineralogical Society of America: Menasha, WI, USA, 1969; pp. 203–209. [Google Scholar]
- London, D.; Burt, D.M. Chemical models for lithium aluminosilicates stabilities in pegmatites and granites. Am. Mineral. 1982, 67, 494–509. [Google Scholar]
- London, D. Magmatic-hydrothermal transition in the Tanco rare-element pegmatite: Evidence from fluid inclusions and phase equilibrium experiments. Am. Mineral. 1986, 71, 376–395. [Google Scholar]
- Edgar, A.D. The α-β-LiAlSi2O6 (spodumene) transition from 5000 to 45000 lb/in2 PH2O. In Proceedings of the Papers and Proceedings of the International Mineralogical Association, 5th General Meeting, Cambridge, England, 30 August–3 September 1966; Mineralogical Society of London: London, UK, 1968; pp. 222–231. [Google Scholar]
- Shannon, R.D. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Crystallogr. 1976, A32, 751–767. [Google Scholar] [CrossRef]
- Dowty, E. Crystal-chemical factors affecting the mobility of ions in minerals. Am. Mineral. 1980, 65, 174–182. [Google Scholar]
- Fortier, S.M.; Giletti, B.J. An empirical model for predicting diffusion coefficients in silicate minerals. Science 1989, 245, 1481–1484. [Google Scholar] [CrossRef]
- Zhao, Z.-F.; Zheng, Y.-F. Diffusion compensation for argon, hydrogen, lead, and strontium in minerals: Empirical relationships to crystal chemistry. Am. Mineral. 2007, 92, 289–308. [Google Scholar] [CrossRef]
- Welsh, A.-M.; Murawski, D.; Prekajski, M.; Vulic, P.; Kremenovic, A. Ionic conductivity in single-crystal LiAlSi2O6: Influence of structure on lithium mobility. Phys. Chem. Miner. 2015, 42, 413–420. [Google Scholar] [CrossRef]
- Li, C.-T. The crystal structure of LiAlSi2O6 III (high quartz solid solution). Z. Kristallogr. 1968, 127, 327–348. [Google Scholar] [CrossRef]
- Xu, H.; Heaney, P.J.; Navrotsky, A.; Topor, L.; Liu, J. Thermochemistry of stuffed quartz-derivative phases along the join LiAlSiO4-SiO2. Am. Mineral. 1999, 84, 1360–1369. [Google Scholar] [CrossRef]
- Welsh, A.-M.; Behrens, H.; Ross, S.; Murawski, D. Structural control of ionic conductivity in LiAlSi2O6 and LiAlSi4O10 glasses and single crystals. Z. Phys. Chem. A. 2012, 226, 491–511. [Google Scholar] [CrossRef]
- Ross, N.L.; Zhao, J.; Slebodnick, C.; Spencer, E.C.; Chakoumakos, B.C. Petalite under pressure: Elastic behavior and phase stability. Am. Mineral. 2015, 100, 714–721. [Google Scholar] [CrossRef]
- Peltosaari, O.; Tanskanen, P.; Heikkinen, E.-P.; Fabritius, T. α → γ → β-phase transformation of spodumene with hybrid microwave and conventional furnaces. Miner. Eng. 2015, 82, 54–60. [Google Scholar] [CrossRef]
- Salakjani, N.K.; Singh, P.; Nikoloski, N. Mineralogical transformations of spodumene concentrate from Greenbushes, Western Australia. Part 1: Conventional heating. Miner. Eng. 2016, 98, 71–79. [Google Scholar] [CrossRef]
- Salakjani, N.K.; Nikoloski, N.; Singh, P. Mineralogical transformations of spodumene concentrate from Greenbushes, Western Australia. Part 2: Microwave heating. Miner. Eng. 2017, 100, 191–199. [Google Scholar] [CrossRef]
- Moore, R.H.; Mann, J.P.; Montoya, A.; Haynes, B.S. In situ synchrotron XRD analysis of the kinetics of spodumene transitions. Phys. Chem. Chem. Phys. 2018, 20, 10753–10761. [Google Scholar] [CrossRef] [PubMed]
- Abdullah, A.A.; Oskierski, H.C.; Altarawneh, M.; Senanayake, G.; Lumpkin, G.; Dlugogorski, B.Z. Phase transformation mechanism of spodumene during its calcination. Miner. Eng. 2019, 140, 105883. [Google Scholar] [CrossRef]
- Dessemond, C.; Soucy, G.; Harveu, J.-F.; Ouzilleau, P. Phase transitions in the α-γ-β spodumene thermodynamic system and impact of γ-spodumene on the efficiency of lithium extraction by acid leaching. Minerals 2020, 10, 519. [Google Scholar] [CrossRef]
- Salakjani, N.K.; Singh, P.; Nikoloski, N. Production of lithium—A literature review part 1: Pretreatment of spodumene. Min. Proc. Ext. Met. Rev. 2020, 41, 335–348. [Google Scholar] [CrossRef]
- Černý, P.; Ferguson, R.B. The Tanco pegmatite Bernic Lake, Manitoba. IV. Petalite and spodumene relations. Can. Mineral. 1972, 11, 660–678. [Google Scholar]
- Lawley, C.J.M.; Petts, D.C.; Jackson, S.E.; Zagorevski, A.; Pearson, D.G.; Kjarsgaard, B.A.; Savard, D.; Tschirhart, V. Precious metal mobility during serpentinization and breakdown of base metal sulphide. Lithos 2020, 354–355, 105278. [Google Scholar] [CrossRef]
- Jackson, S.E. Calibration Strategies for Elemental Analysis by LA-ICP-MS. In Short Course Series, Laser-Ablation-ICP-MS in the Earth Sciences: Current Practices and Outstanding Issues; Sylvester, P., Ed.; Mineralogical Association of Canada: Québec, QC, Canada, 2008; Volume 40, pp. 169–188. ISBN 9780921294498. [Google Scholar]
- Guillong, M.; Hametner, K.; Reusser, E.; Wilson, S.A.; Günther, D. Preliminary characterisation of new glass reference materials (GSA-1G, GSC-1G, GSD-1G and GSE-1G) by laser ablation-inductively coupled plasma-mass spectrometry using 193 nm, 213 nm and 266 nm wavelengths. Geostand. Geoanal. Res. 2005, 29, 315–331. [Google Scholar] [CrossRef]
- Kane, J.S. A history of the development and certification of NIST glass SRMs 610–617. Geostandard Newslett. 1998, 22, 7–13. [Google Scholar] [CrossRef]
- Sharma, S.K.; Simons, B. Raman study of crystalline polymorphs and glasses of spodumene composition quenched from various pressures. Am. Mineral. 1981, 66, 118–126. [Google Scholar]
- Matson, D.W.; Sharma, S.K.; Philpotts, J.A. Raman spectra of some tectosilicates and of glasses along the orthoclase-anorthite and nepheline-anorthite joins. Am. Mineral. 1986, 71, 694–704. [Google Scholar]
- Sprengard, R.; Binder, K.; Brändle, M.; Fotheringham, U.; Sauer, J.; Pannhorst, W. On the interpretation of the experimental Raman spectrum of β-eucryptite LiAlSiO4 from atomistic computer modelling. J. Non-Cryst. Solids 2000, 274, 264–270. [Google Scholar] [CrossRef]
- Anderson, A.J.; Clark, A.H.; Gray, S. The occurrence and origin of zabuleyite (Li2CO3) in spodumene-hosted fluid inclusions: Implications for the internal evolution of rare-element granitic pegmatites. Can. Mineral. 2001, 39, 1513–1527. [Google Scholar] [CrossRef]
- Anderson, A.J. Microthermic behavior of crystal-rich inclusions in spodumene under confining pressure. Can. Mineral. 2019, 57, 853–865. [Google Scholar] [CrossRef]
- Li, J.; Chou, I.-M. Homogenization experiments of crystal-rich inclusions in spodumene from Jiajika lithium deposit, China, under elevated external pressures in a hydrothermal diamond-anvil cell. Geofluids 2017, 2017, 9252913. [Google Scholar] [CrossRef]
- Li, S.; Chou, I.-M. Refinement of the α-β quartz phase boundary based on in situ Raman spectroscopy measurements in hydrothermal diamond-anvil cell and an evaluated equation of state of pure H2O. J. Raman Spectros. 2022, 53, 1471–1482. [Google Scholar] [CrossRef]
- Toplis, M.J.; Dingwell, D.B.; Hess, K.-U.; Lenci, T. Viscosity, fragility, and configurational entropy of melts along the join SiO2-NaAlSiO4. Am. Mineral. 1997, 82, 979–990. [Google Scholar] [CrossRef]
- Berchot, J.-L.; Vivien, D.; Gourier, D.; Thery, J.; Collongues, R. Ion exchange properties of β-eucryptite (LiAlSiO4): EPR investigation on copper-doped single crystals. J. Solid State Chem. 1980, 34, 199–205. [Google Scholar] [CrossRef]
- Baumgartner, B.; Müller, G. Framework distortion by large ions in MAlSi2O6 aluminosilicates with keatite structure. Eur. J. Mineral. 1990, 2, 155–162. [Google Scholar] [CrossRef] [Green Version]












| α-Spodumene | Petalite | |||||
|---|---|---|---|---|---|---|
| Kunzite | Tanco | Bikita | ||||
| N = 40 | 1 σ | N = 40 | 1 σ | N = 40 | 1 σ | |
| Major oxides (wt%) | ||||||
| SiO2 | 64.29 | 0.33 | 64.87 | 0.40 | 78.20 | 0.44 |
| Al2O3 | 27.34 | 0.14 | 27.32 | 0.21 | 16.43 | 0.16 |
| Li2O | 7.98 | 0.04 | 7.83 | 0.12 | 4.90 | 0.02 |
| Minor oxides (ppmw) | ||||||
| Na2O | 575 | 50 | 537 | 61 | 3 | 2 |
| MnO | 183 | 38 | 214 | 118 | <1 | |
| FeO | 50 | 6 | 179 | 35 | 6 | 3 |
| Total (wt%) | 99.69 | 100.12 | 99.53 | |||
| Trace elements (ppmw) * | ||||||
| Be | 0.21 | 0.50 | 6.97 | |||
| Rb | <0.04 | 0.42 | 0.07 | |||
| Sr | <0.004 | 0.04 | 0.05 | |||
| Nb | <0.003 | 0.65 | <0.003 | |||
| Cs | <0.004 | 3.09 | 0.32 | |||
| Ta | 0.05 | 13.8 | 0.01 | |||
| Calculated mineral formulae ** | ||||||
| Si | 1.998 | 2.007 | 4.004 | |||
| Al | 1.002 | 0.996 | 0.992 | |||
| Li | 0.998 | 0.974 | 1.009 | |||
| Na | 0.003 | 0.004 | 0.000 | |||
| Mn | 0.000 | 0.001 | 0.000 | |||
| Fe | 0.000 | 0.001 | 0.000 | |||
| O | 6.000 | 6.000 | 10.000 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thibault, Y.; Gamage McEvoy, J. Alkali-Induced Phase Transition to β-Spodumene along the LiAlSi2O6-LiAlSi4O10 Join. Crystals 2023, 13, 1182. https://doi.org/10.3390/cryst13081182
Thibault Y, Gamage McEvoy J. Alkali-Induced Phase Transition to β-Spodumene along the LiAlSi2O6-LiAlSi4O10 Join. Crystals. 2023; 13(8):1182. https://doi.org/10.3390/cryst13081182
Chicago/Turabian StyleThibault, Yves, and Joanne Gamage McEvoy. 2023. "Alkali-Induced Phase Transition to β-Spodumene along the LiAlSi2O6-LiAlSi4O10 Join" Crystals 13, no. 8: 1182. https://doi.org/10.3390/cryst13081182
APA StyleThibault, Y., & Gamage McEvoy, J. (2023). Alkali-Induced Phase Transition to β-Spodumene along the LiAlSi2O6-LiAlSi4O10 Join. Crystals, 13(8), 1182. https://doi.org/10.3390/cryst13081182






