First Principle Study on Structural, Thermoelectric, and Magnetic Properties of Cubic CdCrO3 Perovskites: A Comprehensive Analysis
Abstract
1. Introduction
2. Computational Method
3. Results and Discussion
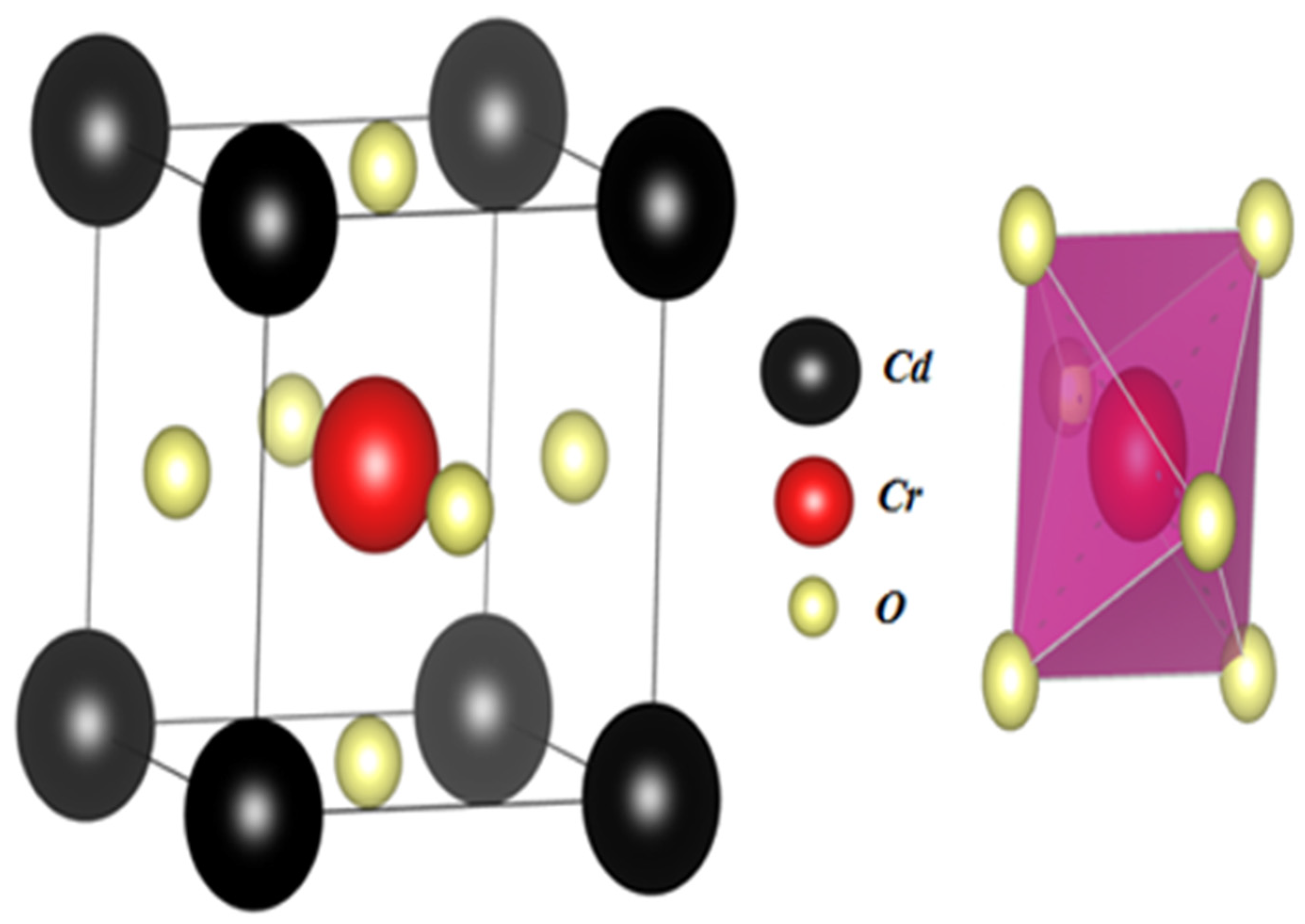
3.1. Structural Properties
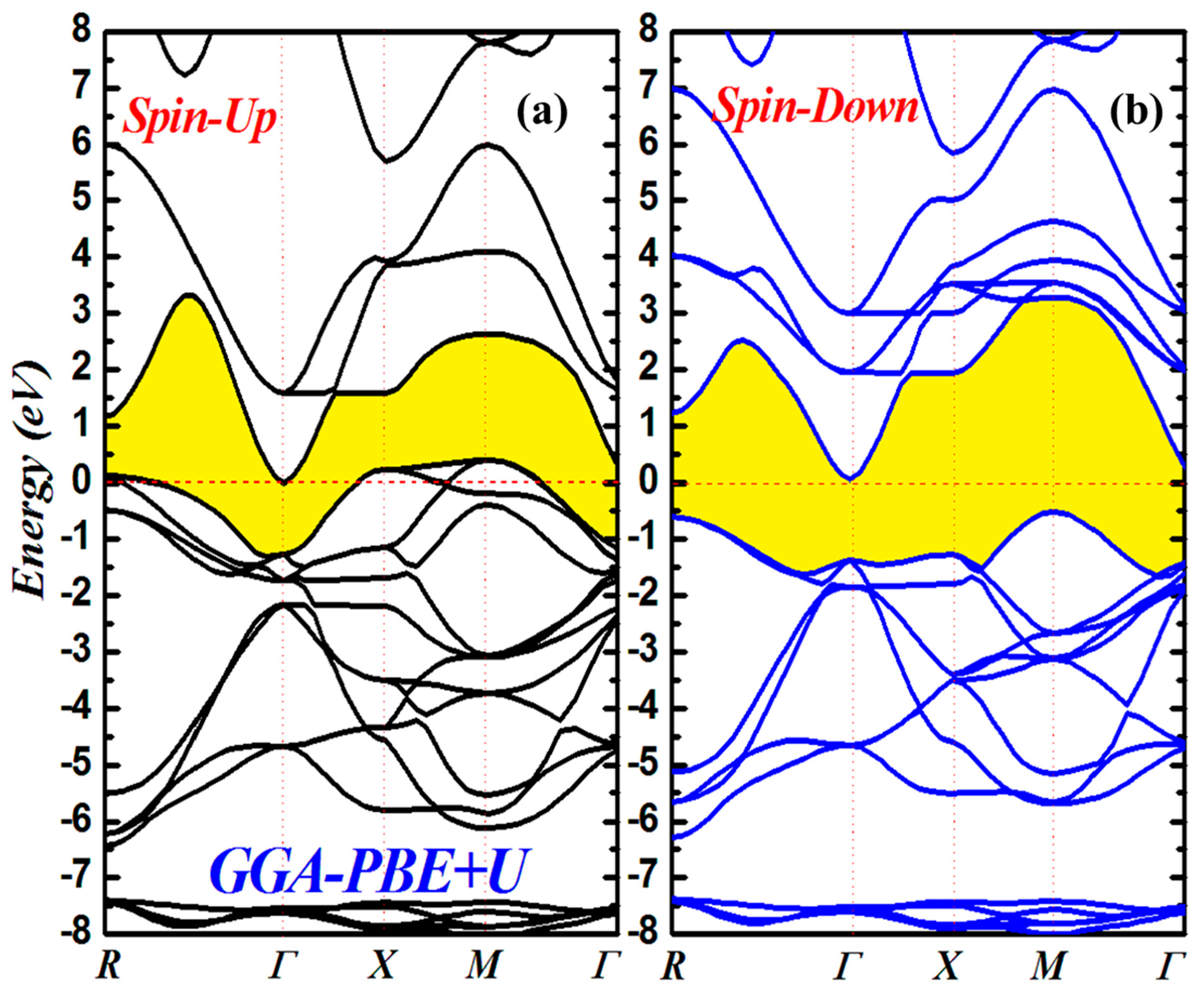
3.2. Electronic Properties
3.3. Magnetic Properties
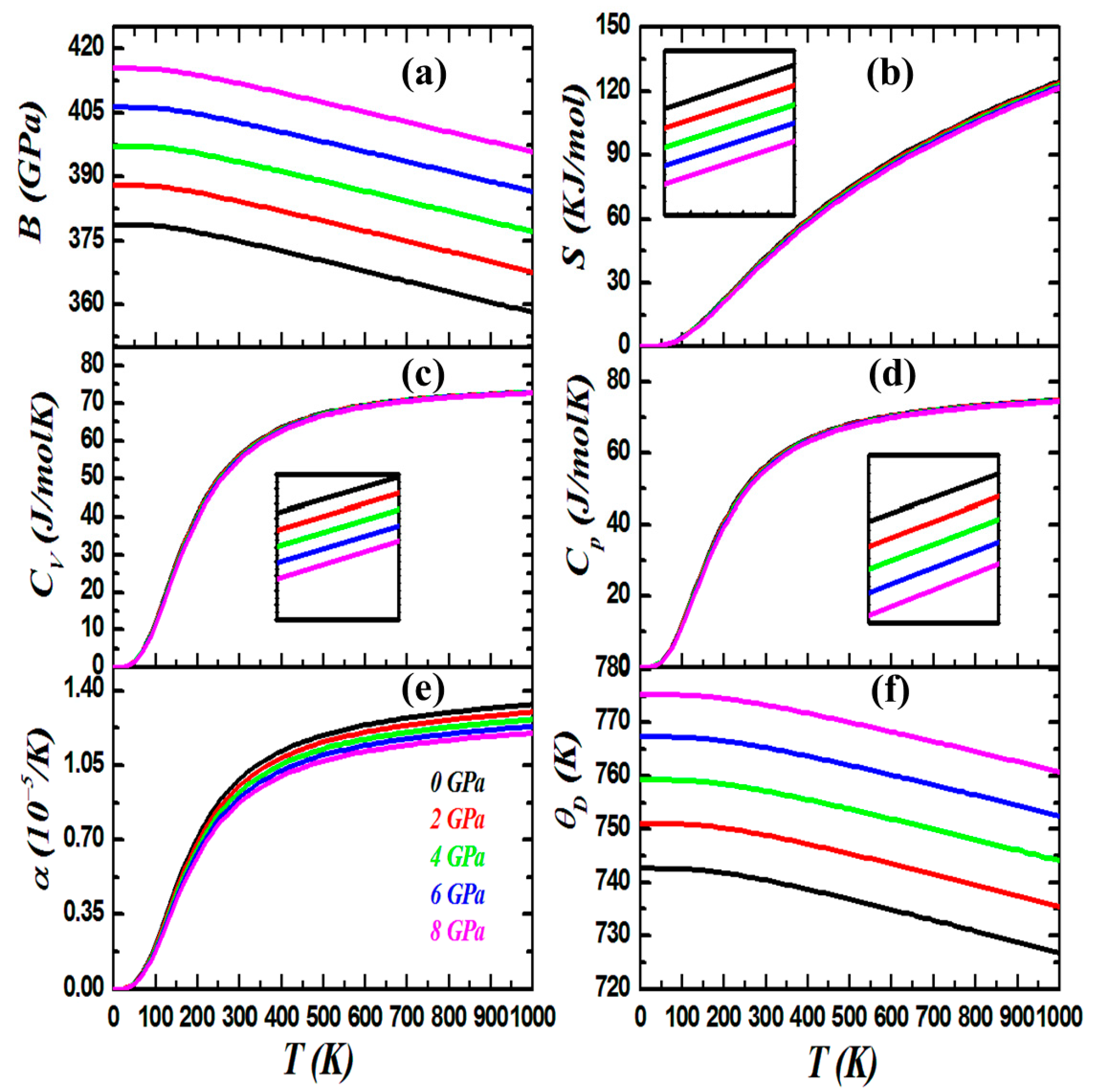
3.4. Thermodynamic Properties
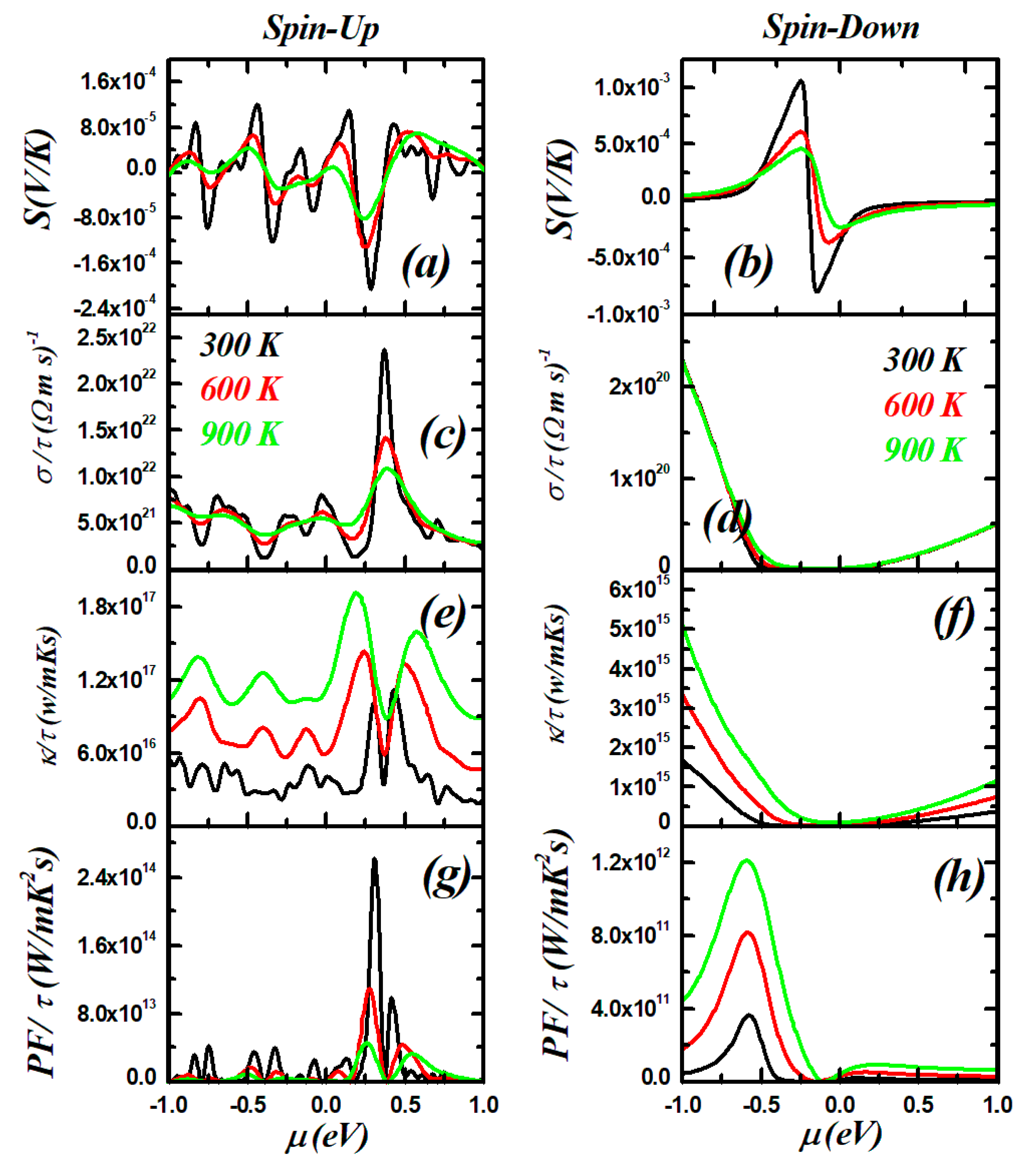
3.5. Thermoelectric Properties
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Attfield, J.P.; Lightfoot, P.; Morris, R.E. Perovskites. Dalton Trans. 2015, 44, 10541–10542. [Google Scholar] [CrossRef] [PubMed]
- Bednorz, J.G.; Müller, K.A. Perovskite-Type Oxides—The New Approach to High-T c Superconductivity. Rev. Mod. Phys. 1988, 60, 585. [Google Scholar] [CrossRef]
- Miyasaka, T.; Kulkarni, A.; Kim, G.M.; Öz, S.; Jena, A.K. Perovskite Solar Cells: Can We Go Organic-free, Lead-free, and Dopant-free? Adv. Energy Mater. 2020, 10, 1902500. [Google Scholar] [CrossRef]
- Behera, D.; Manzoor, M.; Sharma, R.; Salah, M.M.; Stich, I.; Mukherjee, S.K. A Comprehensive First-Principles Investigation of SnTiO3 Perovskite for Optoelectronic and Thermoelectric Applications. Crystals 2023, 13, 408. [Google Scholar] [CrossRef]
- Singh, P.K.; Singh, R.; Singh, V.; Bhattacharya, B.; Khan, Z.H. New Class of Lead Free Perovskite Material for Low-Cost Solar Cell Application. Mater. Res. Bull. 2018, 97, 572–577. [Google Scholar]
- Aboub, Z.; Seddik, T.; Daoudi, B.; Boukraa, A.; Behera, D.; Batouche, M.; Mukherjee, S.K. Impact of La, Ni-Doping on Structural and Electronic Properties of SrTiO3 for Photocatalytic Water Splitting. Inorg. Chem. Commun. 2023, 153, 110871. [Google Scholar] [CrossRef]
- Sakhya, A.P.; Rai, D.P.; Dutta, A.; Thapa, R.K.; Sinha, T.P. Electronic, Optical and Thermoelectric Properties of PrMO3(M=Al, Ga, In) from First-Principles Calculations. RSC Adv. 2016, 6, 59988–59997. [Google Scholar] [CrossRef]
- Vali, R. Lattice Dynamics of Cubic SrZrO3. J. Phys. Chem. Solids 2008, 69, 876–879. [Google Scholar] [CrossRef]
- Abbad, A.; Benstaali, W.; Bentounes, H.A.; Bentata, S.; Benmalem, Y. Search for Half-Metallic Ferromagnetism in Orthorhombic Ce(Fe/Cr)O3 Perovskites. Solid State Commun. 2016, 228, 36–42. [Google Scholar] [CrossRef]
- Mekam, D.; Kacimi, S.; Djermouni, M.; Azzouz, M.; Zaoui, A. Ab Initio Calculations on RE–TM–O3 Perovskites: A Comparative Study of Cation Effect. Results Phys. 2012, 2, 156–163. [Google Scholar] [CrossRef][Green Version]
- Lim, J.B.; Zhang, S.; Shrout, T.R. Modified Pb(Yb, Nb)O3–PbZrO3–PbTiO3 Ternary System for High Temperature Applications. Ceram. Int. 2012, 38, 277–282. [Google Scholar] [CrossRef]
- He, Z.; Peng, J.; Lei, C.; Xie, S.; Zou, D.; Liu, Y. Prediction of Superior Thermoelectric Performance in Unexplored Doped-BiCuSeO via Machine Learning. Mater. Des. 2023, 229, 111868. [Google Scholar] [CrossRef]
- Wang, Z.; He, Z.; Lei, C.; Zou, D.; Liu, Y. Phase Transition Enhanced Thermoelectric Performance for Perovskites: The Case of AgTaO3. Curr. Appl. Phys. 2023, 48, 84–89. [Google Scholar] [CrossRef]
- Yaseen, M.; Ambreen, H.; Iqbal, J.; Shahzad, A.; Zahid, R.; Kattan, N.A.; Ramay, S.M.; Mahmood, A. Electronic, Optical and Magnetic Properties of PrXO3(X=V, Cr): First-Principle Calculations. Philos. Mag. 2020, 100, 3125–3140. [Google Scholar] [CrossRef]
- Rashid, M.; Iqbal, M.A.; Noor, N.A. DFT-MBJ Study of Electronic and Magnetic Properties of Cubic CeCrO3 Compound: An Ab-Initio Investigation. Sci. Inq. Rev. 2017, 1, 27–36. [Google Scholar] [CrossRef]
- Gupta, P. Study of Negative Magnetization, Exchange Bias and Magnetization Switching in Rare Earth Chromites. Ph.D. Thesis, CSIR-National Chemical Laboratory, Pune, India, 2015. [Google Scholar]
- Hohenberg, P.; Kohn, W. Density Functional Theory (DFT). Phys. Rev. 1964, 136, B864. [Google Scholar] [CrossRef]
- Blaha, P.; Schwarz, K.; Sorantin, P.; Trickey, S.B. Full-Potential, Linearized Augmented Plane Wave Programs for Crystalline Systems. Comput. Phys. Commun. 1990, 59, 399–415. [Google Scholar] [CrossRef]
- Blaha, P.; Schwarz, K.; Madsen, G.K.H.; Kvasnicka, D.; Luitz, J. Wien2k. In An Augmented Plane Wave+ Local Orbitals Program for Calculating Crystal Properties; TECH Technological University: Mexico City, Mexico, 2001; Volume 60. [Google Scholar]
- Saxena, A.; Dixit, A.; Behera, D.; Abraham, J.A.; Sharma, R.; Mukherjee, S.K. Insight on Structural, Electronic and Thermoelectric Properties of Perovskite AgBaCl3 by an Ab-Initio for Solar Cell and Renewable Energy. Mater. Today Proc. 2023. [Google Scholar] [CrossRef]
- Manzoor, M.; Behera, D.; Sharma, R.; Iqbal, M.W.; Mukherjee, S.K.; Khenata, R.; Alarfaji, S.S.; Alzahrani, H.A. Investigation of the Structural, Mechanical, Optoelectronic and, Thermoelectric Characteristics of Cubic GeTiO3: An Ab Initio Study. Mater. Today Commun. 2023, 34, 105053. [Google Scholar] [CrossRef]
- Blanco, M.A.; Francisco, E.; Luana, V. GIBBS: Isothermal-Isobaric Thermodynamics of Solids from Energy Curves Using a Quasi-Harmonic Debye Model. Comput. Phys. Commun. 2004, 158, 57–72. [Google Scholar] [CrossRef]
- Madsen, G.K.H.; Singh, D.J. BoltzTraP. A Code for Calculating Band-Structure Dependent Quantities. Comput. Phys. Commun. 2006, 175, 67–71. [Google Scholar] [CrossRef]
- Jain, E.; Behera, D.; Agrawal, Y.; Yugbodh, K.; Abraham, J.A.; Sharma, R.; Mukherjee, S.K. First Principles Investigation on Half Metallic Ferromagnetism Properties of Cubic VLaO3 Compound. Mater. Today Proc. 2023. [Google Scholar] [CrossRef]
- Katsura, T.; Tange, Y. A Simple Derivation of the Birch–Murnaghan Equations of State (EOSs) and Comparison with EOSs Derived from Other Definitions of Finite Strain. Minerals 2019, 9, 745. [Google Scholar] [CrossRef]
- Fedorovskiy, A.E.; Drigo, N.A.; Nazeeruddin, M.K. The Role of Goldschmidt’s Tolerance Factor in the Formation of A2BX6 Double Halide Perovskites and Its Optimal Range. Small Methods 2020, 4, 1900426. [Google Scholar] [CrossRef]
- Behera, D.; Mukherjee, S.K. Theoretical Investigation of the Lead-Free K2InBiX6(X = Cl, Br) Double Perovskite Compounds Using First Principle Calculation. JETP Lett. 2022, 116, 537–546. [Google Scholar] [CrossRef]
- Hossain, K.M.; Hasan, M.Z.; Ali, M.L. Understanding the Influences of Mg Doping on the Physical Properties of SrMoO3 Perovskite. Results Phys. 2020, 19, 103337. [Google Scholar] [CrossRef]
- Marronnier, A.; Lee, H.; Geffroy, B.; Even, J.; Bonnassieux, Y.; Roma, G. Structural Instabilities Related to Highly Anharmonic Phonons in Halide Perovskites. J. Phys. Chem. Lett. 2017, 8, 2659–2665. [Google Scholar] [CrossRef]
- Pan, Y.; Lin, Y.; Liu, G.; Zhang, J. Influence of Transition Metal on the Mechanical and Thermodynamic Properties of IrAl Thermal Barrier Coating. Vacuum 2020, 174, 109203. [Google Scholar] [CrossRef]
- Terkhi, S.; Bentata, R.; Bendahma, F.; Lantri, T.; Bentata, S.; Aziz, Z.; Abbar, B. Half-Metallic Ferromagnetic Behavior of Cubic Lanthanide Based on Perovskite-Type Oxide NdCrO3: First-Principles Calculations. Indian J. Phys. 2021, 95, 833–839. [Google Scholar] [CrossRef]
- Dixit, A.; Behera, D.; Tripathi, S.K.; Srivastava, A.; Sharma, R.; Khenata, R.; Albalawi, H.; Mahmoud, Z.; Mukherjee, S.K. Vibrational, Mechanical, Electronic and Thermodynamic Properties of Rhenium-Based Perovskites XReO3(X=Li, Be) by an Ab-Initio Computation. Mater. Sci. Eng. B 2023, 294, 116545. [Google Scholar] [CrossRef]
- Cococcioni, M.; De Gironcoli, S. Linear Response Approach to the Calculation of the Effective Interaction Parameters in the Method. Phys. Rev. B 2005, 71, 035105. [Google Scholar] [CrossRef]
- Yaakob, M.K.; Hussin, N.H.; Taib, M.F.M.; Kudin, T.I.T.; Hassan, O.H.; Ali, A.M.M.; Yahya, M.Z.A. First Principles LDA+ U Calculations for ZnO Materials. First Princ. LDA+U Calc. ZnO Mater. 2014, 155, 15–22. [Google Scholar] [CrossRef]
- Behera, D.; Dixit, A.; Nahak, B.; Srivastava, A.; Sharma, R.; Khenata, R.; Bin-Omran, S.; Abdelmohsen, S.A.M.; Abdelbacki, A.M.M.; Mukherjee, S.K. Theoretical Insight on the Electronic Band Structure, Mechanical, Vibrational and Thermodynamic Characteristic of Antiperovskites RE3InN (RE=Y and La). Mater. Today Commun. 2023, 35, 105618. [Google Scholar] [CrossRef]
- Behera, D.; Dixit, A.; Kumari, K.; Srivastava, A.; Sharma, R.; Mukherjee, S.K.; Khenata, R.; Boumaza, A.; Bin-Omran, S. Structural, Elastic, Mechanical, and Thermodynamic Characteristic of NaReO3 and KReO3 Perovskite Oxides from First Principles Study. Eur. Phys. J. Plus 2022, 137, 1345. [Google Scholar] [CrossRef]
- Behera, D.; Abraham, J.A.; Sharma, R.; Mukerjee, S.K.; Jain, E. First Principles Study of New D0 Half-Metallic Ferromagnetism in CsBaC Ternary Half-Heusler Alloy. J. Supercond. Nov. Magn. 2022, 35, 3431–3437. [Google Scholar] [CrossRef]
- Behera, D.; Azzouz-Rached, A.; Bouhenna, A.; Salah, M.M.; Shaker, A.; Mukherjee, S.K. First-Principles Studies on the Physical Properties of the Half Heusler RbNbCd and RbNbZn Compounds: A Promising Material for Thermoelectric Applications. Crystals 2023, 13, 618. [Google Scholar] [CrossRef]
- Otero-de-la-Roza, A.; Luaña, V. Gibbs2: A New Version of the Quasi-Harmonic Model Code. I. Robust Treatment of the Static Data. Comput. Phys. Commun. 2011, 182, 1708–1720. [Google Scholar] [CrossRef]
- Verma, A.S.; Kumar, A. Bulk Modulus of Cubic Perovskites. J. Alloys Compd. 2012, 541, 210–214. [Google Scholar] [CrossRef]
- Fitzgerel, R.K.; Verhoek, F.H. The Law of Dulong and Petit. J. Chem. Educ. 1960, 37, 545. [Google Scholar] [CrossRef]
- Inaba, H.; Yamamoto, T. Debye Temperature of Materials. Netsu Sokutei 1983, 10, 132–145. [Google Scholar]
- Sun, J.; Singh, D.J. Thermoelectric Properties of N-Type SrTiO3. APL Mater. 2016, 4, 104803. [Google Scholar] [CrossRef]
- Wang, H.; Su, W.; Liu, J.; Wang, C. Recent Development of N-Type Perovskite Thermoelectrics. J. Mater. 2016, 2, 225–236. [Google Scholar] [CrossRef]
- Jonson, M.; Mahan, G.D. Mott’s Formula for the Thermopower and the Wiedemann-Franz Law. Phys. Rev. B 1980, 21, 4223. [Google Scholar] [CrossRef]
- Behera, D.; Mohammed, B.; Taieb, S.; Mokhtar, B.; Al-Qaisi, S.; Mukherjee, S.K. First-Principle Investigations on Optoelectronics and Thermoelectric Properties of Lead-Free Rb2InSbX6(X=Cl, Br) Double Perovskites: For Renewable Energy Applications. Eur. Phys. J. Plus 2023, 138, 520. [Google Scholar] [CrossRef]
- Seddik, T.; Batouche, M. Bulk to Low Dimensional 2D Thermoelectric Materials: Latest Theoretical Research and Future View. In Progress in Nanoscale and Low-Dimensional Materials and Devices: Properties, Synthesis, Characterization, Modelling and Applications; Springer: Berlin/Heidelberg, Germany, 2022; pp. 571–588. [Google Scholar]
- Moutassem, M.; Seddik, T.; Mohammed, D.E.S.; Batouche, M.; Khachai, H.; Khenata, R.; Ahmed, R.; Srivastava, V.; Bouhemadou, A.; Kushwaha, A.K. Metal to Semiconductor Transition and Figure of Merit Enhancement of Li2CuAs Compound by Na Substitution. Bull. Mater. Sci. 2022, 45, 110. [Google Scholar] [CrossRef]
- Julian, C.L. Theory of Heat Conduction in Rare-Gas Crystals. Phys. Rev. 1965, 137, A128. [Google Scholar] [CrossRef]
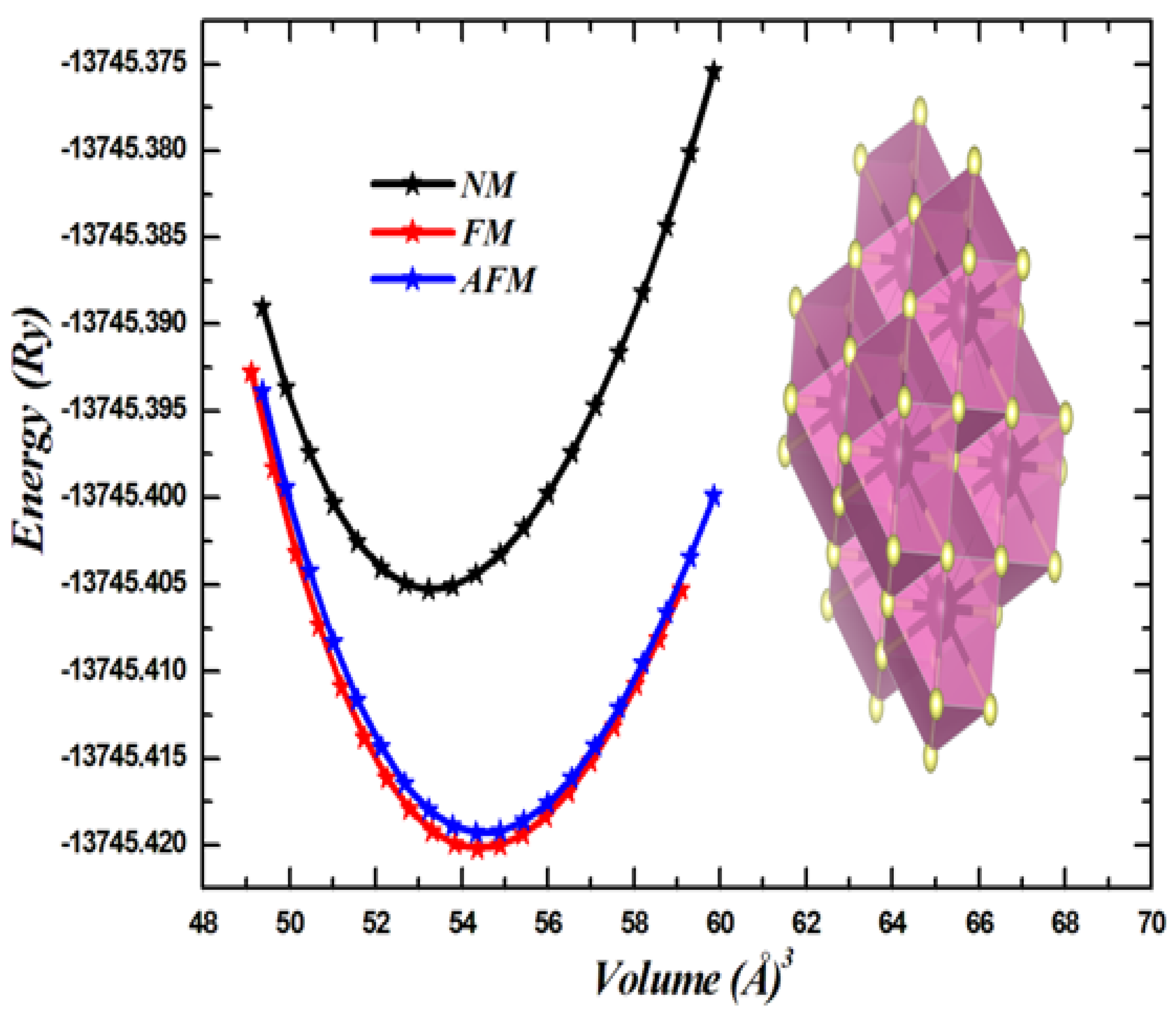



| Compound | States | a | B | B’ | E (Ry) | Ef (eV) | |
|---|---|---|---|---|---|---|---|
| NM | 3.76 | 200.88 | 4.65 | −13,745.405290 | |||
| CdCrO3 | FM | 3.78 | 190.88 | 4.89 | −13,745.420172 | 852 mRy | −4.11 |
| (This study) | AFM | 3.79 | 192.01 | 4.58 | −13,745.419320 | ||
| NM | 3.74 | 226.45 | |||||
| NdCrO3 [31] | FM | 3.80 | 208.93 | ||||
| (other Study) | AFM | 3.95 | 189.90 | ||||
| Experimental | 3.890 |
| MTot | Mcd | MCr | Mo | Mint | Eg (eV) | TC (K) | |
|---|---|---|---|---|---|---|---|
| GGA | 1.99 | 0.045 | 1.67 | −0.004 | 0.292 | 89.46 | |
| Up | 0 | ||||||
| Down | 0 | ||||||
| GGA+U | 1.99 | 0.036 | 1.91 | −0.077 | 0.276 | ||
| Up | 0 | ||||||
| Down | 0.759 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Satapathy, S.; Batouche, M.; Seddik, T.; Salah, M.M.; Maurya, K.K. First Principle Study on Structural, Thermoelectric, and Magnetic Properties of Cubic CdCrO3 Perovskites: A Comprehensive Analysis. Crystals 2023, 13, 1185. https://doi.org/10.3390/cryst13081185
Satapathy S, Batouche M, Seddik T, Salah MM, Maurya KK. First Principle Study on Structural, Thermoelectric, and Magnetic Properties of Cubic CdCrO3 Perovskites: A Comprehensive Analysis. Crystals. 2023; 13(8):1185. https://doi.org/10.3390/cryst13081185
Chicago/Turabian StyleSatapathy, S., Mohammed Batouche, Taieb Seddik, Mostafa M. Salah, and K. K. Maurya. 2023. "First Principle Study on Structural, Thermoelectric, and Magnetic Properties of Cubic CdCrO3 Perovskites: A Comprehensive Analysis" Crystals 13, no. 8: 1185. https://doi.org/10.3390/cryst13081185
APA StyleSatapathy, S., Batouche, M., Seddik, T., Salah, M. M., & Maurya, K. K. (2023). First Principle Study on Structural, Thermoelectric, and Magnetic Properties of Cubic CdCrO3 Perovskites: A Comprehensive Analysis. Crystals, 13(8), 1185. https://doi.org/10.3390/cryst13081185





