Research Progress in the Industrial Crystallization of Citrate—A Review
Abstract
:1. Introduction
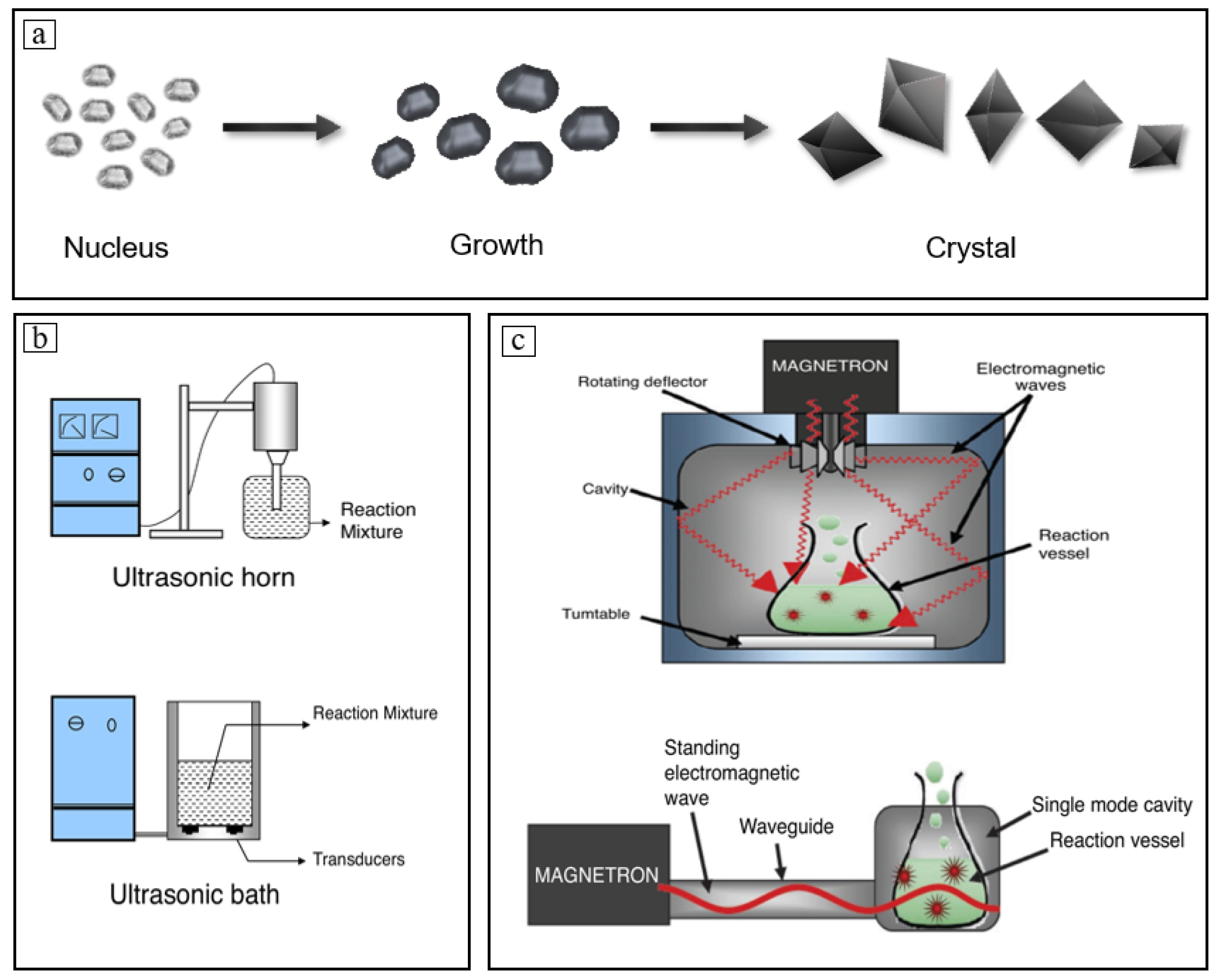
2. Mechanism of Crystallization of Citrate
2.1. Main Methods for Citrate Crystallization
2.2. Formation of Citrate Hydrate
2.3. Nucleation and Growth of Citrate
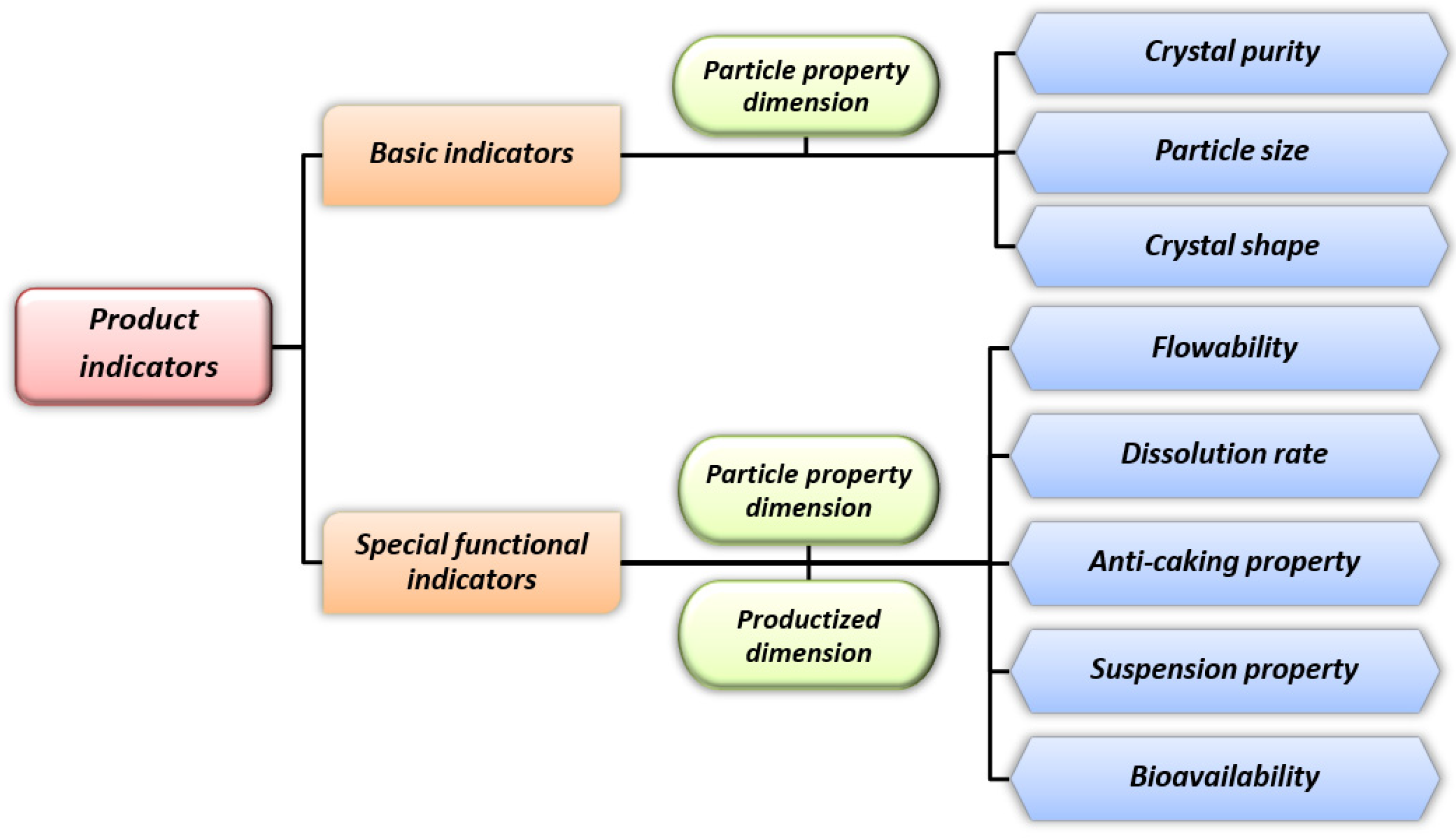
3. Key Product Indicators and Crystallization Control Measures for Citrate Crystals
3.1. Crystal Purity
3.2. Particle Size
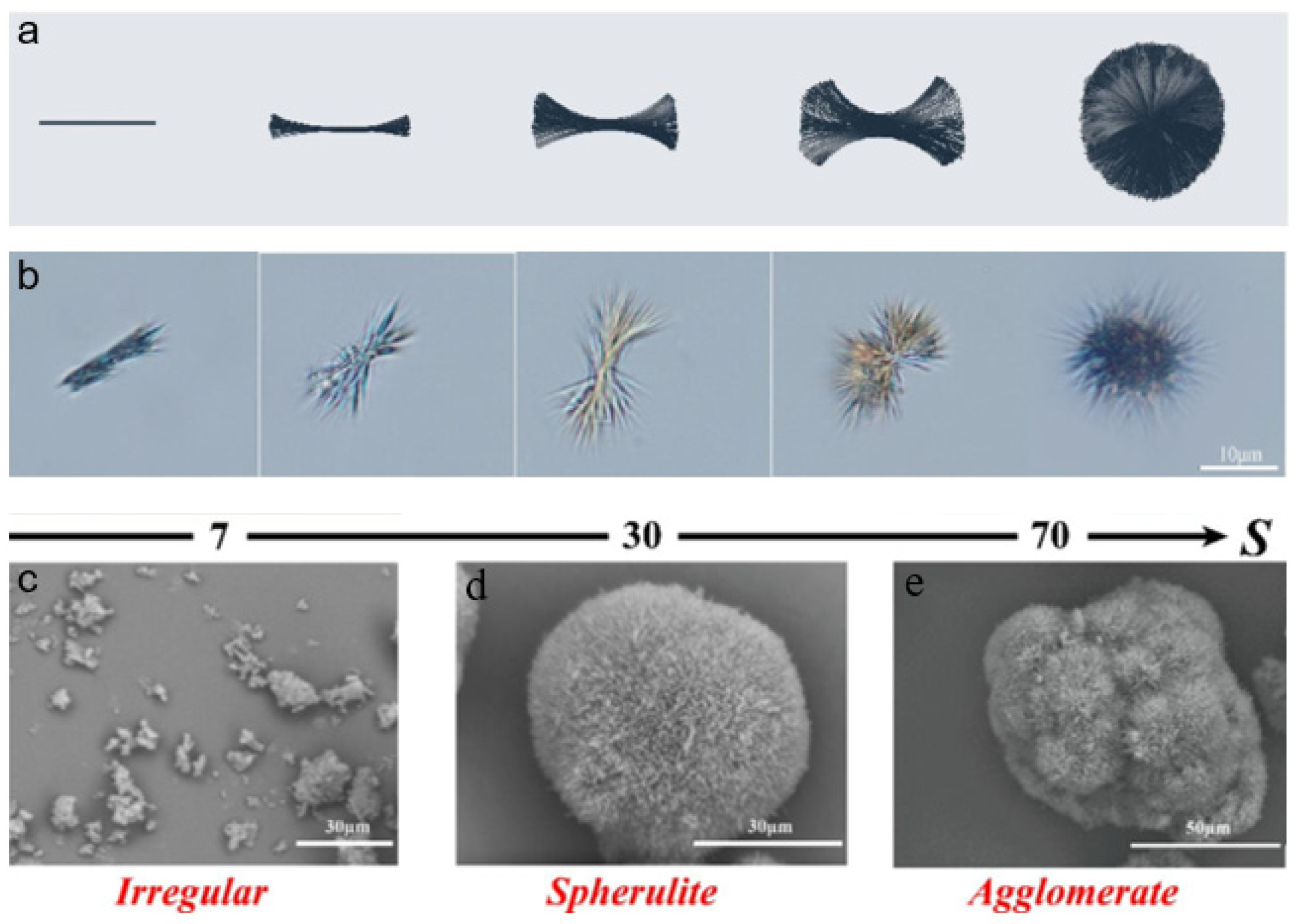
3.3. Crystal Shape
3.4. Special Functional Indicators
4. Industrial Crystallization Equipment for Citrate
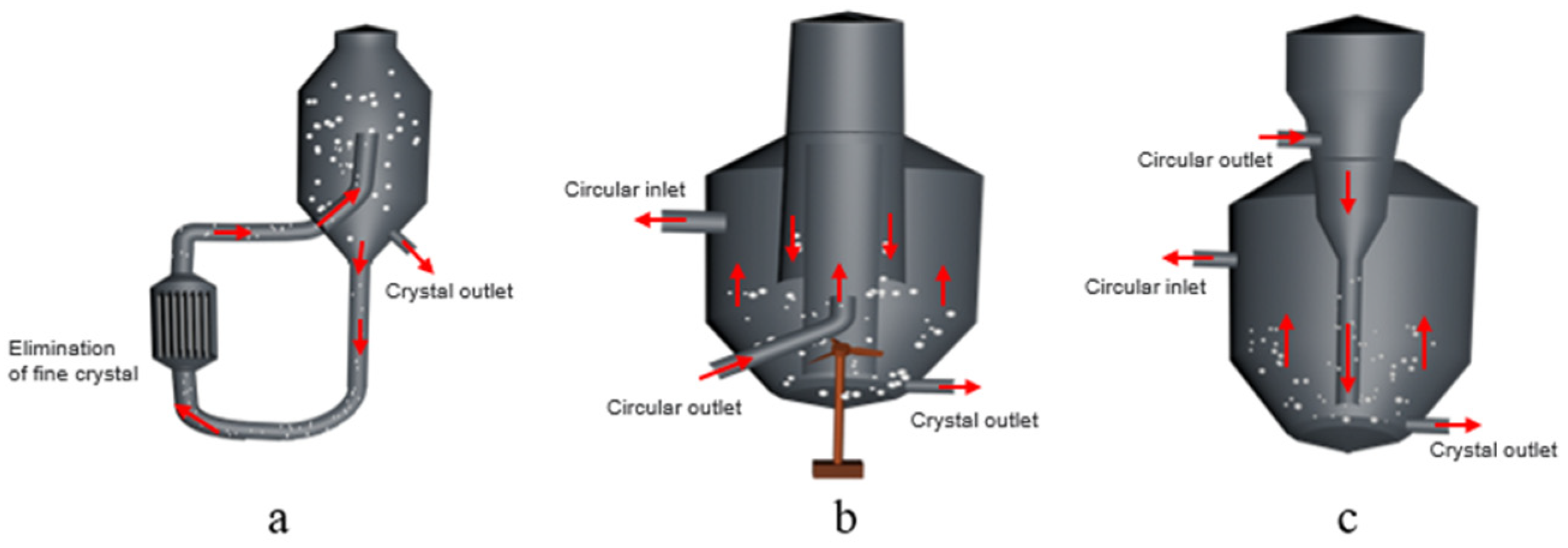
4.1. Crystallizer Device Forms
4.2. Continuous Crystallization
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Canales, B.K. Alkalinizing Agents: A Review of Prescription, Over-the-Counter, and Medical Food Supplements. J. Endourol. 2020, 34, 639. [Google Scholar]
- Hoy, S.M.; Scott, L.J.; Wagstaff, A.J. Sodium picosulfate/magnesium citrate: A review of its use as a colorectal cleanser. Drugs 2009, 69, 123–136. [Google Scholar] [PubMed]
- Philichi, L.; Yuwono, M. A retrospective study comparing polyethylene glycol-electrolyte solution with magnesium citrate for treatment of fecal disimpaction. Gastroenterol. Nurs. 2018, 41, 141–144. [Google Scholar] [CrossRef] [PubMed]
- Dziechciarz, P.; Ruszczyński, M.; Horvath, A. Sodium Picosulphate with Magnesium Citrate versus Polyethylene Glycol for Bowel Preparation in Children: A Systematic Review. Pediatr. Gastroenterol. Hepatol. Nutr. 2022, 25, 228. [Google Scholar] [PubMed]
- Franklin, R.B.; Costello, L.C. Zinc as an anti-tumor agent in prostate cancer and in other cancers. Arch. Biochem. Biophys. 2007, 463, 211–217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Costello, L.C.; Franklin, R.B. A comprehensive review of the role of zinc in normal prostate function and metabolism; and its implications in prostate cancer. Arch. Biochem. Biophys. 2016, 611, 100–112. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Y.; Liu, X.; Si, F.; Huang, L.; Gao, A.; Lin, W.; Hoft, D.F.; Shao, Q.; Peng, G. Citrate promotes excessive lipid biosynthesis and senescence in tumor cells for tumor therapy. Adv. Sci. 2022, 9, 2101553. [Google Scholar]
- Castellani, D.; Giulioni, C.; De Stefano, V.; Brocca, C.; Fuligni, D.; Galosi, A.B.; Teoh, J.Y.-C.; Sarica, K.; Gauhar, V. Dietary management of hypocitraturia in children with urolithiasis: Results from a systematic review. World J. Urol. 2023, 41, 1243–1250. [Google Scholar]
- Spivacow, F.R.; Negri, A.L.; Polonsky, A.; Del Valle, E.E. Long-term treatment of renal lithiasis with potassium citrate. Urology 2010, 76, 1346–1349. [Google Scholar] [CrossRef]
- Doizi, S.; Poindexter, J.R.; Pearle, M.S.; Blanco, F.; Moe, O.W.; Sakhaee, K.; Maalouf, N.M. Impact of potassium citrate vs citric acid on urinary stone risk in calcium phosphate stone formers. J. Urol. 2018, 200, 1278–1284. [Google Scholar]
- Phillips, R.; Hanchanale, V.S.; Myatt, A.; Somani, B.; Nabi, G.; Biyani, C.S. Citrate salts for preventing and treating calcium containing kidney stones in adults. Cochrane Database Syst. Rev. 2015, 2015, CD010057. [Google Scholar]
- Yang, Y.; Li, J.; Wang, L.; Liu, H.; Lai, X. Calcium citrate: An interesting organic calcium biomedical material. Chin. J. Tissue Eng. Res. 2021, 25, 1609. [Google Scholar]
- Icard, P.; Coquerel, A.; Wu, Z.; Gligorov, J.; Fuks, D.; Fournel, L.; Lincet, H.; Simula, L. Understanding the central role of citrate in the metabolism of cancer cells and tumors: An update. Int. J. Mol. Sci. 2021, 22, 6587. [Google Scholar] [PubMed]
- Fiume, M.M.; Heldreth, B.A.; Bergfeld, W.F.; Belsito, D.V.; Hill, R.A.; Klaassen, C.D.; Liebler, D.C.; Marks Jr, J.G.; Shank, R.C.; Slaga, T.J. Safety assessment of citric acid, inorganic citrate salts, and alkyl citrate esters as used in cosmetics. Int. J. Toxicol. 2014, 33, 16S–46S. [Google Scholar] [CrossRef] [PubMed]
- Timpmann, S.; Burk, A.; Medijainen, L.; Tamm, M.; Kreegipuu, K.; Vähi, M.; Unt, E.; Ööpik, V. Dietary sodium citrate supplementation enhances rehydration and recovery from rapid body mass loss in trained wrestlers. Appl. Physiol. Nutr. Metab. 2012, 37, 1028–1037. [Google Scholar]
- Cerullo, G.; Parimbelli, M.; Perna, S.; Pecoraro, M.; Liguori, G.; Negro, M.; D’Antona, G. Sodium citrate supplementation: An updated revision and practical recommendations on exercise performance, hydration status, and potential risks. Transl. Sports Med. 2020, 3, 518–525. [Google Scholar]
- Redant, S.; De Bels, D.; Massaut, J.; Barglazan, D.; Lebitasy, P.M.; Honoré, P.M. Feasibility of citrate dialysis in hyponatremia: A case series. Blood Purif. 2021, 50, 174–179. [Google Scholar] [CrossRef]
- Moseley, K.F.; Weaver, C.M.; Appel, L.; Sebastian, A.; Sellmeyer, D.E. Potassium citrate supplementation results in sustained improvement in calcium balance in older men and women. J. Bone Miner. Res. 2013, 28, 497–504. [Google Scholar]
- Nicar, M.J.; Peterson, R.; Pak, C.Y. Use of potassium citrate as potassium supplement during thiazide therapy of calcium nephrolithiasis. J. Urol. 1984, 131, 430–433. [Google Scholar] [CrossRef]
- Carvalho, M.; Erbano, B.O.; Kuwaki, E.Y.; Pontes, H.P.; Liu, J.W.T.W.; Boros, L.H.; Asinelli, M.O.; Baena, C.P. Effect of potassium citrate supplement on stone recurrence before or after lithotripsy: Systematic review and meta-analysis. Urolithiasis 2017, 45, 449–455. [Google Scholar]
- Palermo, A.; Naciu, A.M.; Tabacco, G.; Manfrini, S.; Trimboli, P.; Vescini, F.; Falchetti, A. Calcium citrate: From biochemistry and physiology to clinical applications. Rev. Endocr. Metab. Disord. 2019, 20, 353–364. [Google Scholar] [CrossRef] [PubMed]
- Gómez, J.M.Q.; Rubió, J.B.; Curiel, M.D.; Pérez, A.D. Calcium citrate and vitamin D in the treatment of osteoporosis. Clin. Drug Investig. 2011, 31, 285–298. [Google Scholar] [CrossRef] [PubMed]
- Tondapu, P.; Provost, D.; Adams-Huet, B.; Sims, T.; Chang, C.; Sakhaee, K. Comparison of the absorption of calcium carbonate and calcium citrate after Roux-en-Y gastric bypass. Obes. Surg. 2009, 19, 1256–1261. [Google Scholar] [CrossRef] [Green Version]
- Schutten, J.C.; Joris, P.J.; Mensink, R.P.; Danel, R.M.; Goorman, F.; Heiner-Fokkema, M.R.; Weersma, R.K.; Keyzer, C.A.; de Borst, M.H.; Bakker, S.J. Effects of magnesium citrate, magnesium oxide and magnesium sulfate supplementation on arterial stiffness in healthy overweight individuals: A study protocol for a randomized controlled trial. Trials 2019, 20, 295. [Google Scholar] [PubMed]
- Schutten, J.C.; Joris, P.J.; Groendijk, I.; Eelderink, C.; Groothof, D.; van der Veen, Y.; Westerhuis, R.; Goorman, F.; Danel, R.M.; de Borst, M.H. Effects of Magnesium Citrate, Magnesium Oxide, and Magnesium Sulfate Supplementation on Arterial Stiffness: A Randomized, Double-Blind, Placebo-Controlled Intervention Trial. J. Am. Heart Assoc. 2022, 11, e021783. [Google Scholar] [PubMed]
- Vermeulen, E.A.; Eelderink, C.; Hoekstra, T.; van Ballegooijen, A.J.; Raijmakers, P.; Beulens, J.W.; de Borst, M.H.; Vervloet, M.G. Reversal Of Arterial Disease by modulating Magnesium and Phosphate (ROADMAP-study): Rationale and design of a randomized controlled trial assessing the effects of magnesium citrate supplementation and phosphate-binding therapy on arterial stiffness in moderate chronic kidney disease. Trials 2022, 23, 769. [Google Scholar]
- Wegmüller, R.; Tay, F.; Zeder, C.; Brnić, M.; Hurrell, R.F. Zinc Absorption by Young Adults from Supplemental Zinc Citrate Is Comparable with That from Zinc Gluconate and Higher than from Zinc Oxide. J. Nutr. 2014, 144, 132–136. [Google Scholar] [CrossRef] [Green Version]
- Sapota, A.; Daragó, A.; Skrzypińska-Gawrysiak, M.; Nasiadek, M.; Klimczak, M.; Kilanowicz, A. The bioavailability of different zinc compounds used as human dietary supplements in rat prostate: A comparative study. Biometals 2014, 27, 495–505. [Google Scholar]
- Sun, X.; Zhang, W.; Li, H.; Zhu, X.; He, D.; Yang, J. Effects of ultrasound on the crystallization of products prepared from lead paste by hydrometallurgical processes. Chem. Ind. Eng. Prog. 2013, 32, 1974–1978. [Google Scholar]
- Zhu, X.F.; Liu, W.C.; Yang, H.Y.; Li, L.; Yang, J.K. Preparation of ultrafine PbO powders from lead paste in spent lead acid battery. Trans. Nonferrous Met. Soc. China 2010, 20, 132–136. [Google Scholar]
- Xiao, Y.; Yang, Y.; Li, J.; Ma, Y.; Wang, H.; Wang, L.; Huang, Y.; Zhang, P.; Zou, Q.; Lai, X. Porous composite calcium citrate/polylactic acid materials with high mineralization activity and biodegradability for bone repair tissue engineering. Int. J. Polym. Mater. Polym. Biomater. 2021, 70, 507–520. [Google Scholar] [CrossRef]
- Koc, B.; Kizildag, S.; Hosgorler, F.; Gumus, H.; Kandis, S.; Ates, M.; Uysal, N. Magnesium citrate increases pain threshold and reduces TLR4 concentration in the brain. Biol. Trace Elem. Res. 2021, 199, 1954–1966. [Google Scholar] [CrossRef] [PubMed]
- Anitua, E.; Zalduendo, M.; Troya, M.; Alkhraisat, M.H. The influence of sodium citrate on the characteristics and biological activity of plasma rich in growth factors. Regen. Med. 2020, 15, 2181–2192. [Google Scholar] [CrossRef] [PubMed]
- Ebrahimi, M.; Fakhr Yasseri, A.; Zareian Baghdadabad, L.; Zahmatkesh, P.; Keshavarz Pakseresht, B.; Khoshchehreh, M.; Namazi Shabestari, A. The Impact of Potassium Citrate on the Kidney Stones Treatment in Rat. Transl. Res. Urol. 2021, 3, 176–181. [Google Scholar]
- Yan, H.; Liu, Y.; Peng, H.; Li, K.; Li, C.; Jiang, S.; Chen, M.; Han, D.; Gong, J. Improving calcium citrate food functions through spherulitic growth in reactive crystallization and a mechanism study. Food Chem. 2023, 404, 134550. [Google Scholar] [CrossRef]
- Sawant, O.; Mahale, S.; Ramchandran, V.; Nagaraj, G.; Bankar, A. Fungal citric acid production using waste materials: A mini-review. J. Microbiol. Biotechnol. Food Sci. 2021, 2021, 821–828. [Google Scholar]
- Luo, H.; Zhou, Y.; Xu, G.; Man, Y.; Wang, J. A Kind of Production Method of Anhydrous Citric Acid Crystal. Patent CN106146292A, 2 April 2015. [Google Scholar]
- Shi, Z. Preparation Method and Application of Anhydrous Citric Acid. Patent CN114315559A, 10 December 2021. [Google Scholar]
- Luo, H.; Xiong, J.; Xu, G.; Gao, Z.; Tao, J. A Kind of Method for Preparing Anhydrous Citric Acid Crystal. Patent CN104177251A, 29 July 2014. [Google Scholar]
- Tippetts, M.; Martini, S. Effect of cooling rate on lipid crystallization in oil-in-water emulsions. Food Res. Int. 2009, 42, 847–855. [Google Scholar] [CrossRef]
- Campos, R.; Narine, S.; Marangoni, A. Effect of cooling rate on the structure and mechanical properties of milk fat and lard. Food Res. Int. 2002, 35, 971–981. [Google Scholar] [CrossRef]
- Cavallo, D.; Gardella, L.; Alfonso, G.C.; Portale, G.; Balzano, L.; Androsch, R. Effect of cooling rate on the crystal/mesophase polymorphism of polyamide 6. Colloid Polym. Sci. 2011, 289, 1073–1079. [Google Scholar] [CrossRef]
- Im, S.H.; Park, O.O. Effect of evaporation temperature on the quality of colloidal crystals at the water−air interface. Langmuir 2002, 18, 9642–9646. [Google Scholar] [CrossRef]
- Aditya, S.; Stephen, J.; Radhakrishnan, M. Utilization of eggshell waste in calcium-fortified foods and other industrial applications: A review. Trends Food Sci. Technol. 2021, 115, 422–432. [Google Scholar]
- Ding, X.-M.; Peng, L.; Wen, F.; Tan, Z.-W.; Mu, Z.-L. Simulated body fluid immersion method for assessing biological characteristics of calcium citrate. Chin. J. Tissue Eng. Res. 2013, 17, 6811. [Google Scholar]
- Oliveira, D.; Benelli, P.; Amante, E. A literature review on adding value to solid residues: Egg shells. J. Clean. Prod. 2013, 46, 42–47. [Google Scholar]
- Ahmed, S.; Gibriel, A.A.; Abdellatif, A.; Ebied, H. Evaluation of food products fortified with oyster shell for the prevention and treatment of osteoporosis. J. Food Sci. Technol. 2015, 52, 6816–6820. [Google Scholar] [CrossRef] [Green Version]
- Zhan, J.; Lu, J.; Wang, D. Review of shell waste reutilization to promote sustainable shellfish aquaculture. Rev. Aquac. 2022, 14, 477–488. [Google Scholar] [CrossRef]
- Xu, Y.; Ye, J.; Zhou, D.; Su, L. Research progress on applications of calcium derived from marine organisms. Sci. Rep. 2020, 10, 18425. [Google Scholar] [CrossRef]
- Mansour, S.A.A. Thermal decomposition of calcium citrate tetrahydrate. Thermochim. Acta 1994, 233, 243–256. [Google Scholar] [CrossRef]
- Mansour, S.A.A. Thermal decomposition of magnesium citrate 14-hydrate. Thermochim. Acta 1994, 233, 231–242. [Google Scholar] [CrossRef]
- Srivastava, A.; Gunjikar, V.G.; Sinha, A.P.B. Thermoanalytical studies of zinc citrate, bismuth citrate and calcium citrate. Thermochim. Acta 1987, 117, 201–217. [Google Scholar] [CrossRef]
- Liu, X.-C.; Kirkensgaard, J.J.K.; Skibsted, L.H. Temperature effects on spontaneous supersaturation of calcium citrate in presence of lactate. Int. Dairy J. 2021, 118, 105023. [Google Scholar] [CrossRef]
- Salami, H.; McDonald, M.A.; Bommarius, A.S.; Rousseau, R.W.; Grover, M.A. In Situ Imaging Combined with Deep Learning for Crystallization Process Monitoring: Application to Cephalexin Production. Org. Process Res. Dev. 2021, 25, 1670–1679. [Google Scholar] [CrossRef]
- Zheng, Y.; Wu, Z. Predictive Control of Batch Crystallization Process Using Machine Learning. IFAC-PapersOnLine 2022, 55, 798–803. [Google Scholar] [CrossRef]
- Feng, H.; Wang, N.; Huang, X.; Wang, T.; Zhou, L.A.; Hao, H.X. Recent progress in melt crystallization. Chem. Eng. Res. Des. 2023, 190, 268–281. [Google Scholar] [CrossRef]
- Hong, W.; Jia, S.; Chen, Y.; Mou, Q.; Gao, Z.; Gong, J. Highly Efficient and Solvent-Free Purification Technique and Model Study of Layer Melt Crystallization. ACS Sustain. Chem. Eng. 2022, 10, 16450–16458. [Google Scholar] [CrossRef]
- Amran, N.A.; Jusoh, M. Effect of Coolant Temperature and Circulation flowrate on the Performance of a Vertical Finned Crystallizer. In Proceedings of the 4th International Conference on Process Engineering and Advanced Materials (ICPEAM 2016), Kuala Lumpur, Malaysia, 15–17 August 2016; pp. 1408–1415. [Google Scholar]
- Sparenberg, M.-C.; Chergaoui, S.; Sang Sefidi, V.; Luis, P. Crystallization control via membrane distillation-crystallization: A review. Desalination 2021, 519, 115315. [Google Scholar] [CrossRef]
- Xu, H.; Zhang, X. A Kind of Preparation Method of Specific Particle Size Potassium Citrate Monohydrate. Patent CN112010750A, 25 August 2020. [Google Scholar]
- Shi, Z. The Invention Relates to a Production Method of Anhydrous Sodium Citrate. Patent CN105037140A, 26 June 2015. [Google Scholar]
- Xu, H.; Zhang, X.; Zhu, Y.; Li, J.; Jia, Q. The Invention Relates to a Preparation Method of Large Granules Anhydrous Sodium Citrate. Patent CN109438225A, 21 November 2018. [Google Scholar]
- Gao, J. Study on Continuous Crystallization Process of Sodium Citrate Hydrate. Master’s Thesis, Tianjin University, Tianjin, China, 2012. [Google Scholar]
- Li, B.; Luo, H.; Xiong, J.; Song, J.; Xu, G. A Kind of Zinc Citrate and Production Method Thereof. Patent CN106854147A, 8 December 2015. [Google Scholar]
- Liu, X.-C.; Skibsted, L.H. Citrate in calcium transport and biomineralisation. Int. Dairy J. 2022, 139, 105561. [Google Scholar] [CrossRef]
- Vavrusova, M.; Skibsted, L.H. Aqueous solubility of calcium citrate and interconversion between the tetrahydrate and the hexahydrate as a balance between endothermic dissolution and exothermic complex formation. Int. Dairy J. 2016, 57, 20–28. [Google Scholar] [CrossRef]
- Liu, X.-C.; Kirkensgaard, J.J.K.; Skibsted, L.H. Hydrates of calcium citrate and their interconversion in relation to calcium bioaccessibility. Food Res. Int. 2021, 140, 109867. [Google Scholar] [CrossRef]
- Qin, Y. Study on Crystallization Process of Citric Acid and Its Calcium Salt. Master’s Thesis, Tianjin University, Tianjin, China, 2014. [Google Scholar]
- Luo, J.; Xiao, G.; Ding, D.; Chong, X.; Ren, J.; Bai, B. Pyrolysis mechanism of magnesium citrate nonahydrate and microstructural evolution during the process. Ceram. Int. 2021, 47, 29607–29619. [Google Scholar] [CrossRef]
- Li, B.; Zhou, Y.; Guizhen, X.; Song, J. A Kind of Magnesium Citrate Crystal and Production Method Thereof. Patent CN106854145A, 4 September 2020. [Google Scholar]
- Shen, Y.; Qiao, X.; Song, Z.; Zhong, S.; Wei, D. Terahertz spectroscopy of citrate Salts: Effects of crystalline state and crystallization water. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2022, 277, 121288. [Google Scholar] [CrossRef]
- Zhu, X.; Wang, X.; Nie, P.; Zhang, W.; Hu, Y.; Yang, J.; Wang, X.; Guo, Y. Thermal decomposition of lead citrate Pb3(C6H5 O7)2·3H2O from recovery spent lead paste by hydrometallurgy process. Trans. Nonferrous Met. Soc. China 2016, 26, 2686–2693. [Google Scholar]
- Cölfen, H. Nonclassical Nucleation and Crystallization. Crystals 2020, 10, 61. [Google Scholar] [CrossRef] [Green Version]
- Han, M.; Li, J.; He, G.; Lin, M.; Xiao, W.; Li, X.; Wu, X.; Jiang, X. Tailored 3D printed micro-crystallization chip for versatile and high-efficiency droplet evaporative crystallization. Lab. Chip. 2019, 19, 767–777. [Google Scholar] [CrossRef]
- Zaykovskaya, A.; Louhi-Kultanen, M. Batch Crystallization of Xylitol by Cooling, Evaporative, and Antisolvent Crystallization. Cryst. Growth Des. 2023, 23, 1813–1820. [Google Scholar] [CrossRef]
- Wang, X.; Wu, L.; Wang, G.; Chen, G. Dynamic Crystallization and Phase Transition in Evaporating Colloidal Droplets. Nano Lett. 2019, 19, 8225–8233. [Google Scholar] [CrossRef]
- Luo, H.; Man, Y.; Xu, G.; Wang, J. A Kind of Production Method of Potassium Citrate Crystal and Potassium Citrate Crystal Produced by the Method. Patent CN106854152A, 8 December 2015. [Google Scholar]
- Gogate, P.R. Intensification of chemical processing applications using ultrasonic and microwave irradiations. Curr. Opin. Chem. Eng. 2017, 17, 9–14. [Google Scholar] [CrossRef]
- Ji, Z.; Wang, J.; Yin, Z.; Hou, D.; Luan, Z. Effect of microwave irradiation on typical inorganic salts crystallization in membrane distillation process. J. Membr. Sci. 2014, 455, 24–30. [Google Scholar] [CrossRef]
- Shi, Z. A Kind of Production Method of Magnesium Citrate. Patent CN114436813A, 4 January 2022. [Google Scholar]
- Li, J.; Zhong, L.; Gao, Y.; Cao, W.; You, Y.; Zhang, P.; Li, J.; Lai, X. Preparation method of nano calcium citrate. Patent CN103755552A, 24 February 2014. [Google Scholar]
- Dowling, R.; Davey, R.J.; Curtis, R.A.; Han, G.; Poornachary, S.K.; Chow, P.S.; Tan, R.B. Acceleration of crystal growth rates: An unexpected effect of tailor-made additives. Chem. Commun. 2010, 46, 5924–5926. [Google Scholar]
- Wu, H.; Wang, J.; Huang, X.; Zhai, L.; Hao, H. Enlarging crystal size of zoxamide by polymeric additives that modulate burst nucleation. J. Mol. Liq. 2022, 357, 119088. [Google Scholar] [CrossRef]
- Rudolph, P.; Kiessling, F.M. Growth and characterization of GaAs crystals produced by the VCz method without boric oxide encapsulation. J. Cryst. Growth 2006, 292, 532–537. [Google Scholar] [CrossRef]
- Liu, Y.; Xing, Q.; Dennis, K.W.; McCallum, R.W.; Lograsso, T.A. Evolution of precipitate morphology during heat treatment and its implications for the superconductivity in K x Fe 1.6+ y Se 2 single crystals. Phys. Rev. B 2012, 86, 144507. [Google Scholar] [CrossRef] [Green Version]
- Fan, G.; Li, T.; Zhao, L.; Zhang, S. Study on purification technology of silicon carbide crystal growth powder. Materials 2022, 15, 8190. [Google Scholar] [CrossRef]
- Kwon, S.-J.; Jazbinsek, M.; Kwon, O.-P.; Günter, P. Crystal growth and morphology control of OH1 organic electrooptic crystals. Cryst. Growth Des. 2010, 10, 1552–1558. [Google Scholar] [CrossRef]
- Duan, J.; Zhao, Y.; He, B.; Tang, Q. High-purity inorganic perovskite films for solar cells with 9.72% efficiency. Angew. Chem. Int. Ed. 2018, 57, 3787–3791. [Google Scholar] [CrossRef] [PubMed]
- Behera, B.C.; Mishra, R.; Mohapatra, S. Microbial citric acid: Production, properties, application, and future perspectives. Food Front. 2021, 2, 62–76. [Google Scholar]
- Luo, G.S.; Shan, X.Y.; Qi, X.; Lu, Y.C. Two-phase electro-electrodialysis for recovery and concentration of citric acid. Sep. Purif. Technol. 2004, 38, 265–271. [Google Scholar] [CrossRef]
- Sun, X.; Lu, H.; Wang, J. Recovery of citric acid from fermented liquid by bipolar membrane electrodialysis. J. Clean. Prod. 2017, 143, 250–256. [Google Scholar] [CrossRef]
- Luo, H.; Cheng, X.; Liu, G.; Zhou, Y.; Lu, Y.; Zhang, R.; Li, X.; Teng, W. Citric acid production using a biological electrodialysis with bipolar membrane. J. Membr. Sci. 2017, 523, 122–128. [Google Scholar] [CrossRef]
- Wang, Q.; Chen, G.Q.; Lin, L.; Li, X.; Kentish, S.E. Purification of organic acids using electrodialysis with bipolar membranes (EDBM) combined with monovalent anion selective membranes. Sep. Purif. Technol. 2021, 279, 119739. [Google Scholar] [CrossRef]
- Wu, J.; Lu, Z.; Tang, Y. The Invention Relates to Water-Soluble Zinc Citrate, Preparation Method and Application Thereof. Patent CN111067106A, 17 December 2019. [Google Scholar]
- Kou, G.; Li, C.; Liu, C.; Zhou, H.; An, F.; Gao, X. Production Process of Amorphous Ultra-Fine Calcium Citrate. Patent CN104529754A, 24 December 2014. [Google Scholar]
- Li, C.; Wei, C.; Zhang, H.; Yu, H.; Jiang, S.; Fan, K. The Invention Relates to a Preparation Method of Calcium Citrate Whisker. Patent CN112409167A, 27 November 2020. [Google Scholar]
- Peng, L.; Dai, H.; Wu, Y.; Peng, Y.; Lu, X. A comprehensive review of phosphorus recovery from wastewater by crystallization processes. Chemosphere 2018, 197, 768–781. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.G.; Rao, K.K. Zinc oxide based photocatalysis: Tailoring surface-bulk structure and related interfacial charge carrier dynamics for better environmental applications. RSC Adv. 2015, 5, 3306–3351. [Google Scholar]
- Yao, J.; Zhu, B.; Yao, J. The Invention Relates to a Synthesis Method of Zinc Citrate Trihydrate. Patent CN104610048A, 11 February 2015. [Google Scholar]
- Gordon, J.; Kazemian, H.; Rohani, S. Rapid and efficient crystallization of MIL-53 (Fe) by ultrasound and microwave irradiation. Microporous Mesoporous Mater. 2012, 162, 36–43. [Google Scholar] [CrossRef]
- Li, Y.; Huang, H.; Xu, G.; Xiong, J.; Xu, X.; Li, C. The Invention Relates to a Production Method of Zinc Citrate Crystal. Patent CN112174802A, 5 July 2019. [Google Scholar]
- Ryu, J.H.; Koo, S.-M.; Yoon, J.-W.; Lim, C.S.; Shim, K.B. Synthesis of nanocrystalline MMoO4 (M=Ni, Zn) phosphors via a citrate complex route assisted by microwave irradiation and their photoluminescence. Mater. Lett. 2006, 60, 1702–1705. [Google Scholar] [CrossRef]
- Tang, X.; Nie, Y.; Jin, Q.; Guo, L.; Zhao, J.; Li, T.; Zhu, Y. Kinetics and mechanism of ultrasonic-assisted magnesium oxide hydration. Ultrason. Sonochem. 2018, 40, 995–1002. [Google Scholar] [CrossRef]
- Tang, B.; Peng, L. The Invention Relates to Refining Process and Realization Device of Sodium Citrate with Controllable Particle Size. Patent CN107188798A, 27 May 2017. [Google Scholar]
- Bergfors, T. Seeds to crystals. J. Struct. Biol. 2003, 142, 66–76. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Shan, B.; Wang, Y.; Zhu, Z.; Yu, Z.-Q.; Ma, C.Y. Progress and opportunities for utilizing seeding techniques in crystallization processes. Org. Process Res. Dev. 2021, 25, 1496–1511. [Google Scholar] [CrossRef]
- Li, W.; Yang, J.; Du, S.; Macaringue, E.; Wang, Y.; Wu, S.; Gong, J. Preparation and Formation Mechanism of l-Valine Spherulites via Evaporation Crystallization. Ind. Eng. Chem. Res. 2021, 60, 6048–6058. [Google Scholar] [CrossRef]
- He, D.; Yang, C.; Wu, Y.; Liu, X.; Xie, W.; Yang, J. PbSO4 Leaching in Citric Acid/Sodium Citrate Solution and Subsequent Yielding Lead Citrate via Controlled Crystallization. Minerals 2017, 7, 93. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Zhong, L.; Gao, Y.; Cao, W.; He, M.; Zou, Q.; Zhang, P.; Lai, X. Fibrous Calcium Citrate and Its Preparation Method and Application. Patent CN104355991A, 4 November 2014. [Google Scholar]
- Li, J.; Zhong, L.; Liu, Y.; Zhou, B.; Liu, Q.; Wu, K.; Zhang, P.; Lai, X. Calcium Citrate in Strip Form and Its Preparation Method and Application. Patent CN104355992A, 4 November 2014. [Google Scholar]
- Li, J.; Zhong, L.; Hou, Y.; Lai, X.; Qiu, K.; Li, G.; Huang, L. Spherical Calcium Citrate, Preparation Method and Application Thereof. Patent CN104402705A, 4 November 2014. [Google Scholar]
- Kawashima, Y.; Okumura, M.; Takenaka, H. Spherical Crystallization: Direct Spherical Agglomeration of Salicylic Acid Crystals During Crystallization. Science 1982, 216, 1127–1128. [Google Scholar] [CrossRef]
- Hu, X.; Zhao, Y.; Xiao, W.; He, G.; Jiang, H.; Ruan, X.; Jiang, X. Improved Spherical Particle Preparation of Ceftriaxone Sodium via Membrane-Assisted Spherical Crystallization. Ind. Eng. Chem. Res. 2023, 62, 4444–4454. [Google Scholar] [CrossRef]
- Chen, K.; Hou, B.; Wu, H.; Huang, X.; Li, F.; Xiao, Y.; Li, J.; Bao, Y.; Hao, H. Hollow and Solid Spherical Azithromycin Particles Prepared by Different Spherical Crystallization Technologies for Direct Tableting. Processes 2019, 7, 276. [Google Scholar] [CrossRef] [Green Version]
- Rasenack, N.; Müller, B.W. Micron-size drug particles: Common and novel micronization techniques. Pharm. Dev. Technol. 2004, 9, 1–13. [Google Scholar] [CrossRef]
- Chen, T.; Zhang, M.; Bhandari, B.; Yang, Z. Micronization and nanosizing of particles for an enhanced quality of food: A review. Crit. Rev. Food Sci. Nutr. 2018, 58, 993–1001. [Google Scholar]
- Garg, V.; Mallick, S.S.; Garcia-Trinanes, P.; Berry, R.J. An investigation into the flowability of fine powders used in pharmaceutical industries. Powder Technol. 2018, 336, 375–382. [Google Scholar] [CrossRef]
- Barone, G.; O’Regan, J.; Kelly, A.L.; O’Mahony, J.A. Physicochemical and bulk handling properties of micronised calcium salts and their application in calcium fortification of whey protein-based solutions. J. Food Eng. 2021, 292, 110213. [Google Scholar] [CrossRef]
- Chen, M.; Wu, S.; Xu, S.; Yu, B.; Shilbayeh, M.; Liu, Y.; Zhu, X.; Wang, J.; Gong, J. Caking of crystals: Characterization, mechanisms and prevention. Powder Technol. 2018, 337, 51–67. [Google Scholar] [CrossRef]
- Terdenge, L.M.; Wohlgemuth, K. Impact of agglomeration on crystalline product quality within the crystallization process chain. Cryst. Res. Technol. 2016, 51, 513–523. [Google Scholar] [CrossRef]
- Zafar, U.; Vivacqua, V.; Calvert, G.; Ghadiri, M.; Cleaver, J.A.S. A review of bulk powder caking. Powder Technol. 2017, 313, 389–401. [Google Scholar] [CrossRef]
- Chen, M.; Zhang, D.; Dong, W.; Luo, Z.; Kang, C.; Li, H.; Wang, G.; Gong, J. Amorphous and humidity caking: A review. Chin. J. Chem. Eng. 2019, 27, 1429–1438. [Google Scholar] [CrossRef]
- Li, J.; Liu, Y.; Gao, Y.; Zhong, L.; Zou, Q.; Lai, X. Preparation and properties of calcium citrate nanosheets for bone graft substitute. Bioengineered 2016, 7, 376–381. [Google Scholar] [CrossRef]
- Li, J.; Li, M.; Liu, Y.; Kang, X.; Wang, F.; Tong, L.; Gao, Y.; Yu, C.; Chen, M.; Gong, J. Rapidly evaluating the caking tendency of sugar alcohols by developing a crystal bridge growth model: A case study of xylitol. Food Chem. 2023, 406, 135051. [Google Scholar] [PubMed]
- Chen, M.; Yu, C.; Yao, M.; Liu, X.; Xu, S.; Tang, W.; Dong, W.; Gong, J. The time and location dependent prediction of crystal caking by a modified crystal bridge growth model and DEM simulation considering particle size and shape. Chem. Eng. Sci. 2020, 214, 115419. [Google Scholar] [CrossRef]
- Li, J.; Guo, S.; Liu, Y.; Yan, H.; Li, M.; Tong, L.; Gao, Y.; Chen, M.; Wu, S.; Gong, J. Model-based design for water-soluble crystals with anti-caking function by the feedback between caking prediction and crystallization control. Chem. Eng. Sci. 2023, 271, 118568. [Google Scholar] [CrossRef]
- France, T.C.; Barone, G.; O’Regan, J.; Kelly, A.L.; O’Mahony, J.A. Physical and colloidal stability of conventional and micronised calcium citrate ingredient powders in the formulation of infant nutritional products. Colloids Surf. B Biointerfaces 2020, 194, 111125. [Google Scholar] [CrossRef] [PubMed]
- Wen, L.; Liu, D.; Hu, J.; Liu, X.; Regenstein, J.M.; Zhou, P. Variation of insoluble calcium salts in protein adsorption and suspension stability when dispersed in sodium caseinate solutions. Food Hydrocoll. 2016, 52, 311–316. [Google Scholar]
- Werner, T.; Kolisek, M.; Vormann, J.; Pilchova, I.; Grendar, M.; Struharnanska, E.; Cibulka, M. Assessment of bioavailability of Mg from Mg citrate and Mg oxide by measuring urinary excretion in Mg-saturated subjects. Magnes. Res. 2019, 32, 63–71. [Google Scholar] [PubMed]
- Rzymski, P.; Pischel, I.; Conrad, F.; Zwingers, T.; Rzymski, P.; Opala, T. The bioavailability of calcium in the form of pyruvate, carbonate, citrate-malate in healthy postmenopausal women. Eur. Food Res. Technol. = Z. Lebensm. Unters. Forsch. A 2016, 242, 45–50. [Google Scholar] [CrossRef] [Green Version]
- Fujita, T. Calcium bioavailability from heated oyster shell-seaweed calcium (active absorbable algae calcium) as assessed by urinary calcium excretion. J. Bone Miner. Metab. 1996, 14, 31–34. [Google Scholar] [CrossRef]
- Esuoso, K.O. Influence of nitrilotriacetic acid and 8-hydroxyquinoline on the production of citric acid from molasses using Aspergillus niger. J. Ferment. Bioeng. 1994, 77, 693–695. [Google Scholar] [CrossRef]
- Ye, T. Principles and Applications of Chemical Crystallization Processes; Beijing University of Technology Press: Beijing, China, 2006; pp. 121–188. [Google Scholar]
- Ji, Z. Inorganic Salt Continuous Crystallization Device. Patent CN203816265U, 10 September 2014. [Google Scholar]
- Jiang, X.; Xiang, G.; Xiang, G. Multifunctional Multi-Effect Automatic Continuous Evaporation Crystallization Process and Crystallization Equipment. Patent CN101306260B, 22 February 2012. [Google Scholar]
- Li, J.; Zhang, H.; Hu, H. The Invention Provides a Feed Grade Calcium Citrate Premixed Production Packaging. Patent CN215540031U, 18 January 2022. [Google Scholar]
- Gong, J.; Zhang, D.; Zhang, M.; Hou, B.; Yin, Q.; Wang, J. A Multi-Stage Vacuum Adiabatic Flash Continuous Crystallization Method and Equipment. Patent CN105435482B, 26 June 2018. [Google Scholar]
- Wng, Q. DTB evaporative crystallizer technology design solution and application. China Chem. Equip. 2017, 19, 21–23+20. [Google Scholar]
- Feng, K.; Pan, B.; Ge, J.; Yuan, C.; Liu, L. A Kind of Potassium Citrate Processing Equipment. Patent CN215429025U, 7 January 2022. [Google Scholar]
- Zhao, S.; Xu, K.; Luo, G. A System and Method for the Production of Potassium Citrate. Patent CN113372213A, 10 September 2021. [Google Scholar]
- Wang, T.; Lu, H.; Wang, J.; Xiao, Y.; Zhou, Y.; Bao, Y.; Hao, H. Recent progress of continuous crystallization. J. Ind. Eng. Chem. 2017, 54, 14–29. [Google Scholar] [CrossRef]
- Ma, Y.; Wu, S.; Macaringue, E.G.J.; Zhang, T.; Gong, J.; Wang, J. Recent progress in continuous crystallization of pharmaceutical products: Precise preparation and control. Org. Process Res. Dev. 2020, 24, 1785–1801. [Google Scholar] [CrossRef]
- McGinty, J.; Yazdanpanah, N.; Price, C.; Ter Horst, J.H.; Sefcik, J. Nucleation and crystal growth in continuous crystallization. In The Handbook of Continuous Crystallization; Royal Society of Chemistry: London, UK, 2020. [Google Scholar]
- Cai, Z.; Feng, J.; Wang, Z.; Ye, W.; Wu, Y.; Chen, S. Method for Producing Potassium Citrate by Continuous Concentration and Crystallization and Apparatus for Realizing the Method. Patent CN101050173A, 5 April 2006. [Google Scholar]
- Li, C.; An, F.; Kong, Y.; Liu, J.; Ren, S.; Zhou, H. A Kind of Production Method of Sodium Citrate. Patent CN103044239A, 17 December 2012. [Google Scholar]
- Li, J.; Wang, J.; Li, J. A Kind of Technology of On-Line Continuous Production of Calcium Citrate. Patent CN114262265A, 17 December 2021. [Google Scholar]
- Orehek, J.; Teslic, D.; Likozar, B. Continuous crystallization processes in pharmaceutical manufacturing: A review. Org. Process Res. Dev. 2020, 25, 16–42. [Google Scholar] [CrossRef]
| Name | Crystallization Method | Raw Materials | Temperature | pH |
|---|---|---|---|---|
| Sodium citrate | Evaporation crystallization, cooling crystallization | Citric acid, sodium source (sodium hydroxide and sodium carbonate) | Approx 85 °C | 7–9 |
| Potassium citrate | Evaporation crystallization, cooling crystallization | Citric acid, potassium source (potassium bicarbonate and potassium hydroxide) | 70–80 °C | 5.5–9.5 |
| Calcium citrate | Reaction crystallization | Citric acid, calcium sources (chemical calcium sources such as calcium carbonate, calcium hydroxide, calcium chloride, calcium nitrate, and natural biological calcium sources such as shells, egg shells, shrimp head shells, cow bone, etc.) | No more than 110 °C | 3–5 |
| Magnesium citrate | Reaction crystallization | Citric acid, magnesium source (magnesium oxide, magnesium hydroxide, magnesium carbonate, and magnesium bicarbonate) | 60–90 °C | 5–8 |
| Zinc citrate | Reaction crystallization | Citric acid, zinc source (zinc oxide, zinc carbonate, zinc nitrate) | 40–80 °C | 4–7 |
| Lead citrate | Cooling crystallization | Citric acid, sodium citrate, lead source (spent lead paste, lead sulfate) | 35–95 °C | <6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, Y.; Qin, X.; Yan, H.; Li, J.; Li, C.; Lian, M.; Wei, X.; Shen, R.; Chen, M.; Li, K.; et al. Research Progress in the Industrial Crystallization of Citrate—A Review. Crystals 2023, 13, 1186. https://doi.org/10.3390/cryst13081186
Ma Y, Qin X, Yan H, Li J, Li C, Lian M, Wei X, Shen R, Chen M, Li K, et al. Research Progress in the Industrial Crystallization of Citrate—A Review. Crystals. 2023; 13(8):1186. https://doi.org/10.3390/cryst13081186
Chicago/Turabian StyleMa, Yanyu, Xueyou Qin, Hui Yan, Junjie Li, Chengwei Li, Mingke Lian, Xuemei Wei, Runpu Shen, Mingyang Chen, Kangli Li, and et al. 2023. "Research Progress in the Industrial Crystallization of Citrate—A Review" Crystals 13, no. 8: 1186. https://doi.org/10.3390/cryst13081186
APA StyleMa, Y., Qin, X., Yan, H., Li, J., Li, C., Lian, M., Wei, X., Shen, R., Chen, M., Li, K., & Gong, J. (2023). Research Progress in the Industrial Crystallization of Citrate—A Review. Crystals, 13(8), 1186. https://doi.org/10.3390/cryst13081186








