Research Progress on the Molecular Mechanism of Polymorph Nucleation in Solution: A Perspective from Research Mentality and Technique
Abstract
:1. Introduction
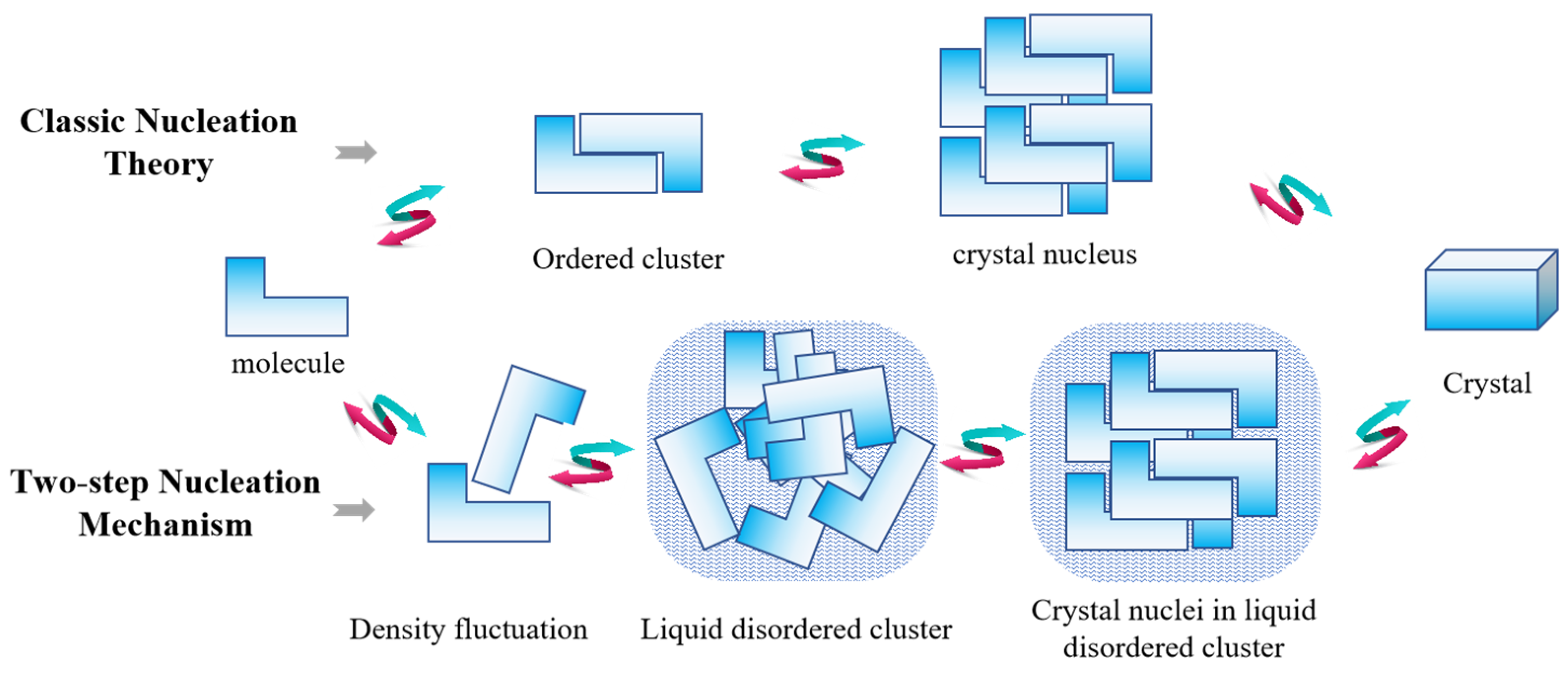
2. Development of Crystal Nucleation Theory and Its Defects
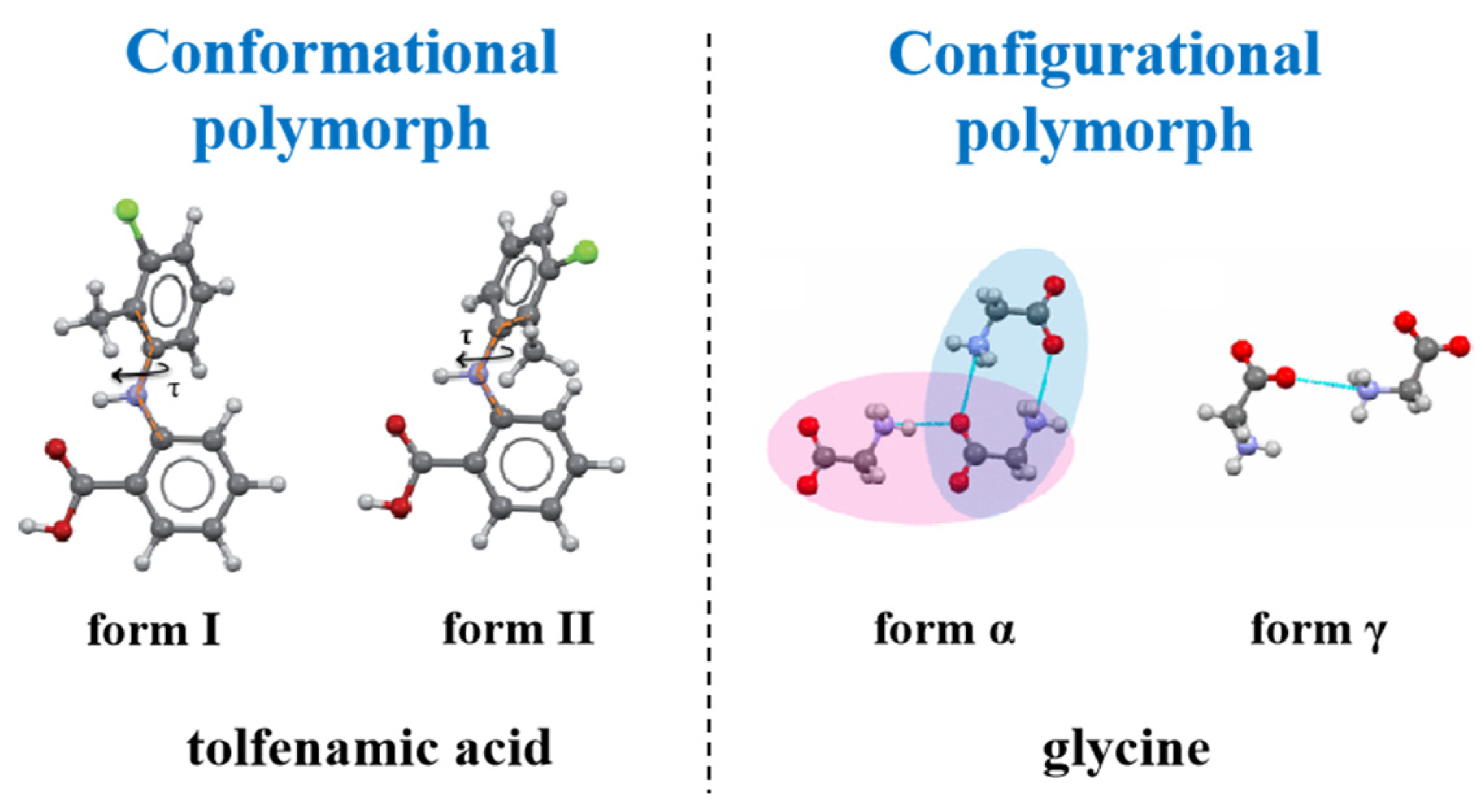
3. Categorization of Organic Small Molecule Polymorphs
4. Research Mentality and Technique of Molecular Pathways of Polymorphic Nucleation in Solution
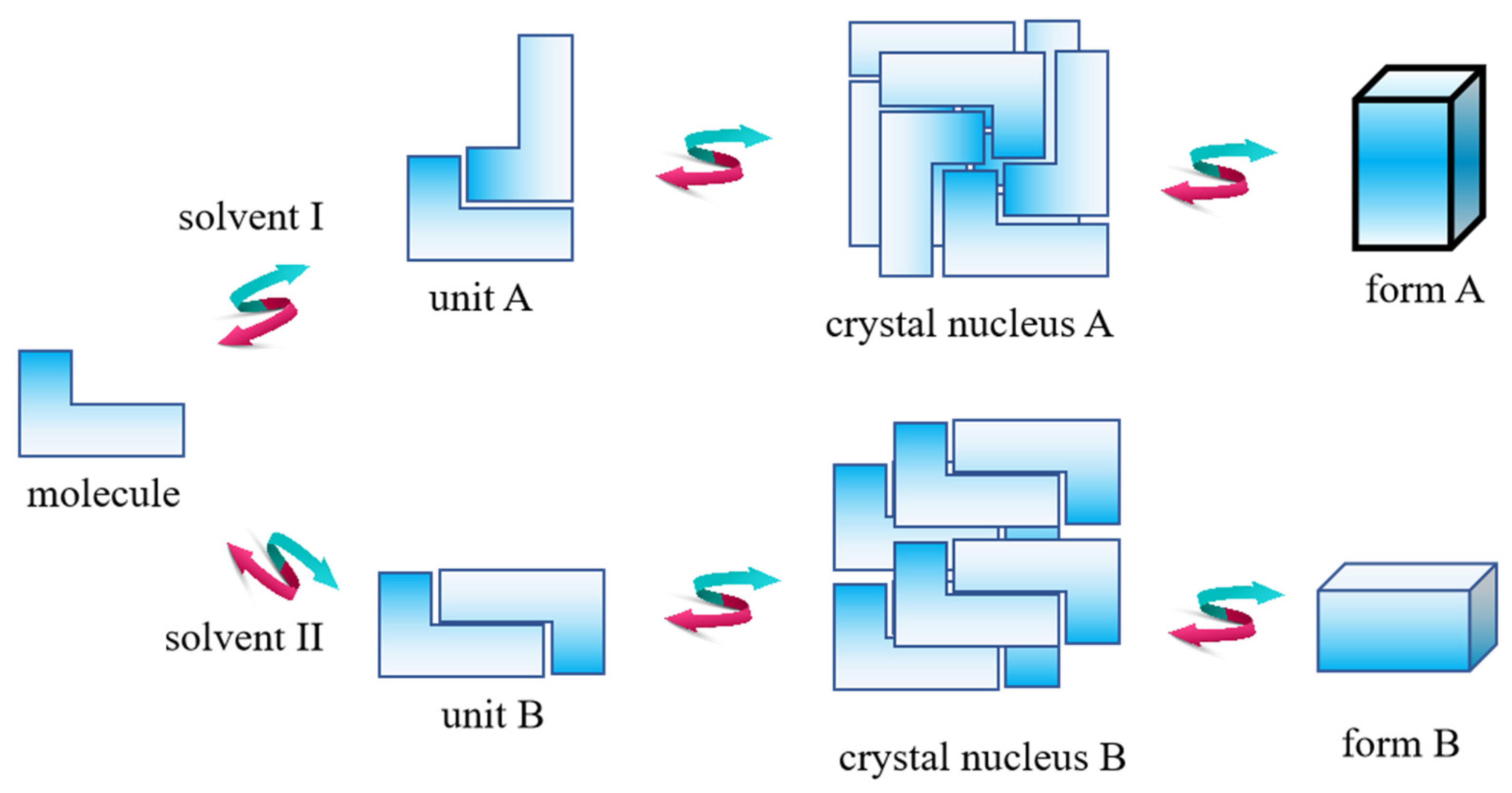
4.1. Research on Nucleation Pathways Based on Configurational Polymorphs
4.2. Research on Nucleation Pathways Based on Conformational Polymorphs
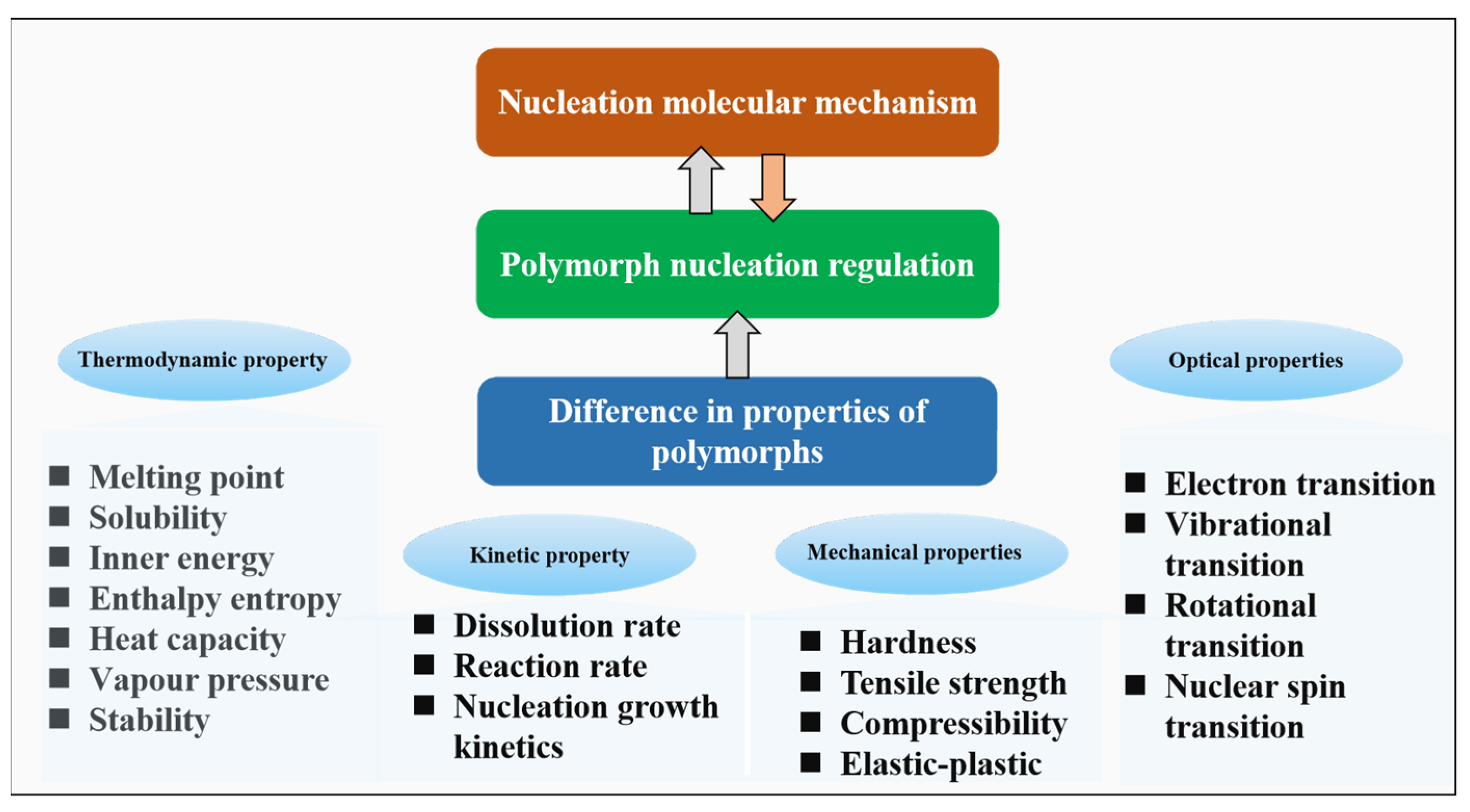
4.3. The Regulation of Polymorphic Nucleation Based on Molecular Mechanisms
4.4. The Progress and Application of Relevant Research Tools
4.4.1. Experimental Analysis Methods
4.4.2. Computational Simulation Methods
5. Conclusions and Prospects
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Mitscherlich, E. On the Relationship Between Crystalline Form and Chemical Composition. I. Note on Arsenates and Phosphates. Ann. Chim. Phys. 1822, 19, 350–419. [Google Scholar]
- Liebig, J.; Wohler, F. Untersuchungen iiber das Radical der Benzoesaure. Pharm. Anntiinflam. 1832, 249, 514. [Google Scholar]
- David, W.; Shankland, K.; Pulham, C.; Blagden, N.; Davey, R.; Song, M. Polymorphism in Benzamide. Angew. Chem. 2005, 117, 7194–7197. [Google Scholar] [CrossRef]
- McCrone, W.C. Polymorphism. Phys. Chem. Solid Stat. 1965, 2, 725–767. [Google Scholar]
- Kraus, E.H. Chemische Krystallographie. Science 1907, 25, 143–144. [Google Scholar] [CrossRef]
- Cruz-Cabeza, A.J.; Bernstein, J. Conformational Polymorphism. Chem. Rev. 2014, 114, 2170–2191. [Google Scholar] [CrossRef]
- Thomas, S.P.; Spackman, M.A. The Polymorphs of ROY: A Computational Study of Lattice Energies and Conformational Energy Differences. Aust. J. Chem. 2018, 71, 279. [Google Scholar] [CrossRef] [Green Version]
- Hollingsworth, M.D. Crystal Engineering: From Structure to Function. Science 2002, 295, 2410–2413. [Google Scholar] [CrossRef]
- Thakuria, R.; Thakur, T.S. Crystal Polymorphism in Pharmaceutical Science. Compr. Supramol. Chem. II 2017, 5, 283–309. [Google Scholar] [CrossRef]
- Chemburkar, S.R.; Bauer, J.; Deming, K.; Spiwek, H.; Patel, K.; Morris, J.; Henry, R.; Spanton, S.; Dziki, W.; Porter, W. Dealing with the Impact of Ritonavir Polymorphs on the Late Stages of Bulk Drug Process Development. Org. Process Res. Dev. 2000, 4, 413–417. [Google Scholar] [CrossRef]
- Peschar, R.; Pop, M.M.; De Ridder, D.J.; van Mechelen, J.B.; Driessen, R.A.; Schenk, H. Crystal Structures of 1, 3-distearoyl-2-oleoylglycerol and Cocoa Butter in the β (V) Phase Reveal the Driving Force Behind the Occurrence of Fat Bloom on Chocolate. J. Phys. Chem. B 2004, 108, 15450–15453. [Google Scholar] [CrossRef]
- Desiraju, G.R. Crystal Engineering: From Molecule to Crystal. J. Am. Chem. Soc. 2013, 135, 9952–9967. [Google Scholar] [CrossRef] [PubMed]
- Naumov, P.; Chizhik, S.; Panda, M.K.; Nath, N.K.; Boldyreva, E. Mechanically Responsive Molecular Crystals. Chem. Rev. 2015, 115, 12440–12490. [Google Scholar] [CrossRef]
- Mishra, M.K.; Sanphui, P.; Ramamurty, U.; Desiraju, G.R. Solubility-Hardness Correlation in Molecular Crystals: Curcumin and Sulfathiazole Polymorphs. Cryst. Growth Des. 2014, 14, 3054–3061. [Google Scholar] [CrossRef]
- Wang, Y.; Hu, X.; Ge, L.; Liu, Z.; Xu, M.; Peng, Y.; Li, B.; Yang, Y.; Li, S.; Xie, X.; et al. Research Progress in Capping Diamond Growth on GaN HEMT: A Review. Crystals 2023, 13, 500. [Google Scholar] [CrossRef]
- Llinas, A.; Goodman, J.M. Polymorph Control: Past, Present and Future. Drug Discov. Today 2008, 13, 198–210. [Google Scholar] [CrossRef] [PubMed]
- Mullin, J.W. Crystallization; Elsevier: Amsterdam, The Netherlands, 2001. [Google Scholar]
- Davey, R.J.; Schroeder, S.L.; ter Horst, J.H. Nucleation of Organic Crystals—A Molecular Perspective. Angew. Chem. 2013, 52, 2166–2179. [Google Scholar] [CrossRef]
- Erdemir, D.; Lee, A.Y.; Myerson, A.S. Nucleation of Crystals from Solution: Classical and Two-Step Models. Acc. Chem. Res. 2009, 42, 621–629. [Google Scholar] [CrossRef]
- Tang, W. Study on Molecular Mechanism of Organic Crystal Nucleation. Ph.D. Thesis, Tianjin University, Tianjin, China, 2017. [Google Scholar]
- Gebauer, D.; Cölfen, H. Prenucleation Clusters and Non-Classical Nucleation. Nano Today 2011, 6, 564–584. [Google Scholar] [CrossRef] [Green Version]
- Pons Siepermann, C.A.; Myerson, A.S. Inhibition of Nucleation Using a Dilute, Weakly Hydrogen-Bonding Molecular Additive. Cryst. Growth Des. 2018, 18, 3584–3595. [Google Scholar] [CrossRef]
- Betts, F.; Posner, A. An X-ray Radial Distribution Study of Amorphous Calcium Phosphate. Mater. Res. Bull. 1974, 9, 353–360. [Google Scholar] [CrossRef]
- Onuma, K.; Ito, A. Cluster Growth Model for Hydroxyapatite. Chem. Mater. 1998, 10, 3346–3351. [Google Scholar] [CrossRef]
- Gebauer, D.; Völkel, A.; Cölfen, H. Stable Prenucleation Calcium Carbonate Clusters. Science 2008, 322, 1819–1822. [Google Scholar] [CrossRef] [Green Version]
- Gebauer, D.; Kellermeier, M.; Gale, J.D.; Bergström, L.; Cölfen, H. Pre-nucleation Clusters as Solute Precursors in Crystallisation. Chem. Soc. Rev. 2014, 43, 2348–2371. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wallace, A.F.; Hedges, L.O.; Fernandez-Martinez, A.; Raiteri, P.; Gale, J.D.; Waychunas, G.A.; Whitelam, S.; Banfield, J.F.; De Yoreo, J.J. Microscopic Evidence for Liquid-Liquid Separation in Supersaturated CaCO3 Solutions. Science 2013, 341, 885–889. [Google Scholar] [CrossRef] [Green Version]
- Vekilov, P.G. Dense Liquid Precursor for the Nucleation of Ordered Solid Phases from Solution. Cryst. Growth Des. 2004, 4, 671–685. [Google Scholar] [CrossRef]
- Kellermeier, M.; Rosenberg, R.; Moise, A.; Anders, U.; Przybylski, M.; Cölfen, H. Amino Acids Form Prenucleation Clusters: ESI-MS as A Fast Detection Method in Comparison to Analytical Ultracentrifugation. Faraday Discuss. 2012, 159, 23–45. [Google Scholar] [CrossRef] [Green Version]
- Zhou, J.; Zhou, Y.; Tang, W. Molecular Mechanism of Organic Crystal Nucleation: A Perspective of Solution Chemistry and Polymorphism. Crystals 2022, 12, 980. [Google Scholar] [CrossRef]
- Vekilov, P.G. Nucleation. Cryst. Growth Des. 2010, 10, 5007–5019. [Google Scholar] [CrossRef]
- Myerson, A.S.; Trout, B.L. Nucleation from Solution. Science 2013, 341, 855–856. [Google Scholar] [CrossRef]
- Davey, R.; Allen, K.; Blagden, N.; Cross, W.; Lieberman, H.; Quayle, M.; Righini, S.; Seton, L.; Tiddy, G. Crystal Engineering–Nucleation, the Key Step. CrystEngComm 2002, 4, 257–264. [Google Scholar] [CrossRef]
- Aitipamula, S.; Banerjee, R.; Bansal, A.K.; Biradha, K.; Cheney, M.L.; Choudhury, A.R.; Desiraju, G.R.; Dikundwar, A.G.; Dubey, R.; Duggirala, N. Polymorphs, Salts, and Cocrystals: What’s in a Name? Cryst. Growth Des. 2012, 12, 2147–2152. [Google Scholar] [CrossRef]
- Nangia, A. Conformational Polymorphism in Organic Crystals. Acc. Chem. Res. 2008, 41, 595–604. [Google Scholar] [CrossRef]
- Kitamura, M. Strategy for Control of Crystallization of Polymorphs. CrystEngComm 2009, 11, 949–964. [Google Scholar] [CrossRef]
- Chai, Y.; Wang, L.; Bao, Y.; Teng, R.; Liu, Y.; Xie, C. Investigating the Solvent Effect on Crystal Nucleation of Etoricoxib. Cryst. Growth Des. 2019, 19, 1660–1667. [Google Scholar] [CrossRef]
- Lobato Silva, L.F.; Paschoal, W.; Pinheiro, G.S.; da Silva Filho, J.G.; Freire, P.T.C.; de Sousa, F.F.; Moreira, S.G.C. Understanding the Effect of Solvent Polarity on the Polymorphism of Octadecanoic Acid through Spectroscopic Techniques and DFT Calculations. CrystEngComm 2019, 21, 297–309. [Google Scholar] [CrossRef]
- Chadwick, K.; Davey, R.J.; Dent, G.; Pritchard, R.G.; Hunter, C.A.; Musumeci, D. Cocrystallization: A Solution Chemistry Perspective and the Case of Benzophenone and Diphenylamine. Cryst. Growth Des. 2009, 9, 1990–1999. [Google Scholar] [CrossRef]
- Parveen, S.; Davey, R.J.; Dent, G.; Pritchard, R.G. Linking Solution Chemistry to Crystal Nucleation: The Case of Tetrolic Acid. Chem. Commun. 2005, 12, 1531–1533. [Google Scholar] [CrossRef]
- Steendam, R.R.E.; Khandavilli, U.B.R.; Keshavarz, L.; Frawley, P.J. Solution versus Crystal Hydration: The Case of γ-Amino Acid Pregabalin. Cryst. Growth Des. 2019, 8, 4483–4488. [Google Scholar] [CrossRef]
- Fucke, K.; McIntyre, G.J.; Lemee-Cailleau, M.H.; Wilkinson, C.; Edwards, A.J.; Howard, J.A.; Steed, J.W. Insights into the Crystallisation Process from Anhydrous, Hydrated and Solvated Crystal Forms of Diatrizoic Acid. Chemistry 2015, 21, 1036–1047. [Google Scholar] [CrossRef] [Green Version]
- Mukherjee, A.; Dixit, K.; Sarma, S.P.; Desiraju, G.R. Aniline–phenol recognition: From solution through supramolecular synthons to cocrystals. IUCrJ 2014, 1, 228–239. [Google Scholar] [CrossRef] [Green Version]
- Hansen, T.B.; Taris, A.; Rong, B.-G.; Grosso, M.; Qu, H. Polymorphic Behavior of Isonicotinamide in Cooling Crystallization from Various Solvents. J. Cryst. Growth 2016, 450, 81–90. [Google Scholar] [CrossRef]
- Zhang, T.; Liu, Y.; Du, S.; Wu, S.; Han, D.; Liu, S.; Gong, J. Polymorph Control by Investigating the Effects of Solvent and Supersaturation on Clopidogrel Hydrogen Sulfate in Reactive Crystallization. Cryst. Growth Des. 2017, 17, 6123–6131. [Google Scholar] [CrossRef]
- Liu, S.; Xu, S.; Tang, W.; Yu, B.; Hou, B.; Gong, J. Revealing the Roles of Solvation in D-mannitol’s Polymorphic Nucleation. CrystEngComm 2018, 20, 7435–7445. [Google Scholar] [CrossRef]
- Lohani, S.; Nesmelova, I.V.; Suryanarayanan, R.; Grant, D.J.W. Spectroscopic Characterization of Molecular Aggregates in Solutions: Impact on Crystallization of Indomethacin Polymorphs from Acetonitrile and Ethanol. Cryst. Growth Des. 2011, 11, 2368–2378. [Google Scholar] [CrossRef]
- Liu, Y.; Gabriele, B.; Davey, R.J.; Cruz-Cabeza, A.J. Concerning Elusive Crystal Forms: The Case of Paracetamol. J. Am. Chem. Soc. 2020, 142, 6682–6689. [Google Scholar] [CrossRef]
- Black, J.F.B.; Cruz-Cabeza, A.J.; Davey, R.J.; Willacy, R.D.; Yeoh, A. The Kinetic Story of Tailor-made Additives in Polymorphic Systems: New Data and Molecular Insights for p-Aminobenzoic Acid. Cryst. Growth Des. 2018, 18, 7518–7525. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.; Shi, P.; Yang, Y.; Sun, P.; Wang, Y.; Xu, S.; Gong, J. Tuning Crystallization and Stability of the Metastable Polymorph of DL-methionine by A Structurally Similar Additive. CrystEngComm 2019, 21, 3731–3739. [Google Scholar] [CrossRef]
- Zhang, K.; Xu, S.; Liu, S.; Tang, W.; Fu, X.; Gong, J. Novel Strategy to Control Polymorph Nucleation of Gamma Pyrazinamide by Preferred Intermolecular Interactions during Heterogeneous Nucleation. Cryst. Growth Des. 2018, 18, 4874–4879. [Google Scholar] [CrossRef]
- Diao, Y.; Myerson, A.S.; Hatton, T.A.; Trout, B.L. Surface Design for Controlled Crystallization: The Role of Surface Chemistry and Nanoscale Pores in Heterogeneous Nucleation. Langmuir 2011, 27, 5324–5334. [Google Scholar] [CrossRef] [PubMed]
- Garetz, B.A.; Matic, J.; Myerson, A.S. Polarization Switching of Crystal Structure in the Nonphotochemical Light-Induced Nucleation of Supersaturated Aqueous Glycine Solutions. Phys. Rev. Lett. 2002, 89, 175501. [Google Scholar] [CrossRef] [PubMed]
- Davey, R.J.; Blagden, N.; Righini, S.; Alison, H.; Quayle, M.J.; Fuller, S. Crystal Polymorphism as a Probe for Molecular Self-assembly During Nucleation from Solutions the Case of 2,6-Dihydroxybenzoic Acid. Cryst. Growth Des. 2001, 1, 59–65. [Google Scholar] [CrossRef]
- Davey, R.J.; Dent, G.; Mughal, R.K.; Parveen, S. Concerning the Relationship between Structural and Growth Synthons in Crystal Nucleation: Solution and Crystal Chemistry of Carboxylic Acids As Revealed through IR Spectroscopy. Cryst. Growth Des. 2006, 6, 1788–1796. [Google Scholar] [CrossRef]
- Kulkarni, S.A.; McGarrity, E.S.; Meekes, H.; ter Horst, J.H. Isonicotinamide Self-association: The Link between Solvent and Polymorph Nucleation. Chem. Commun. 2012, 48, 4983–4985. [Google Scholar] [CrossRef] [Green Version]
- Hamad, S.; Moon, C.; Catlow, C.R.A.; Hulme, A.T.; Price, S.L. Kinetic Insights into the Role of the Solvent in the Polymorphism of 5-Fluorouracil from Molecular Dynamics Simulations. J. Phys. Chem. B 2006, 110, 3323–3329. [Google Scholar] [CrossRef]
- Spitaleri, A.; Hunter, C.A.; McCabe, J.F.; Packer, M.J.; Cockroft, S.L. A 1H NMR Study of Crystal Nucleation in Solution. CrystEngComm 2004, 6, 489–493. [Google Scholar] [CrossRef]
- Hunter, C.A.; McCabe, J.F.; Spitaleri, A. Solvent Effects of the Structures of Prenucleation Aggregates of Carbamazepine. CrystEngComm 2012, 14, 7115–7117. [Google Scholar] [CrossRef]
- Chiarella, R.A.; Gillon, A.L.; Burton, R.C.; Davey, R.J.; Sadiq, G.; Auffret, A.; Cioffi, M.; Hunter, C.A. The Nucleation of Inosine: The Impact of Solution Chemistry on the Appearance of Polymorphic and Hydrated Crystal Forms. Faraday Discuss. 2007, 136, 179–193. [Google Scholar] [CrossRef]
- Ouyang, J.; Chen, J.; Rosbottom, I.; Chen, W.; Guo, M.; Heng, J.Y. Supersaturation and solvent dependent nucleation of carbamazepine polymorphs during rapid cooling crystallization. CrystEngComm 2021, 23, 813–823. [Google Scholar] [CrossRef]
- Wang, M.; Wang, J.; Wang, N.; Song, W.; Huang, X.; Wang, T.; Hao, H. Molecular Assembly and Nucleation Kinetics during Nucleation of 3, 5-Dinitrobenzoic Acid. J. Phys. Chem. A 2023, 127, 3862–3872. [Google Scholar] [CrossRef]
- Li, X.; Wang, N.; Huang, Y.; Xing, J.; Huang, X.; Ferguson, S.; Wang, T.; Zhou, L.; Hao, H. The role of solute conformation, solvent–solute and solute–solute interactions in crystal nucleation. AIChE J. 2023, 69, e18144. [Google Scholar] [CrossRef]
- Tang, W.; Mo, H.; Zhang, M.; Parkin, S.; Gong, J.; Wang, J.; Li, T. Persistent Self-Association of Solute Molecules in Solution. J. Phys. Chem. B 2017, 121, 10118–10124. [Google Scholar] [CrossRef]
- Du, W.; Cruz-Cabeza, A.J.; Woutersen, S.; Davey, R.J.; Yin, Q. Can the Study of Self-assembly in Solution Lead to A Good Model for the Nucleation Pathway? The Case of Tolfenamic Acid. Chem. Sci. 2015, 6, 3515–3524. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mattei, A.; Li, T. Polymorph Formation and Nucleation Mechanism of Tolfenamic Acid in Solution: An Investigation of Pre-nucleation Solute Association. Pharm. Res. 2012, 29, 460–470. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Tang, W.; Du, S.; Xu, S.; Shi, P.; Wu, S.; Gong, J. Surprising Effect of Carbon Chain Length on Inducing Ability of Additives: Elusive Form-II of γ-Aminobutyric Acid (GABA) Induced by Sodium Carboxylate Additives. Cryst. Growth Des. 2019, 19, 3825–3833. [Google Scholar] [CrossRef]
- Ryu, Y.; Aya, T.; Isao, A.; Hyuma, M.; Kentaro, Y.; Hiroyuki, K. Solvent-Dependent Conformational Switching of N-Phenylhydroxamic Acid and Its Application in Crystal Engineering. Cryst. Growth Des. 2006, 6, 2007–2010. [Google Scholar]
- Ischenko, V.; Englert, U.; Jansen, M. Conformational Dimorphism of 1,1,3,3,5,5-Hexachloro-1,3,5-Trigermacyclohexane: Solvent-induced Crystallization of a Metastable Polymorph Containing Boat-shaped Molecules. Chem. Eur. J. 2005, 11, 1375–1383. [Google Scholar] [CrossRef]
- Oparin, R.D.; Vaksler, Y.A.; Krestyaninov, M.A.; Idrissi, A.; Shishkina, S.V.; Kiselev, M.G. Polymorphism and Conformations of Mefenamic Acid in Supercritical Carbon Dioxide. J. Supercrit. Fluid 2019, 152, 104547. [Google Scholar] [CrossRef]
- Belov, K.; Dyshin, A.; Krestyaninov, M.; Efimov, S.; Khodov, I.; Kiselev, M. Conformational Preferences of Tolfenamic Acid in DMSO-CO2 Solvent System by 2D NOESY. J. Mol. Liq. 2022, 367, 120481. [Google Scholar] [CrossRef]
- Khodov, I.A.; Belov, K.V.; Krestyaninov, M.A.; Dyshin, A.A.; Kiselev, M.G. Investigation of the Spatial Structure of Flufenamic Acid in Supercritical Carbon Dioxide Media via 2D NOESY. Materials 2023, 16, 1524. [Google Scholar] [CrossRef]
- Li, X.; Wang, N.; Yang, J.; Huang, Y.; Ji, X.; Huang, X.; Wang, T.; Wang, H.; Hao, H. Molecular Conformational Evolution Mechanism during Nucleation of Crystals in solution. IUCrJ 2020, 7, 542–556. [Google Scholar] [CrossRef] [PubMed]
- Back, K.R.; Davey, R.J.; Grecu, T.; Hunter, C.A.; Taylor, L.S. Molecular Conformation and Crystallization: The Case of Ethenzamide. Cryst. Growth Des. 2012, 12, 6110–6117. [Google Scholar] [CrossRef]
- Shi, P.; Xu, S.; Du, S.; Rohani, S.; Liu, S.; Tang, W.; Jia, L.; Wang, J.; Gong, J. Insight into Solvent-Dependent Conformational Polymorph Selectivity: The Case of Undecanedioic Acid. Cryst. Growth Des. 2018, 18, 5947–5956. [Google Scholar] [CrossRef]
- Zeng, Q.; Mukherjee, A.; Muller, P.; Rogers, R.D.; Myerson, A.S. Exploring the Role of Ionic Liquids to Tune the Polymorphic Outcome of Organic Compounds. Chem. Sci. 2018, 9, 1510–1520. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caridi, A.; Kulkarni, S.A.; Di Profio, G.; Curcio, E.; ter Horst, J.H. Template-Induced Nucleation of Isonicotinamide Polymorphs. Cryst. Growth Des. 2014, 14, 1135–1141. [Google Scholar] [CrossRef]
- Kulkarni, S.A.; Weber, C.C.; Myerson, A.S.; ter Horst, J.H. Self-association during Heterogeneous Nucleation onto Well-defined Templates. Langmuir 2014, 30, 12368–12375. [Google Scholar] [CrossRef] [Green Version]
- Bora, P.; Saikia, B.; Sarma, B. Oriented Crystallization on Organic Monolayers to Control Concomitant Polymorphism. Chem. Eur. J. 2020, 26, 699–710. [Google Scholar] [CrossRef]
- Abramov, Y.A.; Zell, M.; Krzyzaniak, J.F. Toward a Rational Solvent Selection for Conformational Polymorph Screening. In Chemical Engineering in the Pharmaceutical Industry: Active Pharmaceutical Ingredients; Wiley: Hoboken, NJ, USA, 2019; pp. 519–532. [Google Scholar]
- Shi, P.; Xu, S.; Yang, H.; Wu, S.; Tang, W.; Wang, J.; Gong, J. Use of Additives to Regulate Solute Aggregation and Direct Conformational Polymorph Nucleation of Pimelic Acid. IUCrJ 2021, 8, 161–167. [Google Scholar] [CrossRef] [PubMed]
- Voronin, A.P.; Vasilev, N.A.; Surov, A.O.; Churakov, A.V.; Perlovich, G.L. Exploring the Solid Form Landscape of the Antifungal Drug Isavuconazole: Crystal Structure Analysis, Phase Transformation Behavior and Dissolution Performance. CrystEngComm 2021, 23, 8513–8526. [Google Scholar] [CrossRef]
- Surov, A.O.; Voronin, A.P.; Drozd, K.V.; Volkova, T.V.; Vasilev, N.; Batov, D.; Churakov, A.V.; Perlovich, G.L. Extending the Range of Nitrofurantoin Solid Forms: Effect of Molecular and Crystal Structure on Formation Thermodynamics and Physicochemical Properties. Cryst. Growth Des. 2022, 22, 2569–2586. [Google Scholar]
- Surov, A.O.; Ramazanova, A.G.; Voronin, A.P.; Drozd, K.V.; Churakov, A.V.; Perlovich, G.L. Virtual Screening, Structural Analysis, and Formation Thermodynamics of Carbamazepine Cocrystals. Pharmaceutics 2023, 15, 836. [Google Scholar] [CrossRef]
- Jia, L.; Xu, S.; Liu, S.; Du, S.; Wu, S.; Gong, J. Polymorphs of Daidzein and Intermolecular Interaction Effect on Solution Crystallization. CrystEngComm 2017, 19, 7146–7153. [Google Scholar] [CrossRef]
- Zhu, X.; Hu, C.T.; Yang, J.; Joyce, L.A.; Qiu, M.; Ward, M.D.; Kahr, B. Manipulating Solid Forms of Contact Insecticides for Infectious Disease Prevention. J. Am. Chem. Soc. 2019, 141, 16858–16864. [Google Scholar] [CrossRef] [PubMed]
- Shtukenberg, A.G.; Zhu, Q.; Carter, D.J.; Vogt, L.; Hoja, J.; Schneider, E.; Song, H.; Pokroy, B.; Polishchuk, I.; Tkatchenko, A.; et al. PowdeSr Diffraction and Crystal Structure Prediction Identify Four New Coumarin Polymorphs. Chem. Sci. 2017, 8, 4926–4940. [Google Scholar] [CrossRef] [Green Version]
- Shtukenberg, A.G.; Hu, C.T.; Zhu, Q.; Schmidt, M.U.; Xu, W.; Tan, M.; Kahr, B. The Third Ambient Aspirin Polymorph. Cryst. Growth Des. 2017, 17, 3562–3566. [Google Scholar] [CrossRef]
- Sekharan, S.; Liu, X.; Yang, Z.; Liu, X.; Deng, L.; Ruan, S.; Abramov, Y.; Sun, G.; Li, S.; Zhou, T. Selecting a Stable Solid form of Remdesivir Using Microcrystal Electron Diffraction and Crystal Structure Prediction. RSC Adv. 2021, 11, 17408–17412. [Google Scholar] [CrossRef]
- Li, S.; Tang, W.; Li, M.; Wang, L.; Yang, Y.; Gong, J. Understanding the Role of Citric Acid on the Crystallization Pathways of Calcium Oxalate Hydrates. Cryst. Growth Des. 2019, 19, 3139–3147. [Google Scholar] [CrossRef]
- Li, S.; Tang, W.; Shi, P.; Li, M.; Sun, J.; Gong, J. A New Perspective of Gallic Acid on Calcium Oxalate Nucleation. Cryst. Growth Des. 2020, 20, 3173–3181. [Google Scholar] [CrossRef]
- Yau, S.-T.; Vekilov, P.G. Quasi-planar Nucleus Structure in Apoferritin Crystallization. Nature 2000, 406, 494–497. [Google Scholar] [CrossRef]
- Khamar, D.; Zeglinski, J.; Mealey, D.; Rasmuson, A.C. Investigating the Role of Solvent-solute Interaction in Crystal Nucleation of Salicylic Acid from Organic Solvents. J. Am. Chem. Soc. 2014, 136, 11664–11673. [Google Scholar] [CrossRef]
- Tang, W.; Mo, H.; Zhang, M.; Gong, J.; Wang, J.; Li, T. Glycine’s pH-dependent Polymorphism: A Perspective from Self-association in Solution. Cryst. Growth Des. 2017, 17, 5028–5033. [Google Scholar] [CrossRef]
- Tang, W.; Zhang, M.; Mo, H.; Gong, J.; Wang, J.; Li, T. Higher-Order Self-Assembly of Benzoic Acid in Solution. Cryst. Growth Des. 2017, 17, 5049–5053. [Google Scholar] [CrossRef]
- Pienack, N.; Bensch, W. In-situ Monitoring of the Formation of Crystalline Solids. Angew. Chem. 2011, 50, 2014–2034. [Google Scholar] [CrossRef] [PubMed]
- Nolting, D.; Ottosson, N.; Faubel, M.; Hertel, I.V.; Winter, B. Pseudoequivalent Nitrogen Atoms in Aqueous Imidazole Distinguished by Chemical Shifts in Photoelectron Spectroscopy. J. Am. Chem. Soc. 2008, 130, 8150–8151. [Google Scholar] [CrossRef]
- Burton, R.C.; Ferrari, E.S.; Davey, R.J.; Finney, J.L.; Bowron, D.T. The Relationship between Solution Structure and Crystal Nucleation: A Neutron Scattering Study of Supersaturated Methanolic Solutions of Benzoic Acid. J. Phys. Chem. B 2010, 114, 8807–8816. [Google Scholar] [CrossRef]
- Katsuno, H.; Kimura, Y.; Yamazaki, T.; Takigawa, I. Early Detection of Nucleation Events From Solution in LC-TEM by Machine Learning. Front. Chem. 2022, 10, 818230. [Google Scholar] [CrossRef]
- Kimura, Y.; Katsuno, H.; Yamazaki, T. Possible Embryos and Precursors of Crystalline Nuclei of Calcium Carbonate Observed by Liquid-cell Transmission Electron Microscopy. Faraday Discuss. 2022, 235, 81–94. [Google Scholar] [CrossRef]
- Yan, T.; Xi, D.; Fang, Q.; Zhang, Y.; Wang, J.; Wang, X. High-Pressure Polymorphism in Hydrogen-Bonded Crystals: A Concise Review. Crystals 2022, 12, 739. [Google Scholar] [CrossRef]
- Yang, H.; Yang, Y.; Jia, L.; Tang, W.; Xu, S.; Du, S.; Li, M.; Gong, J. The Phase Transformation and Formation Mechanism of Isostructural Solvates: A Case Study of Azoxystrobin. Cryst. Growth Des. 2019, 19, 1550–1558. [Google Scholar] [CrossRef]
- Di Tommaso, D. The Molecular Self-association of Carboxylic Acids in Solution: Testing the Validity of the Link Hypothesis Using A Quantum Mechanical Continuum Solvation Approach. CrystEngComm 2013, 15, 6564–6577. [Google Scholar] [CrossRef] [Green Version]
- Mattei, A.; Li, T. Nucleation of Conformational Polymorphs: A Computational Study of Tolfenamic Acid by Explicit Solvation. Cryst. Growth Des. 2014, 14, 2709–2713. [Google Scholar] [CrossRef]
- Shi, P.; Ma, Y.; Han, D.; Du, S.; Zhang, T.; Li, Z. Uncovering the Solubility Behavior of Vitamin B6 Hydrochloride in Three Aqueous Binary Solvents by Thermodynamic Analysis and Molecular Dynamic Simulation. J. Mol. Liq. 2019, 283, 584–595. [Google Scholar] [CrossRef]
- Lynch, M.B.; Lawrence, S.E.; Nolan, M. Predicting Nucleation of Isonicotinamide from the Solvent-Solute Interactions of Isonicotinamide in Common Organic Solvents. J. Phys. Chem. A 2018, 122, 3301–3312. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Ji, X.; Cheng, X.; Li, D.; Wang, T.; Huang, X.; Wang, N.; Yin, Q.; Hao, H. Effect of the Solvent on the Morphology of Sulfamerazine Crystals and its Molecular Mechanism. CrystEngComm 2022, 24, 5497–5506. [Google Scholar] [CrossRef]
- Abramov, Y.A.; Li, B.; Chang, C.; Zeng, Q.; Sun, G.; Gobbo, G. Uncertainty Distribution of Crystal Structure Prediction. Cryst. Growth Des. 2021, 21, 5496–5502. [Google Scholar] [CrossRef]
- Abramov, Y.; Sun, G.; Zhou, Y.; Yang, M.; Zeng, Q.; Shen, Z. Solid-form Transition Temperature Prediction from A Virtual Polymorph Screening: A reality check. Cryst. Growth Des. 2019, 19, 7132–7137. [Google Scholar] [CrossRef]
- Abramov, Y.A.; Zhang, P.; Zeng, Q.; Yang, M.; Liu, Y.; Sekharan, S. Computational Insights into Kinetic Hindrance Affecting Crystallization of Stable Forms of Active Pharmaceutical Ingredients. Cryst. Growth Des. 2020, 20, 1512–1525. [Google Scholar] [CrossRef]
- Sun, G.; Jin, Y.; Li, S.; Yang, Z.; Shi, B.; Chang, C.; Abramov, Y.A. Virtual Coformer Screening by Crystal Structure Predictions: Crucial Role of Crystallinity in Pharmaceutical Cocrystallization. J. Phys. Chem. Lett. 2020, 11, 8832–8838. [Google Scholar] [CrossRef]
- Yuan, J.; Liu, X.; Wang, S.; Chang, C.; Zeng, Q.; Song, Z.; Jin, Y.; Zeng, Q.; Sun, G.; Ruan, S. Virtual Coformer Screening by A Combined Machine Learning and Physics-based Approach. CrystEngComm 2021, 23, 6039–6044. [Google Scholar] [CrossRef]
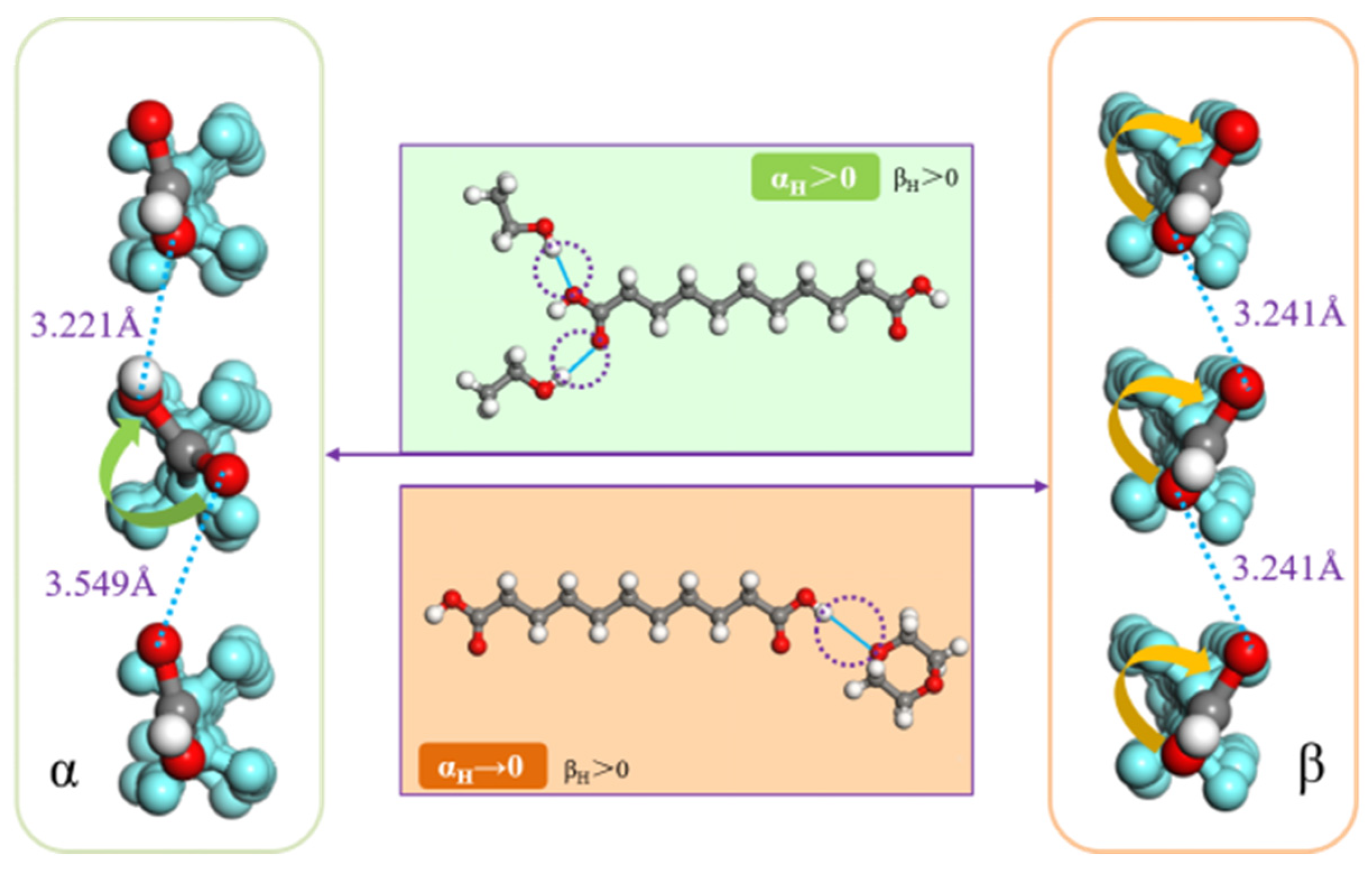
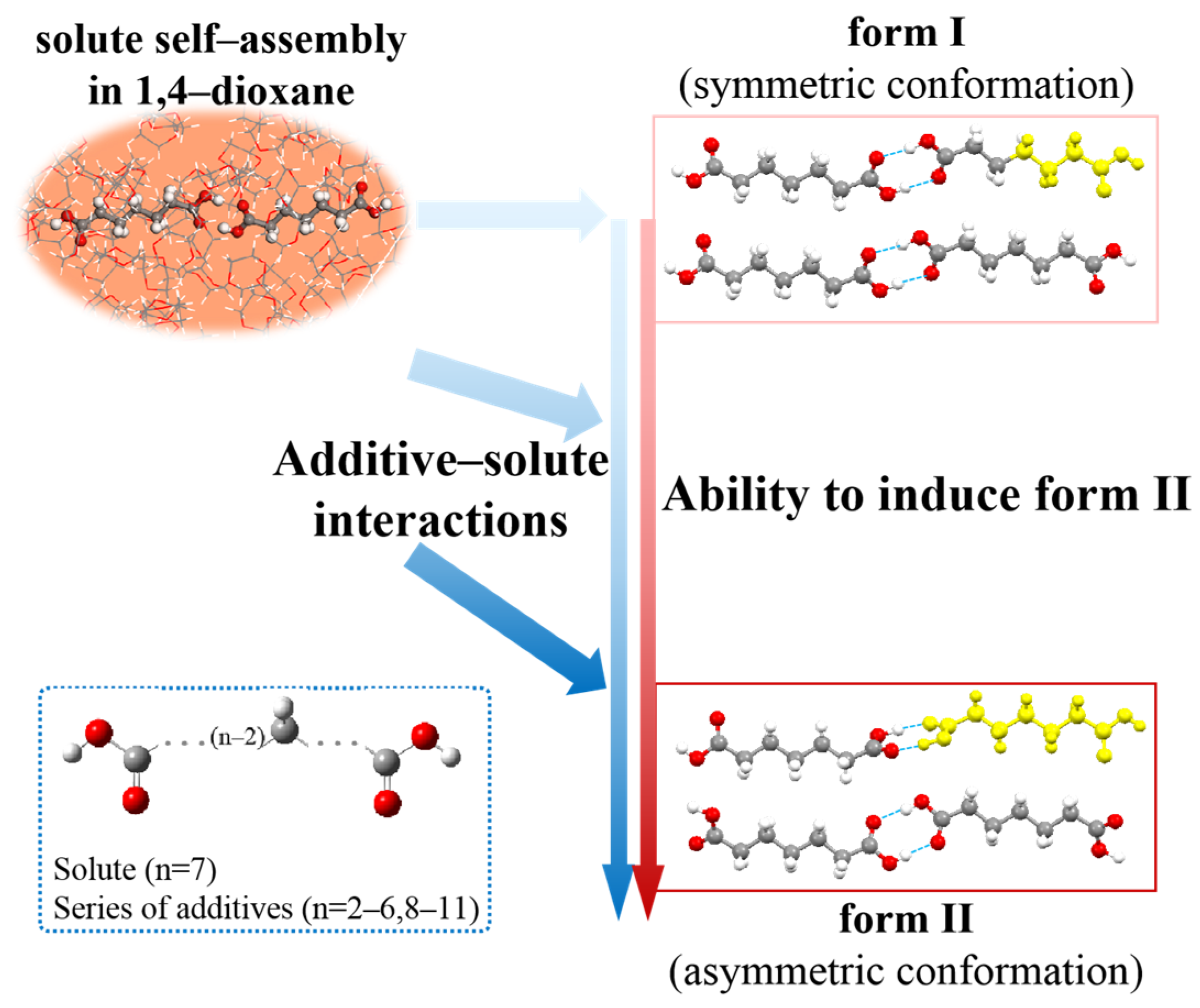
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, P.; Han, Y.; Zhu, Z.; Gong, J. Research Progress on the Molecular Mechanism of Polymorph Nucleation in Solution: A Perspective from Research Mentality and Technique. Crystals 2023, 13, 1206. https://doi.org/10.3390/cryst13081206
Shi P, Han Y, Zhu Z, Gong J. Research Progress on the Molecular Mechanism of Polymorph Nucleation in Solution: A Perspective from Research Mentality and Technique. Crystals. 2023; 13(8):1206. https://doi.org/10.3390/cryst13081206
Chicago/Turabian StyleShi, Peng, Ying Han, Zhenxing Zhu, and Junbo Gong. 2023. "Research Progress on the Molecular Mechanism of Polymorph Nucleation in Solution: A Perspective from Research Mentality and Technique" Crystals 13, no. 8: 1206. https://doi.org/10.3390/cryst13081206



