Influence of Growth Time and Temperature on Optical Characteristics and Surface Wetting in Nano-Crystalline Graphene Deposited by PECVD Directly on Silicon Dioxide
Abstract
:1. Introduction
2. Materials and Methods
2.1. Film Deposition Method
2.2. Surface Characterization
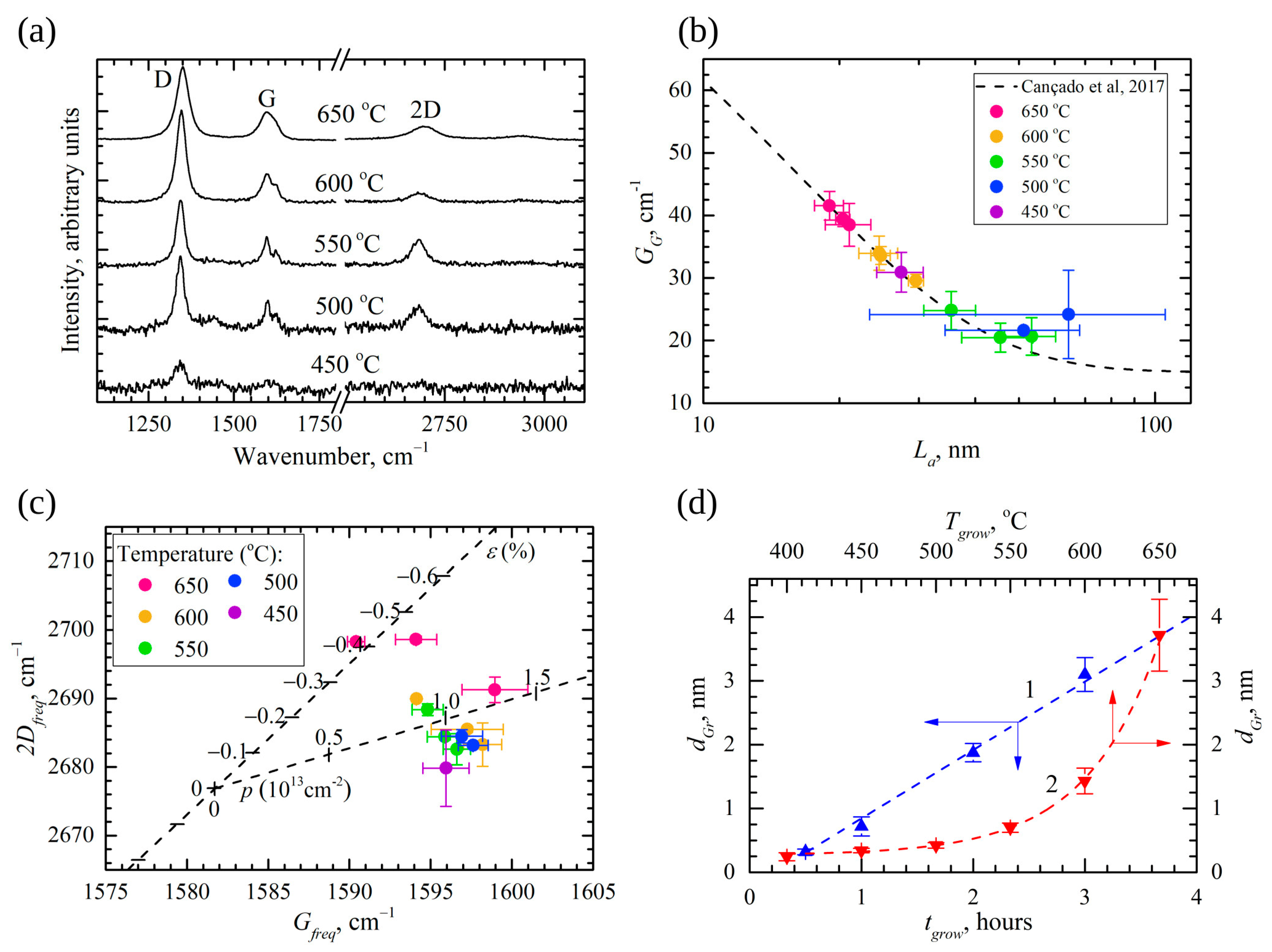
2.3. Raman Spectroscopy
2.4. Optical Measurements
2.5. Contact Angle Tests
3. Results
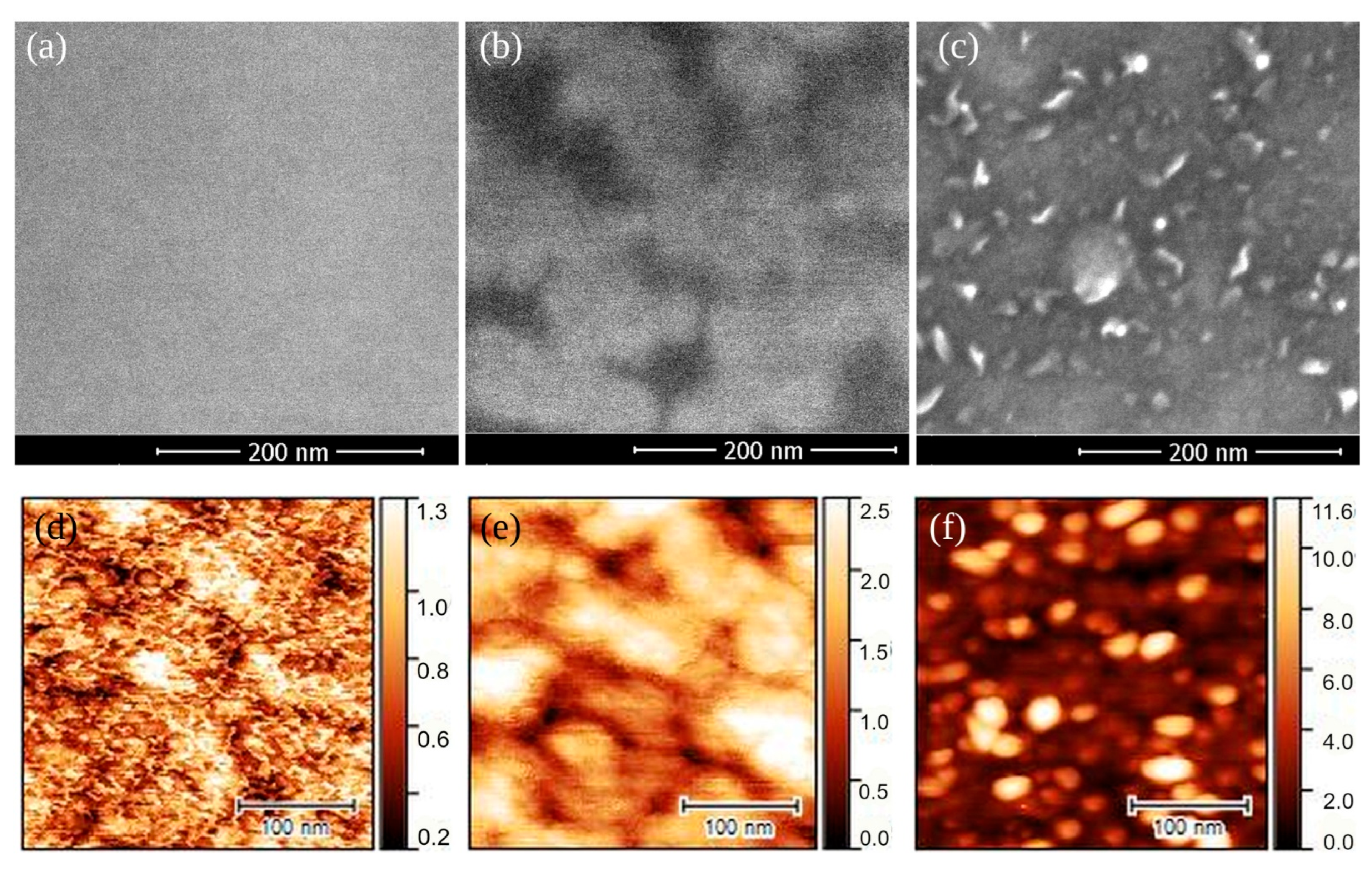
3.1. Arrangement and Composition
3.2. Optical Reflectance
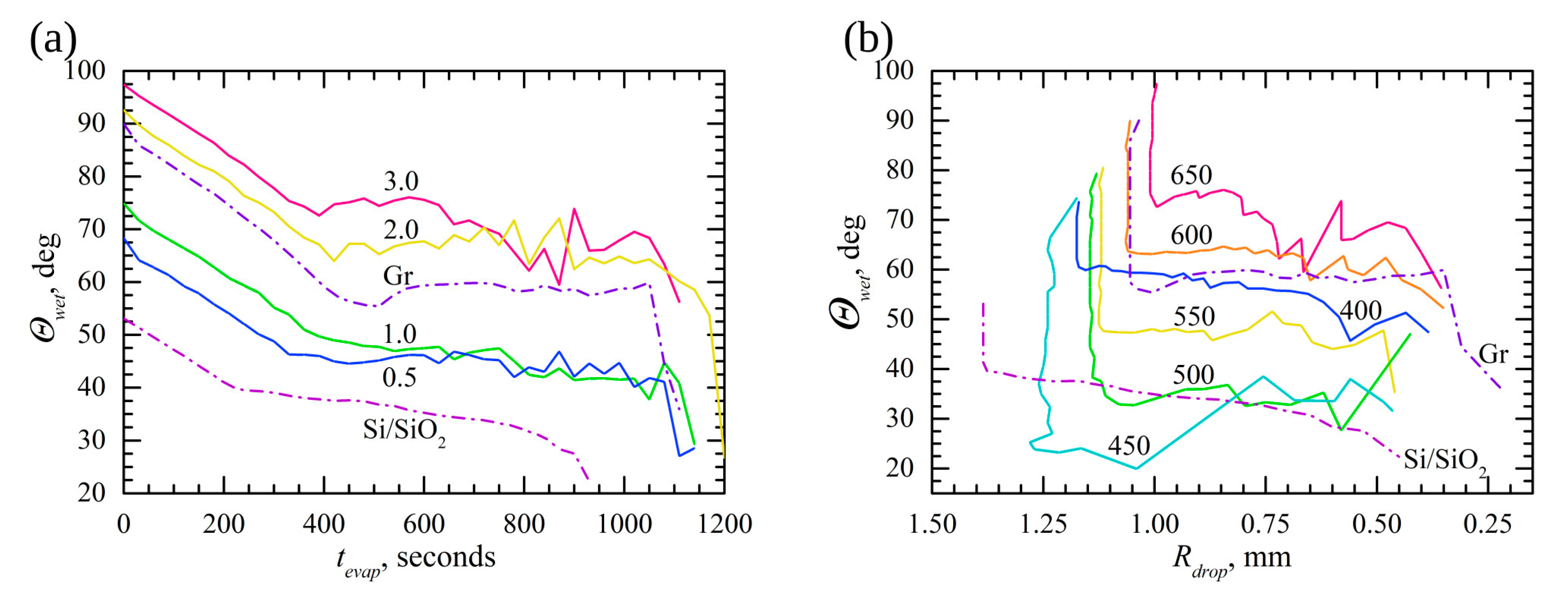
3.3. Droplet Evaporation Dynamics
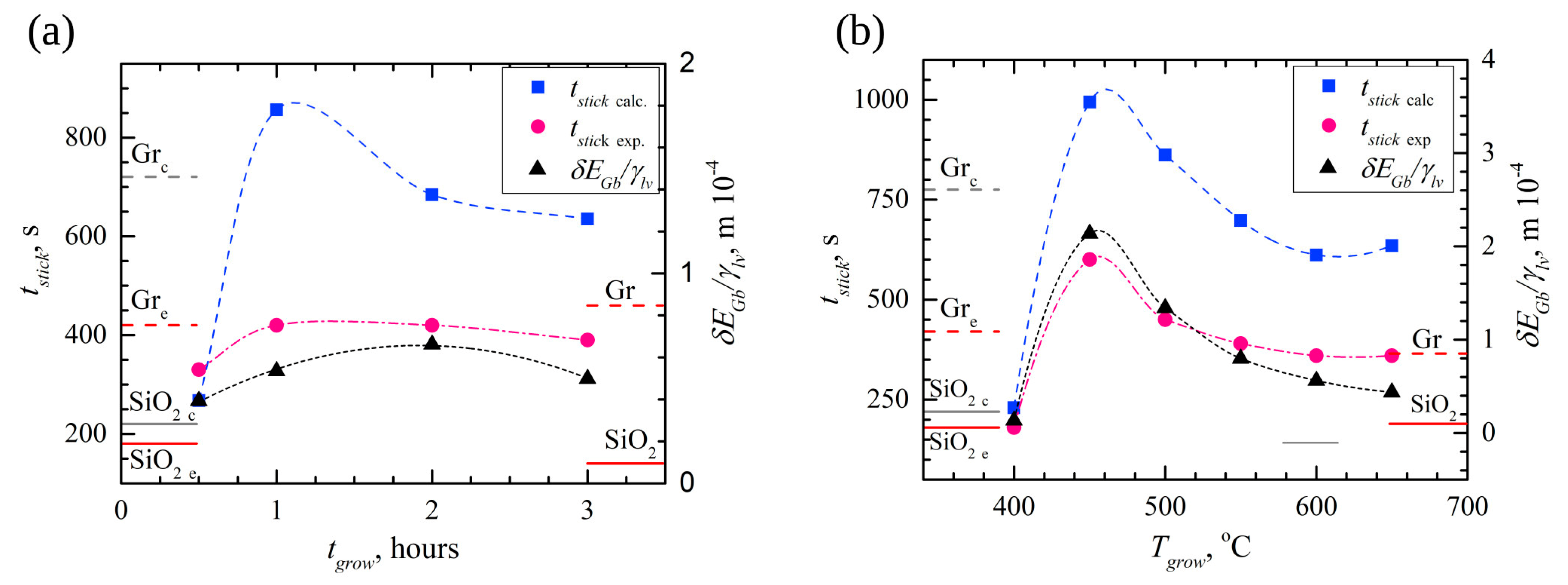
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Marchena, M.; Song, Z.; Senaratne, W.; Li, C.; Liu, X.; Baker, D.; Ferrer, J.C.; Mazumder, P.; Soni, K.; Lee, R.; et al. Direct Growth of 2D and 3D Graphene Nano-Structures over Large Glass Substrates by Tuning a Sacrificial Culate Layer. 2D Mater. 2017, 4, 025088. [Google Scholar] [CrossRef] [Green Version]
- Dong, Y.; Guo, S.; Mao, H.; Xu, C.; Xie, Y.; Deng, J.; Wang, L.; Du, Z.; Xiong, F.; Sun, J. In Situ Growth of CVD Graphene Directly on Dielectric Surface toward Application. ACS Appl. Electron. Mater. 2020, 2, 238–246. [Google Scholar] [CrossRef]
- Li, M.; Liu, D.; Wei, D.; Song, X.; Wei, D.; Wee, A.T.S. Controllable Synthesis of Graphene by Plasma-Enhanced Chemical Vapor Deposition and Its Related Applications. Adv. Sci. 2016, 3, 1600003. [Google Scholar] [CrossRef]
- Yi, K.; Liu, D.; Chen, X.; Yang, J.; Wei, D.; Liu, Y.; Wei, D. Plasma-Enhanced Chemical Vapor Deposition of Two-Dimensional Materials for Applications. Acc. Chem. Res. 2021, 54, 1011–1022. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.; Islam, S.M.; Ahmed, S.; Kumar, R.R.; Habib, M.R.; Huang, K.; Hu, M.; Yu, X.; Yang, D. Direct CVD Growth of Graphene on Technologically Important Dielectric and Semiconducting Substrates. Adv. Sci. 2018, 5, 1800050. [Google Scholar] [CrossRef] [Green Version]
- Muñoz, R.; Martínez, L.; López-Elvira, E.; Munuera, C.; Huttel, Y.; García-Hernández, M. Direct Synthesis of Graphene on Silicon Oxide by Low Temperature Plasma Enhanced Chemical Vapor Deposition. Nanoscale 2018, 10, 12779–12787. [Google Scholar] [CrossRef]
- Meškinis; Vasiliauskas, A.; Guobienė, A.; Talaikis, M.; Niaura, G.; Gudaitis, R. The Direct Growth of Planar and Vertical Graphene on Si(100) via Microwave Plasma Chemical Vapor Deposition: Synthesis Conditions Effects. RSC Adv. 2022, 12, 18759–18772. [Google Scholar] [CrossRef]
- Bekdüz, B.; Kaya, U.; Langer, M.; Mertin, W.; Bacher, G. Direct Growth of Graphene on Ge(100) and Ge(110) via Thermal and Plasma Enhanced CVD. Sci. Rep. 2020, 10, 12938. [Google Scholar] [CrossRef]
- Li, C.; Zheng, C.; Cao, F.; Zhang, Y.; Xia, X. The Development Trend of Graphene Derivatives. J. Electron. Mater. 2022, 51, 4107–4114. [Google Scholar] [CrossRef]
- Yeh, N.C.; Hsu, C.C.; Bagley, J.; Tseng, W.S. Single-Step Growth of Graphene and Graphene-Based Nanostructures by Plasma-Enhanced Chemical Vapor Deposition. Nanotechnology 2019, 30, 162001. [Google Scholar] [CrossRef] [Green Version]
- Zhou, F.; Shan, J.; Cui, L.; Qi, Y.; Hu, J.; Zhang, Y.; Liu, Z. Direct Plasma-Enhanced-Chemical-Vapor-Deposition Syntheses of Vertically Oriented Graphene Films on Functional Insulating Substrates for Wide-Range Applications. Adv. Funct. Mater. 2022, 32, 2202026. [Google Scholar] [CrossRef]
- Lukša, A.; Nargelienė, V.; Treideris, M.; Bukauskas, V.; Talaikis, M.; Šetkus, A. Stages of Self-Arrangement in Growth of Nanostructured Graphene Films Related to the Flow of Ionized Species during Plasma-Enhanced Chemical Vapor Deposition. J. Vac. Sci. Technol. A 2023, 41, 53402. [Google Scholar] [CrossRef]
- Shin, K.W.; Cho, Y.; Lee, Y.; Lee, H.; Nam, S.G.; Byun, K.E.; Lee, C.S.; Park, S.; Shin, H.J. Study of Selective Graphene Growth on Non-Catalytic Hetero-Substrates. 2D Mater. 2020, 7, 011002. [Google Scholar] [CrossRef]
- Gray, A.; Balooch, M.; Allegret, S.; De Gendt, S.; Wang, W.E. Optical Detection and Characterization of Graphene by Broadband Spectrophotometry. J. Appl. Phys. 2008, 104, 053109. [Google Scholar] [CrossRef]
- Ghamsari, B.G.; Tosado, J.; Yamamoto, M.; Fuhrer, M.S.; Anlage, S.M. Measuring the Complex Optical Conductivity of Graphene by Fabry-Pérot Reflectance Spectroscopy. Sci. Rep. 2016, 6, 34166. [Google Scholar] [CrossRef] [Green Version]
- Bruna, M.; Borini, S. Optical Constants of Graphene Layers in the Visible Range. Appl. Phys. Lett. 2009, 94, 031901. [Google Scholar] [CrossRef]
- Barrutia, L.; Ochoa-Martínez, E.; Gabás, M.; Centeno, A.; Zurutuza, A.; Rey-Stolle, I.; Algora, C. Evidence of Decreased Optical Absorption of Chemical Vapor Deposition Graphene Multilayers Deposited on Semiconductor Structures. ACS Photonics 2022, 9, 868–872. [Google Scholar] [CrossRef]
- Cheon, S.; Kihm, K.D.; Kim, H.G.; Lim, G.; Park, J.S.; Lee, J.S. How to Reliably Determine the Complex Refractive Index (RI) of Graphene by Using Two Independent Measurement Constraints. Sci. Rep. 2014, 4, 6364. [Google Scholar] [CrossRef] [Green Version]
- Li, W.; Cheng, G.; Liang, Y.; Tian, B.; Liang, X.; Peng, L.; Hight Walker, A.R.; Gundlach, D.J.; Nguyen, N.V. Broadband Optical Properties of Graphene by Spectroscopic Ellipsometry. Carbon 2016, 99, 348–353. [Google Scholar] [CrossRef] [Green Version]
- Kim, D.; Pugno, N.M.; Buehler, M.J.; Ryu, S. Solving the Controversy on the Wetting Transparency of Graphene. Sci. Rep. 2015, 5, 15526. [Google Scholar] [CrossRef] [Green Version]
- Parobek, D.; Liu, H. Wettability of Graphene. 2D Mater. 2015, 2, 032001. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, Y.; Abidi, N.; Cabrales, L. Wettability and Surface Free Energy of Graphene Films. Langmuir 2009, 25, 11078–11081. [Google Scholar] [CrossRef] [PubMed]
- Leroy, F.; Liu, S.; Zhang, J. Parametrizing Nonbonded Interactions from Wetting Experiments via the Work of Adhesion: Example of Water on Graphene Surfaces. J. Phys. Chem. C 2015, 119, 28470–28481. [Google Scholar] [CrossRef]
- Taherian, F.; Marcon, V.; Van Der Vegt, N.F.A.; Leroy, F. What Is the Contact Angle of Water on Graphene? Langmuir 2013, 29, 1457–1465. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Alvarado, B. Water Wettability of Graphene and Graphite, Optimization of Solid-Liquid Interaction Force Fields, and Insights from Mean-Field Modeling. J. Chem. Phys. 2019, 151, 114701. [Google Scholar] [CrossRef]
- Dreier, L.B.; Liu, Z.; Narita, A.; Van Zadel, M.J.; Müllen, K.; Tielrooij, K.J.; Backus, E.H.G.; Bonn, M. Surface-Specific Spectroscopy of Water at a Potentiostatically Controlled Supported Graphene Monolayer. J. Phys. Chem. C 2019, 123, 24031–24038. [Google Scholar] [CrossRef] [Green Version]
- Cançado, L.G.; Da Silva, M.G.; Martins Ferreira, E.H.; Hof, F.; Kampioti, K.; Huang, K.; Pénicaud, A.; Achete, C.A.; Capaz, R.B.; Jorio, A. Disentangling Contributions of Point and Line Defects in the Raman Spectra of Graphene-Related Materials. 2D Mater. 2017, 4, 025039. [Google Scholar] [CrossRef]
- Lee, J.E.; Ahn, G.; Shim, J.; Lee, Y.S.; Ryu, S. Optical Separation of Mechanical Strain from Charge Doping in Graphene. Nat. Commun. 2012, 3, 1024. [Google Scholar] [CrossRef] [Green Version]
- Bormashenko, E.; Musin, A.; Zinigrad, M. Evaporation of Droplets on Strongly and Weakly Pinning Surfaces and Dynamics of the Triple Line. Colloids Surfaces A Physicochem. Eng. Asp. 2011, 385, 235–240. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lukša, A.; Bukauskas, V.; Nargelienė, V.; Treideris, M.; Talaikis, M.; Selskis, A.; Suchodolskis, A.; Šetkus, A. Influence of Growth Time and Temperature on Optical Characteristics and Surface Wetting in Nano-Crystalline Graphene Deposited by PECVD Directly on Silicon Dioxide. Crystals 2023, 13, 1243. https://doi.org/10.3390/cryst13081243
Lukša A, Bukauskas V, Nargelienė V, Treideris M, Talaikis M, Selskis A, Suchodolskis A, Šetkus A. Influence of Growth Time and Temperature on Optical Characteristics and Surface Wetting in Nano-Crystalline Graphene Deposited by PECVD Directly on Silicon Dioxide. Crystals. 2023; 13(8):1243. https://doi.org/10.3390/cryst13081243
Chicago/Turabian StyleLukša, Algimantas, Virginijus Bukauskas, Viktorija Nargelienė, Marius Treideris, Martynas Talaikis, Algirdas Selskis, Artūras Suchodolskis, and Arūnas Šetkus. 2023. "Influence of Growth Time and Temperature on Optical Characteristics and Surface Wetting in Nano-Crystalline Graphene Deposited by PECVD Directly on Silicon Dioxide" Crystals 13, no. 8: 1243. https://doi.org/10.3390/cryst13081243
APA StyleLukša, A., Bukauskas, V., Nargelienė, V., Treideris, M., Talaikis, M., Selskis, A., Suchodolskis, A., & Šetkus, A. (2023). Influence of Growth Time and Temperature on Optical Characteristics and Surface Wetting in Nano-Crystalline Graphene Deposited by PECVD Directly on Silicon Dioxide. Crystals, 13(8), 1243. https://doi.org/10.3390/cryst13081243






