Hydrothermal Synthesis and Crystal Structure of Vesuvianite Compounds, Ca19Al13Si18O71(OH)7 and Sr19Fe12Ge19O72(OH)6
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis
2.2. Single Crystal X-ray Diffraction
2.3. Additional Characterization
3. Results and Discussion
3.1. Synthesis
3.2. Structural Analysis
3.2.1. Space Group Assignment
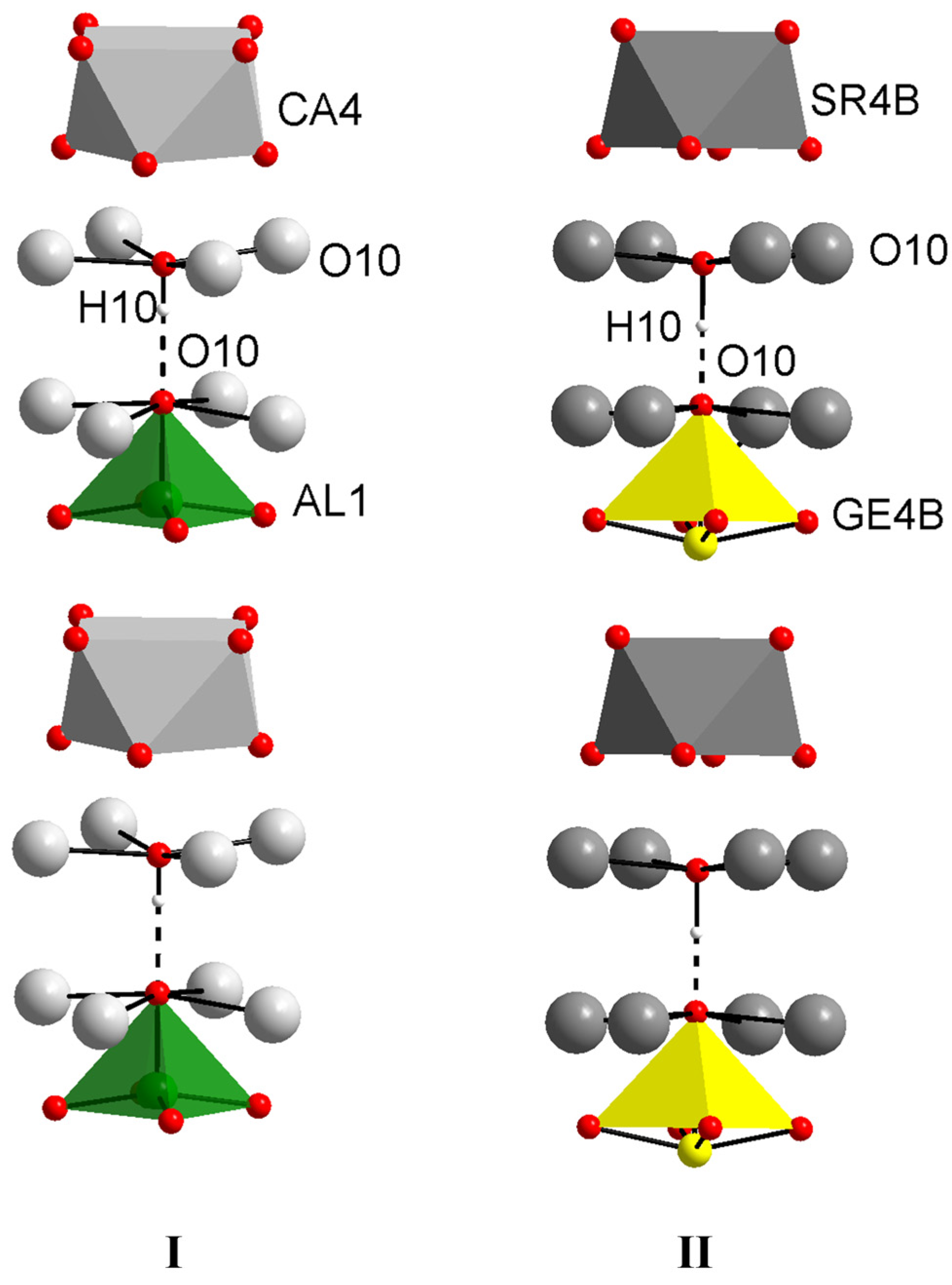
3.2.2. Structural Features
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Groat, L.; Hawthorne, F.; Ercit, T.S. The Chemistry of Vesuvianite. Can. Mineral. 1992, 30, 19–48. [Google Scholar]
- Allen, F.M.; Burnham, C.W. A Comprehensive Structure-Model for Vesuvianite; Symmetry Variations and Crystal Growth. Can. Mineral. 1992, 30, 1–18. [Google Scholar]
- Ito, J.; Arem, J.E. Idocrase: Synthesis, Phase Relations and Crystal Chemistry. Am. Mineral. 1970, 55, 880–912. [Google Scholar]
- Fitzgerald, S.; Leavens, P.B.; Nelen, J.A. Chemical Variation in Vesuvianite. Mineral. Petrol. 1992, 46, 163–178. [Google Scholar] [CrossRef]
- Groat, L.; Hawthorne, F.; Ercit, T.S. Excess Y-Group Cations in the Crystal Structure of Vesuvianite. Can. Mineral. 1994, 32, 497–504. [Google Scholar]
- Groat, L.A.; Hawthorne, F.C.; Ercit, T.S. The Incorporation of Boron into the Vesuvianite Structure. Can. Mineral. 1994, 32, 505–523. [Google Scholar]
- Moiseev, M.M.; Panikorovskii, T.L.; Aksenov, S.M.; Mazur, A.S.; Mikhailova, J.A.; Yakovenchuk, V.N.; Bazai, A.V.; Ivanyuk, G.Y.; Agakhanov, A.A.; Shilovskikh, V.V.; et al. Insights into crystal chemistry of the vesuvianite-group: Manaevite-(Ce), a new mineral with complex mechanisms of its hydration. Phys. Chem. Mineral. 2020, 47, 18. [Google Scholar] [CrossRef]
- Groat, L.A.; Evans, R.J.; Cempírek, J.; McCammon, C.; Houzar, S. Fe-rich and As-bearing vesuvianite and wiluite from Kozlov, Czech Republic. Am. Mineral. 2013, 98, 1330–1337. [Google Scholar] [CrossRef]
- Groat, L.A.; Evans, R.J. Crystal Chemistry of Bi- and Mn-Bearing Vesuvianite from Langban, Sweden. Am. Mineral. 2012, 97, 1627–1634. [Google Scholar] [CrossRef]
- Rucklidge, J.C.; Kocman, V.; Whitlow, S.H.; Gabe, E.J. The Crystal Structures of Three Canadian Vesuvianites. Can. Mineral. 1975, 13, 15–21. [Google Scholar]
- Arem, J.E. Crystal Chemistry and Structure of Idocrase; Harvard University: Cambridge, MA, USA, 1970. [Google Scholar]
- Groat, L.A. The Crystal Chemistry of Vesuvianite. Ph.D. Thesis, University of Manitoba, Winnipeg, MA, USA, 1988. [Google Scholar]
- Allen, F.M. Structural and Chemical Variations of Vesuvianite. Ph.D. Thesis, Harvard University, Cambridge, MA, USA, 1985. [Google Scholar]
- Groat, L.A.; Hawthorne, F.C.; Ercit, T.S.; Putnis, A. The Symmetry of Vesuvianite. Can. Mineral. 1993, 31, 617–635. [Google Scholar]
- Gnos, E.; Armbruster, T. Relationship among Metamorphic Grade, Vesuvianite “Rod Polytypism”, and Vesuvianite Composition. Am. Mineral. 2006, 91, 862–870. [Google Scholar] [CrossRef]
- Armbruster, T.; Gnos, E. P4/n and P4nc Long-Range Ordering in Low-Temperature Vesuvianites. Am. Mineral. 2000, 85, 563–569. [Google Scholar] [CrossRef]
- Panikorovskii, T.L.; Krivovichev, S.V.; Zolotarev, A.A.J.; Antonov, A.A. Crystal chemistry of low-symmetry (P4nc) vesuvianite from the Kharmankul’ Cordon (South Urals, Russia). Zap. Ross. Mineral. Obsh. 2016, 145, 94–104. [Google Scholar]
- McMillen, C.D.; Kolis, J.W. Hydrothermal Synthesis as a Route to Mineralogically-Inspired Structures. Dalton Trans. 2016, 45, 2772–2784. [Google Scholar] [CrossRef] [PubMed]
- Sanjeewa, L.D.; McGuire, M.A.; Garlea, V.O.; Hu, L.; Chumanov, G.; McMillen, C.D.; Kolis, J.W. Hydrothermal Synthesis and Characterization of Brackebuschite-type Transition Metal Vanadate: Ba2M(VO4)2(OH), M = V3+, Mn3+ and Fe3+ with Interesting Jahn Teller and Spin Liquid Behavior. Inorg. Chem. 2015, 54, 7014–7020. [Google Scholar] [CrossRef]
- Sanjeewa, L.D.; Garlea, V.O.; McGuire, M.A.; McMillen, C.D.; Cao, H.; Kolis, J.W. Structural and magnetic characterization of the new one-dimensional S = 5/2 antiferromagnetic chain system SrMn(VO4)(OH). Phys. Rev. B 2016, 93, 224407. [Google Scholar] [CrossRef]
- Terry, R.; McMillen, C.D.; Kolis, J.W. Hydrothermal Single Crystal Growth and Structural Investigation of the Nepheline and Kalsilite Stuffed Tridymite Species. J. Chem. Crystallogr. 2023, 53, 25–37. [Google Scholar] [CrossRef]
- Warren, B.E.; Modell, D.I. The Structure of Vesuvianite Ca10Al4(Mg,Fe)2Si9O10(OH)4. Z. Für Krist.-Cryst. Mater. 1931, 78, 422–432. [Google Scholar] [CrossRef]
- Smart, M.M.; Smith Pellizzeri, T.M.; Morrison, G.; McMillen, C.D.; zur Loye, H.-C.; Kolis, J.W. Ferrite Materials Containing Kagomé Layers: Chemistry of Ba2Fe11Ge2O22 and K2Co4V9O22 Hexaferrites. Chem. Mater. 2021, 33, 2258–2266. [Google Scholar] [CrossRef]
- Smart, M.M.; McMillen, C.D.; Ivey, K.; Kolis, J.W. Chemistry of Transition Metal Silicates and Germanates: The Largest Metal Polygermanate, K11Mn21Ge32O86(OH)9(H2O) with a 76 Å Ordered Lattice. Inorg. Chem. 2020, 59, 16804–16808. [Google Scholar] [CrossRef]
- Apex3 (version 2017.3); Bruker AXS Inc.: Madison, WI, USA, 2015.
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Galuskin, E.; Galuskina, I.; Stadnicka, K.; Armbruster, T.; Kozanecki, M. The Crystal Structure of Si-Deficient, OH-Substituted, Boron-Bearing Vesuvianite from the Wiluy River, Sakha-Yakutia, Russia. Can. Mineral. 2007, 45, 239–248. [Google Scholar] [CrossRef]
- Paluszkiewicz, C.; Żabiński, W. Vibrational Spectroscopy as a Tool for Discrimination of High and Low Vesuvianite. Vib. Spectrosc. 2004, 35, 77–80. [Google Scholar] [CrossRef]
- Groat, L.A.; Hawthorne, F.C.; Ercit, T.S. The Role of Fluorine in Vesuvianite: A Crystal-Structure Study. Can. Mineral. 1992, 30, 1065–1075. [Google Scholar]
- Ohkawa, N.; Yoshiasa, A.; Yakeno, S. Crystal Chemistry of Vesuvianite: Site Preferences of Square Pyramidal Coordinated Sites. Am. Mineral. 1992, 77, 945–953. [Google Scholar]
- Panikorovskii, T.L.; Chukanov, N.V.; Rusakov, V.S.; Shilovskikh, V.V.; Mazur, A.S.; Balassone, G.; Ivanyuk, G.Y.; Krivovichev, S.V. Vesuvianite from the Somma-Vesuvius Complex: New Data and Revised Formula. Minerals 2017, 7, 248. [Google Scholar] [CrossRef]
- Lager, G.A.; Xie, Q.; Ross, F.K.; Rossman, G.R.; Armbruster, T.; Rotella, F.J.; Shultz, A.J. Hydrogen-Atom Positions in P4/nnc Vesuvianite. Can. Mineral. 1999, 37, 763–768. [Google Scholar]
- Valley, J.W.; Peacor, D.R.; Bowman, J.R.; Essene, E.J.; Allard, M.J. Crystal Chemistry of a Mg-Vesuvianite and Implications of Phase Equilibria in the System CaO-MgO-Al2O3 -SiO2-H2O-CO2. J. Metamorph. Geol. 1985, 3, 137–153. [Google Scholar] [CrossRef]
- Shannon, R.D. Revised Effective Ionic Radii and Systematic Studies of Interatomic Distances in Halides and Chalcogenides. Acta Crystallogr. A 1976, 32, 751–767. [Google Scholar] [CrossRef]
- Phillips, B.L.; Allen, F.M.; Kirkpatrick, R.J. High-Resolution Solid-State 27Al NMR Spectroscopy of Mg-Rich Vesuvianite. Am. Mineral. 1987, 72, 1190–1194. [Google Scholar]
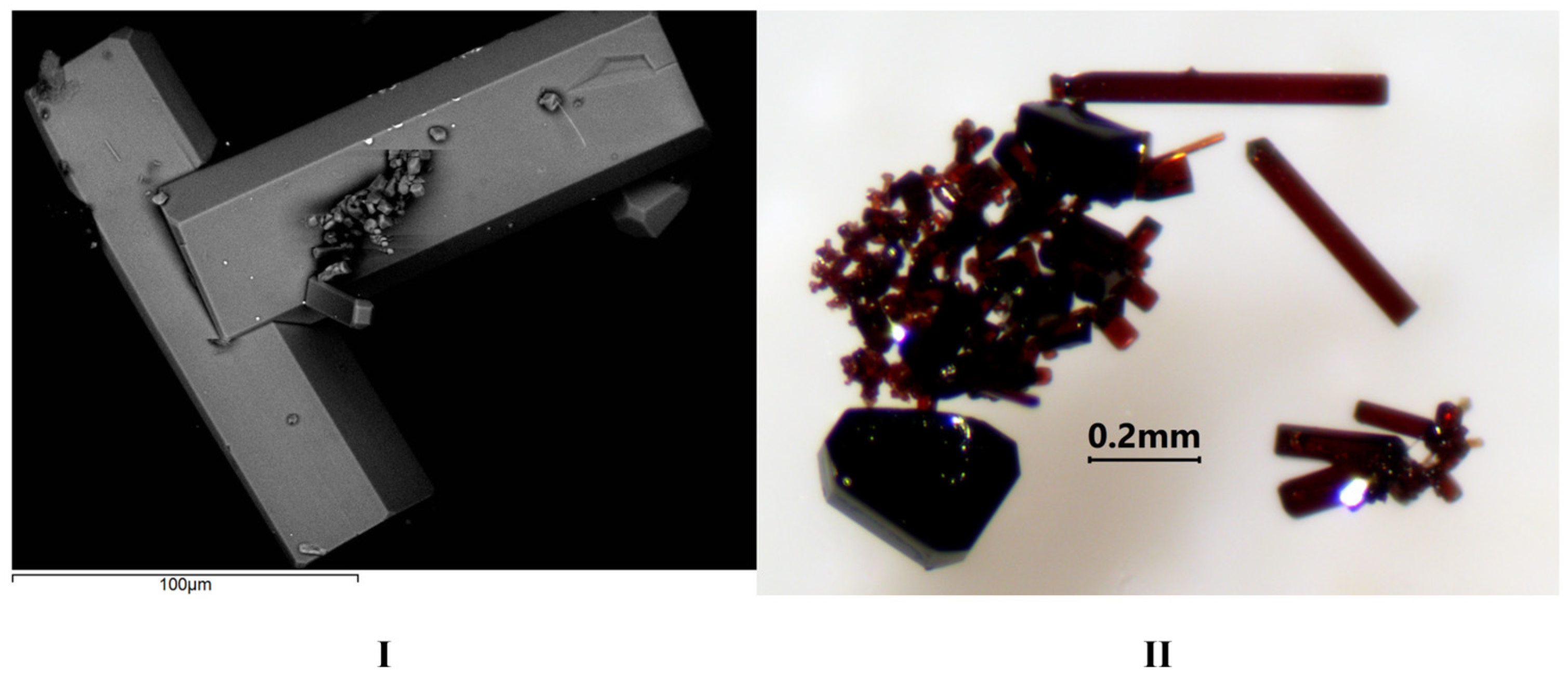
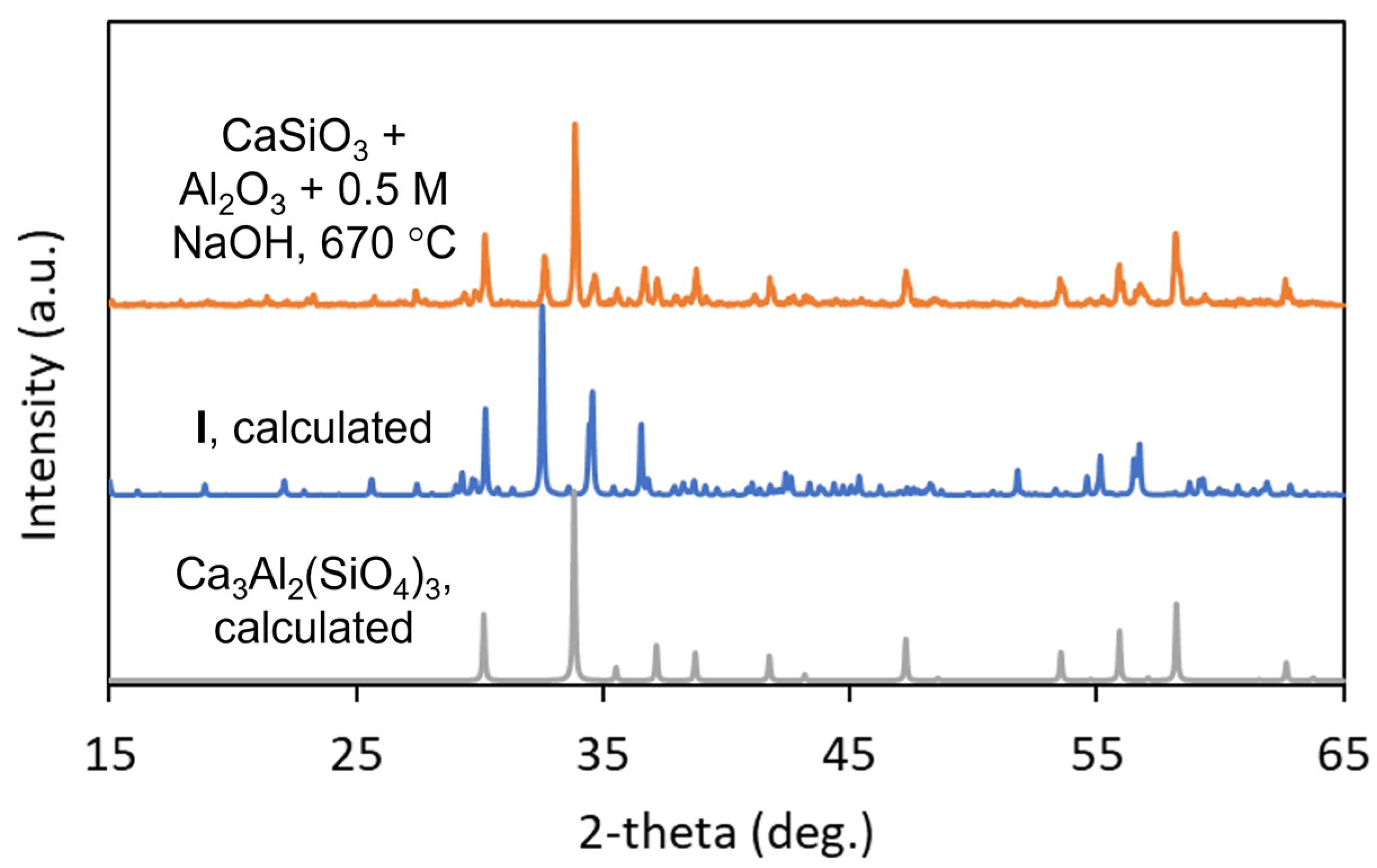
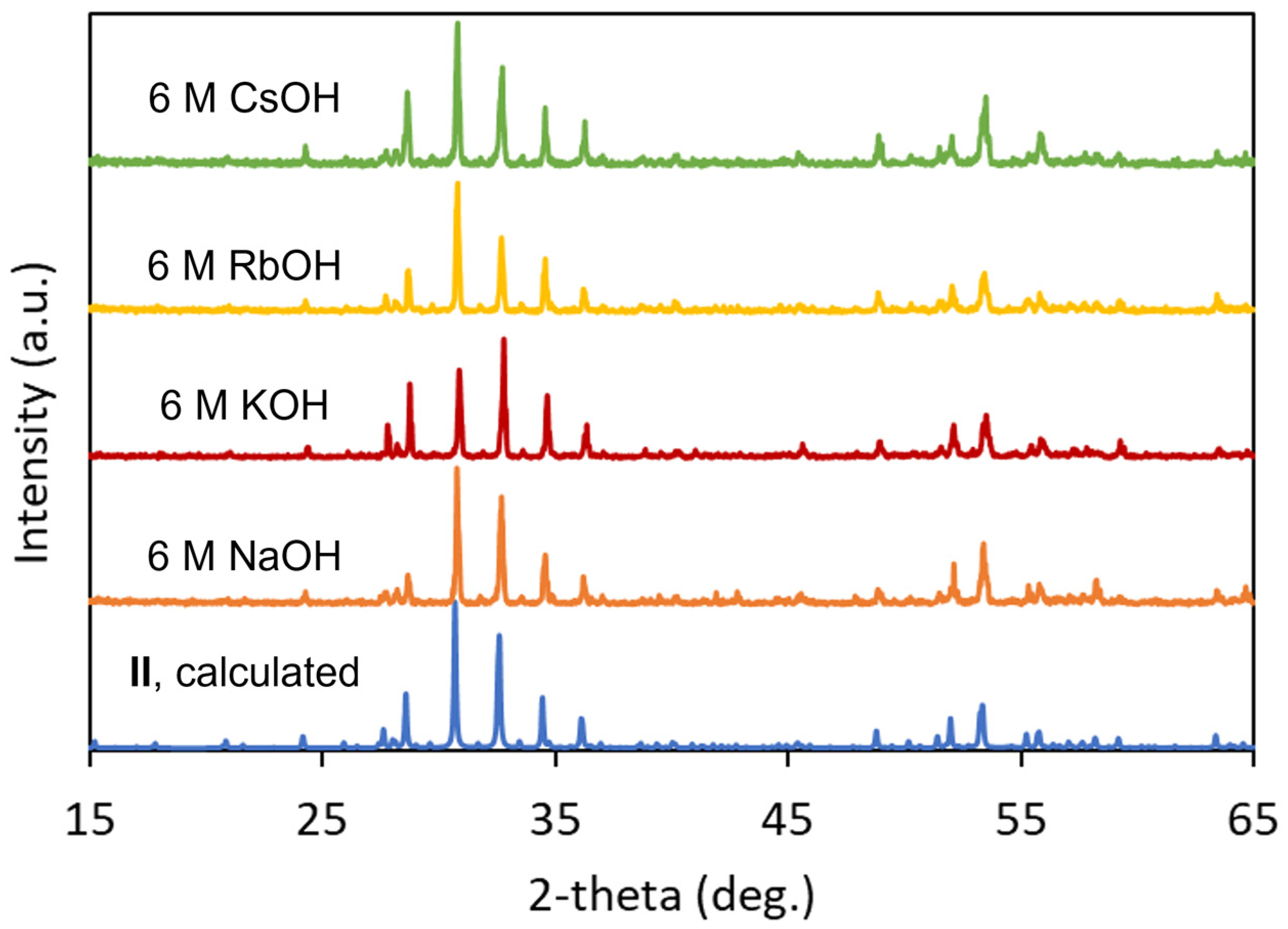
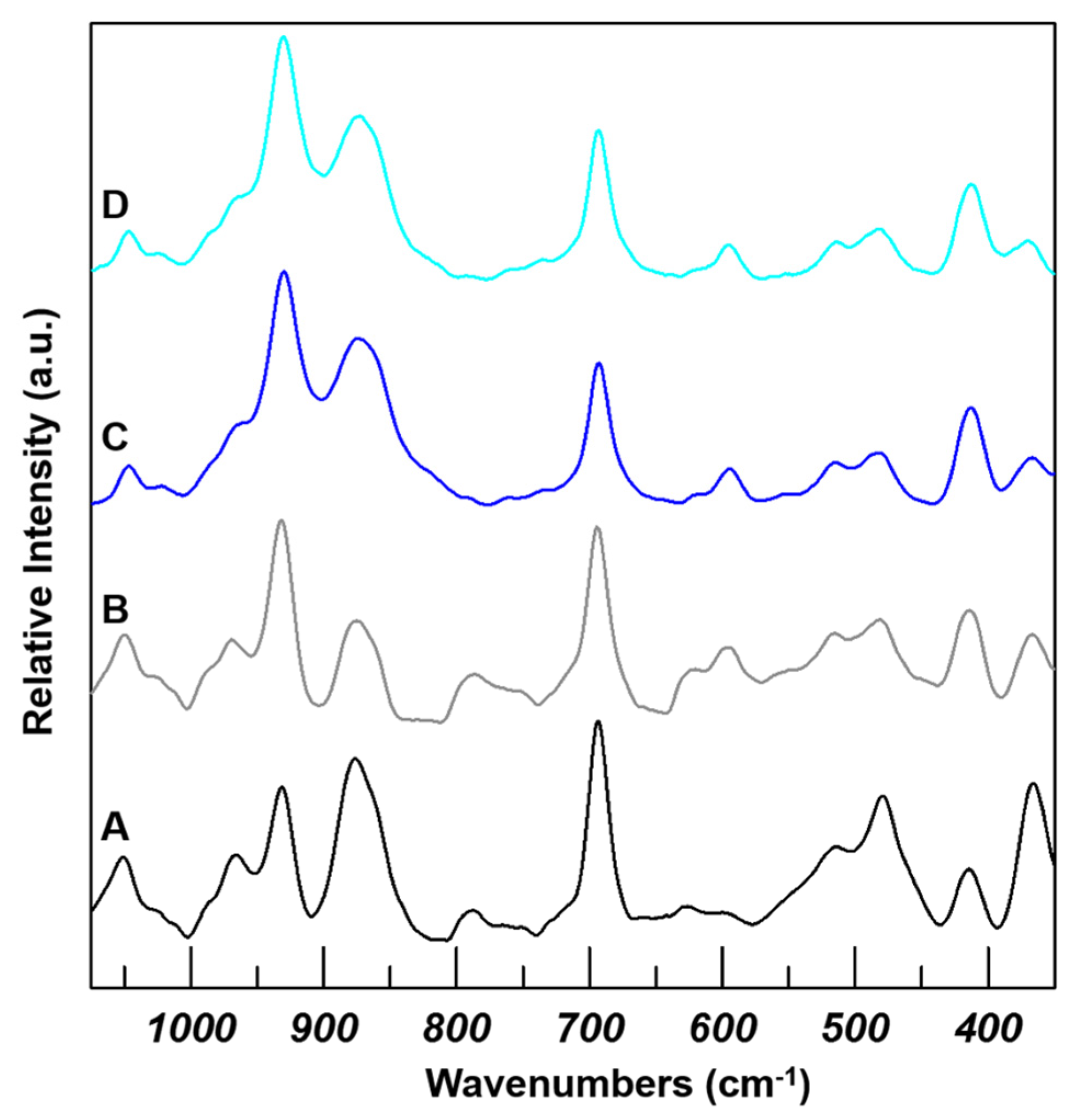

| I | II | |
|---|---|---|
| Empirical Formula | Ca19Al13Si18O78H7 | Sr19Fe12Ge19O78H6 |
| F. W. (g/mol) | 2872.94 | 4968.24 |
| Temperature (K) | 296(2) | 105(2) |
| Crystal System | Tetragonal | Tetragonal |
| Space Group | P4/nnc (no. 126) | P4/nnc (no. 126) |
| a (Å) | 15.5429(16) | 16.4498(8) |
| c (Å) | 11.8210(12) | 12.4779(6) |
| Volume (Å3) | 2855.7(7) | 3376.5(4) |
| Z | 2 | 2 |
| D(calcd) (mg/m3) | 3.341 | 4.887 |
| Wavelength (Å) | 0.71073 | 0.71073 |
| μ, mm−1 | 2.494 | 25.793 |
| F(000) | 2864 | 4544 |
| Crystal Size (mm) | 0.055 × 0.061 × 0.101 | 0.114 × 0.085 × 0.055 |
| θ range,° | 2.621 to 30.530 | 3.215 to 30.469 |
| Reflections Collected | 2188 | 2579 |
| Independent Reflections | 1958 | 2149 |
| R indices (I > 2σ(I)) | R1 = 0.0337 a wR2 = 0.0782 b | R1 = 0.0461 a wR2 = 0.0694 b |
| R indices (all data) | R1 = 0.0404 a wR2 = 0.0803 b | R1 = 0.0624 a wR2 = 0.0727 b |
| Goodness-of-fit on F2 | 1.228 | 1.164 |
| Ca19Al13Si18O71(OH)7 P4/nnc | Sr19Fe12Ge19O72(OH)6 P4/nnc | N’chwaning P4nc | Asbestos P4/n | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Symmetry | n-- | -n- | --c | n-- | -n- | --c | n-- | -n- | --c | n-- | -n- | --c |
| N | 332 | 624 | 375 | 1328 | 1828 | 1355 | 333 | 422 | 268 | 405 | 664 | 426 |
| N I>3σ | 5 | 141 | 98 | 1 | 39 | 13 | 140 | 125 | 68 | 5 | 270 | 177 |
| <I> | 0.1 | 0.4 | 0.5 | 0.3 | 0.4 | 0.4 | 1.1 | 0.7 | 0.7 | 0.2 | 1.5 | 1.5 |
| <I/σ> | 0.6 | 2.1 | 2.4 | 0.5 | 0.8 | 0.7 | 3.0 | 2.1 | 2.1 | 0.9 | 4.1 | 4.6 |
| Site | Atom | x/a | y/b | z/c | Ueq (Å2) | sof |
|---|---|---|---|---|---|---|
| X1 | Ca1 | 0.250000 | 0.750000 | 0.750000 | 0.0089(2) | 1 |
| X2 | Ca2 | 0.68930(4) | 0.45589(4) | 0.62048(5) | 0.00824(12) | 1 |
| X3 | Ca3 | 0.39865(5) | 0.31832(5) | 0.88814(7) | 0.01636(15) | 1 |
| X4 | Ca4 | 0.750000 | 0.750000 | 0.6500(2) | 0.0107(4) | 0.5 |
| Y1 | Al1 | 0.750000 | 0.750000 | 0.5350(4) | 0.0118(6) | 0.5 |
| Y2 | Al2 | 0.500000 | 0.500000 | 0.500000 | 0.0069(2) | 1 |
| Y3 | Al3 | 0.37885(6) | 0.61264(6) | 0.62594(8) | 0.00720(16) | 1 |
| Z1 | Si1 | 0.250000 | 0.750000 | 0.500000 | 0.0060(3) | 1 |
| Z2 | Si2 | 0.31920(6) | 0.54145(6) | 0.87110(7) | 0.00617(15) | 1 |
| Z3 | Si3 | 0.58384(6) | 0.65099(6) | 0.63484(7) | 0.00760(15) | 1 |
| O1 | O1 | 0.32742(14) | 0.71949(15) | 0.58569(19) | 0.0082(4) | 1 |
| O2 | O2 | 0.34061(15) | 0.61747(15) | 0.77819(19) | 0.0093(4) | 1 |
| O3 | O3 | 0.22184(14) | 0.54881(15) | 0.92346(19) | 0.0085(4) | 1 |
| O4 | O4 | 0.39337(14) | 0.56251(15) | 0.97038(19) | 0.0087(4) | 1 |
| O5 | O5 | 0.48577(16) | 0.67069(15) | 0.6775(2) | 0.0113(4) | 1 |
| O6 | O6 | 0.62081(18) | 0.72815(16) | 0.5592(2) | 0.0179(5) | 1 |
| O7 | O7 | 0.32773(15) | 0.44477(15) | 0.8219(2) | 0.0118(4) | 1 |
| O8 | O8 | 0.59127(14) | 0.56069(14) | 0.56660(19) | 0.0089(4) | 1 |
| O9 | O9 | 0.64494(14) | 0.64494(14) | 0.750000 | 0.0105(5) | 1 |
| O10 | O10 | 0.750000 | 0.750000 | 0.3672(5) | 0.0191(9) | 1 |
| H10 | H10 | 0.750000 | 0.750000 | 0.2900(19) | 0.050 | 0.5 |
| O11 | O11 | 0.43844(15) | 0.50514(15) | 0.63642(19) | 0.0094(4) | 1 |
| H11 | H11 | 0.457(7) | 0.474(6) | 0.715(7) | 0.08(3) | 0.75 |
| Site | Atom | x/a | y/b | z/c | Ueq (Å2) | sof |
|---|---|---|---|---|---|---|
| X1 | Sr1 | 0.250000 | 0.750000 | 0.250000 | 0.0033(2) | 1 |
| X2 | Sr2 | 0.45670(3) | 0.80989(3) | 0.61902(5) | 0.00307(12) | 1 |
| X3 | Sr3 | 0.67853(4) | 0.60061(4) | 0.38584(5) | 0.00668(13) | 1 |
| X4a | Sr4a | 0.750000 | 0.750000 | 0.652(5) | 0.0094(14) | 0.2 |
| X4b | Sr4b | 0.750000 | 0.750000 | 0.650(3) | 0.0084(10) | 0.3 |
| Y1a | Ge4a | 0.750000 | 0.750000 | 0.5316(5) | 0.0113(9) | 0.3 |
| Y1b | Ge4b | 0.750000 | 0.750000 | 0.5936(10) | 0.0132(13) | 0.2 |
| Y2 | Fe2 | 0.500000 | 1.000000 | 0.500000 | 0.0052(2) | 1 |
| Y3 | Fe3 | 0.38831(5) | 0.87928(5) | 0.37237(8) | 0.00393(17) | 1 |
| Z1 | Ge1 | 0.250000 | 0.750000 | 0.500000 | 0.0054(3) | 1 |
| Z2 | Ge2 | 0.45893(4) | 0.68107(4) | 0.36976(5) | 0.00250(13) | 1 |
| Z3 | Ge3 | 0.65076(4) | 0.91725(4) | 0.63439(5) | 0.00414(14) | 1 |
| O1 | O1 | 0.2811(3) | 0.8297(3) | 0.4128(4) | 0.0027(8) | 1 |
| O2 | O2 | 0.3793(3) | 0.6592(3) | 0.2776(4) | 0.0041(9) | 1 |
| O3 | O3 | 0.4504(3) | 0.7807(3) | 0.4218(4) | 0.0050(9) | 1 |
| O4 | O4 | 0.4374(3) | 0.6071(3) | 0.4723(3) | 0.0031(9) | 1 |
| O5 | O5 | 0.6690(3) | 1.0164(3) | 0.6815(4) | 0.0068(9) | 1 |
| O6 | O6 | 0.7312(3) | 0.8823(3) | 0.5571(4) | 0.0133(11) | 1 |
| O7 | O7 | 0.5566(3) | 0.6748(3) | 0.3192(4) | 0.0059(9) | 1 |
| O8 | O8 | 0.5606(3) | 0.9073(3) | 0.5627(4) | 0.0049(9) | 1 |
| O9 | O9 | 0.6473(3) | 0.8527(3) | 0.750000 | 0.0073(12) | 1 |
| O10 | O10 | 0.750000 | 0.750000 | 0.3676(12) | 0.026(3) | 1 |
| H10 | H10 | 0.750000 | 0.750000 | 0.28(4) | 0.050 | 0.5 |
| O11 | O11 | 0.4942(3) | 0.9381(3) | 0.3622(3) | 0.0043(8) | 1 |
| H11 | H11 | 0.497800 | 0.969600 | 0.303400 | 0.050 | 0.625 |
| Atom | O1 | O2 | O3 | O4 | O5 | O6 | O7 | O8 | O9 | O10 | O11 | Sum |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ca1 | 0.37 (x4→) | 0.23 (x4→) | 2.40 | |||||||||
| Ca2 | 0.25 | 0.29 | 0.32 | 0.28 | 0.38, 0.27 | 0.38 | 2.17 | |||||
| Ca3 | 0.27 | 0.25 | 0.32, 0.23, 0.19 | 0.18 | 0.20 (x4↓) | 0.23 | 1.87 | |||||
| Ca4 | 0.40 (x4→) | 0.18 (x4→) | 2.32 | |||||||||
| Al1 | 0.31 (x4→) | 0.37 | 1.61 | |||||||||
| Al2 | 0.41 (x2→) | 0.50 (x2→) | 0.50 (x2→) | 2.82 | ||||||||
| Al3 | 0.46 | 0.47 | 0.42 | 0.31 | 0.37 | 0.45 | 2.48 | |||||
| Si1 | 0.95 (x4→) | 3.80 | ||||||||||
| Si2 | 0.94 | 0.96 | 0.87 | 1.02 | 3.79 | |||||||
| Si3 | 0.97 | 1.06 | 1.00 | 0.90 (x2↓) | 3.93 | |||||||
| Sum | 2.03 | 1.93 | 1.97 | 1.87 | 1.99 | 2.02 | 1.76 | 2.06 | 1.98 | 1.17 | 1.18 |
| Atom | O1 | O2 | O3 | O4 | O5 | O6 | O7 | O8 | O9 | O10 | O11 | Sum |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sr1 | 0.38 (x4→) | 0.26 (x4→) | 2.56 | |||||||||
| Sr2 | 0.27 | 0.30 | 0.35 | 0.27 | 0.37, 0.31 | 0.41 | 2.28 | |||||
| Sr3 | 0.27 | 0.26 | 0.37, 0.24, 0.23 | 0.21 | 0.19 (x4↓) | 0.26 | 2.03 | |||||
| Sr4a | 0.37 (x4→) | 0.22 (x4→) | 2.36 | |||||||||
| Sr4b | 0.37 (x4→) | 0.21 (x4→) | 2.32 | |||||||||
| Ge4a | 0.28 (x4→) | 0.43 | 1.55 | |||||||||
| Ge4b | 0.26 (x4→) | 1.04 | ||||||||||
| Fe2 | 0.43 (x2→) | 0.55 (x2→) | 0.52 (x2→) | 3.00 | ||||||||
| Fe3 | 0.51 | 0.55 | 0.50 | 0.34 | 0.43 | 0.52 | 2.85 | |||||
| Ge1 | 0.92 (x4→) | 3.68 | ||||||||||
| Ge2 | 0.91 | 0.95 | 0.87 | 1.05 | 3.78 | |||||||
| Ge3 | 0.97 | 1.04 | 1.02 | 0.88 (x2↓) | 3.91 | |||||||
| Sum | 2.08 | 1.76 | 2.07 | 2.18 | 2.34 | 2.58 | 1.89 | 2.19 | 2.19 | 1.19 | 1.30 |
| Bond | Length | Bond | Length |
|---|---|---|---|
| Ca1-O1 (x4) | 2.3338(19) | Al1-O10 | 1.977(6) |
| Ca1-O2 (x4) | 2.5168(19) | Al1-O6 (x4) | 2.057(2) |
| Ca2-O8 | 2.3202(19) | Al2-O11 (x2) | 1.877(19) |
| Ca2-O5 | 2.326(2) | Al2-O8 (x2) | 1.8760(19) |
| Ca2-O3 | 2.384(2) | Al2-O4 (x2) | 1.9539(19) |
| Ca2-O2 | 2.426(2) | ||
| Ca2-O4 | 2.445(2) | Al3-O2 | 1.898(2) |
| Ca2-O5 | 2.450(2) | Al3-O1 | 1.902(2) |
| Ca2-O1 | 2.484(2) | Al3-O11 | 1.914(2) |
| Al3-O3 | 1.942(2) | ||
| Ca3-O7 | 2.385(2) | Al3-O5 | 1.984(2) |
| Ca3-O3 | 2.453(2) | Al3-O4 | 2.054(2) |
| Ca3-O6 | 2.478(3) | ||
| Ca3-O7 | 2.505(2) | Si1-O1 (x4) | 1.6438(18) |
| Ca3-O11 | 2.512(2) | ||
| Ca3-O10 | 2.5550(8) | Si2-O7 | 1.617(2) |
| Ca3-O7 | 2.588(3) | Si2-O3 | 1.639(2) |
| Ca3-O8 | 2.612(2) | Si2-O2 | 1.647(2) |
| Si2-O4 | 1.677(2) | ||
| Ca4-O6 (x4) | 2.304(3) | ||
| Ca4-O9 (x4) | 2.592(3) | Si3-O6 | 1.603(3) |
| Si3-O8 | 1.623(2) | ||
| Si3-O5 | 1.636(2) | ||
| Si3-O9 | 1.6634(12) |
| Bond | Length | Bond | Length |
|---|---|---|---|
| Sr1-O1 (x4) | 2.471(4) | Sr4b-O6 (x4) | 2.49(2) |
| Sr1-O5 (x4) | 2.621(4) | Sr4b-O9 (x4) | 2.69(2) |
| Sr2-O8 | 2.446(4) | Ge4b-O6 (x4) | 2.245(6) |
| Sr2-O5 | 2.485(5) | ||
| Sr2-O3 | 2.509(5) | Fe2-O8 (x2) | 1.982(5) |
| Sr2-O2 | 2.569(5) | Fe2-O11 (x2) | 2.000(4) |
| Sr2-O4 | 2.607(4) | Fe2-O4 (x2) | 2.070(4) |
| Sr2-O5 | 2.551(5) | ||
| Sr2-O1 | 2.601(4) | Fe3-O2 | 1.981(5) |
| Sr2-O6 | 3.048(5) | Fe3-O11 | 1.997(5) |
| Fe3-O1 | 2.007(5) | ||
| Sr3-O7 | 2.490(5) | Fe3-O3 | 2.013(5) |
| Sr3-O3 | 2.598(5) | Fe3-O5 | 2.070(5) |
| Sr3-O6 | 2.617(5) | Fe3-O4 | 2.163(5) |
| Sr3-O7 | 2.652(5) | ||
| Sr3-O11 | 2.614(4) | Ge1-O1 (x4) | 1.779(4) |
| Sr3-O10 | 2.7338(14) | ||
| Sr3-O7 | 2.660(5) | Ge2-O7 | 1.729(5) |
| Sr3-O8 | 2.701(5) | Ge2-O3 | 1.769(5) |
| Sr3-O6 | 3.190(5) | Ge2-O2 | 1.780(4) |
| Ge2-O4 | 1.801(4) | ||
| Sr4a-O6 (x4) | 2.49(3) | ||
| Sr4a-O9 (x4) | 2.69(3) | Ge3-O6 | 1.735(5) |
| Ge3-O8 | 1.740(4) | ||
| Ge4a-O10 | 2.046(16) | Ge3-O5 | 1.760(5) |
| Ge4a-O6 (x4) | 2.221(6) | Ge3-O9 | 1.792(3) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Smart, M.M.; Moore, C.A.; McMillen, C.D.; Kolis, J.W. Hydrothermal Synthesis and Crystal Structure of Vesuvianite Compounds, Ca19Al13Si18O71(OH)7 and Sr19Fe12Ge19O72(OH)6. Crystals 2023, 13, 1257. https://doi.org/10.3390/cryst13081257
Smart MM, Moore CA, McMillen CD, Kolis JW. Hydrothermal Synthesis and Crystal Structure of Vesuvianite Compounds, Ca19Al13Si18O71(OH)7 and Sr19Fe12Ge19O72(OH)6. Crystals. 2023; 13(8):1257. https://doi.org/10.3390/cryst13081257
Chicago/Turabian StyleSmart, Megan M., Cheryl A. Moore, Colin D. McMillen, and Joseph W. Kolis. 2023. "Hydrothermal Synthesis and Crystal Structure of Vesuvianite Compounds, Ca19Al13Si18O71(OH)7 and Sr19Fe12Ge19O72(OH)6" Crystals 13, no. 8: 1257. https://doi.org/10.3390/cryst13081257
APA StyleSmart, M. M., Moore, C. A., McMillen, C. D., & Kolis, J. W. (2023). Hydrothermal Synthesis and Crystal Structure of Vesuvianite Compounds, Ca19Al13Si18O71(OH)7 and Sr19Fe12Ge19O72(OH)6. Crystals, 13(8), 1257. https://doi.org/10.3390/cryst13081257






