Solubility and Crystallization of Glucosamine Hydrochloride in Water with the Presence of Additives
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
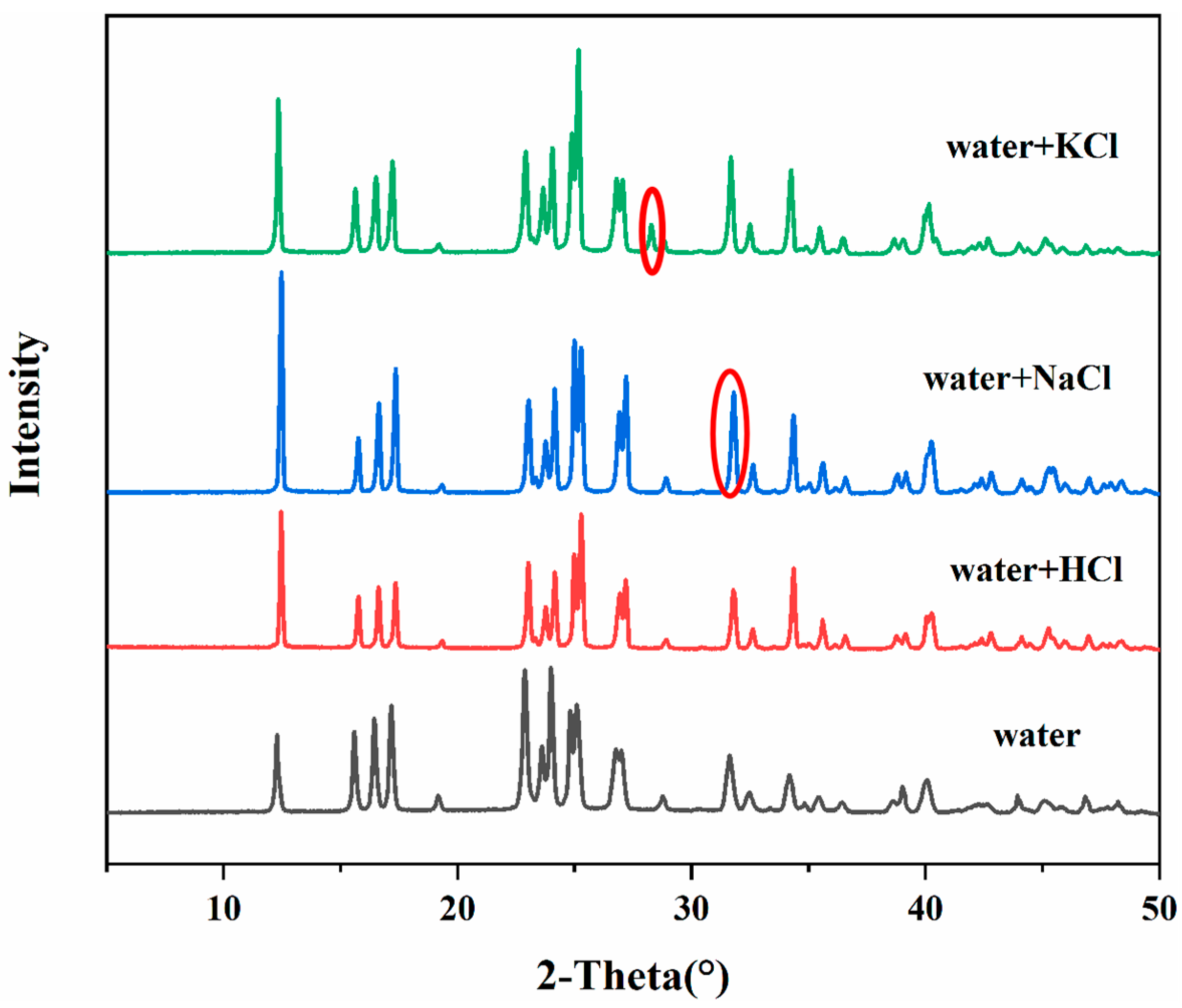
2.2. Powder X-ray Diffraction Measurement
2.3. Infrared Spectroscopy Measurement
2.4. Solubility Measurements
2.5. The Cooling Crystallization of GAH with the Presence of Additives
3. Thermodynamic Models
3.1. Modified Apelblat Model
3.2. Van’t Hoff Model
4. Results and Discussion
4.1. PXRD Analysis
4.2. Infrared Spectroscopic Analysis
4.3. Experimental Solubility Data
4.4. Data Correlation
4.5. Effects of Additive on GAH Crystal Morphology
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Tamai, Y.; Miyatake, K.; Okamoto, Y.; Takamori, Y.; Sakamoto, H.; Minami, S. Enhanced healing of cartilaginous injuries by glucosamine hydrochloride. Carbohydr. Polym. 2002, 48, 369–378. [Google Scholar] [CrossRef]
- Jamialahmadi, K.; Arasteh, O.; Matbou Riahi, M.; Mehri, S.; Riahi-Zanjani, B.; Karimi, G. Protective effects of glucosamine hydrochloride against free radical-induced erythrocytes damage. Environ. Toxicol. Pharmacol. 2014, 38, 212–219. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Sha, J.; Chang, Y.; Cao, Z.; Hu, X.; Li, Y.; Li, T.; Ren, B. Solubility measurement, model evaluation and Hansen solubility parameter of ipriflavone in three binary solvents. J. Chem. Thermodyn. 2021, 152, 106285. [Google Scholar] [CrossRef]
- Sang, J.; Wang, H.; Jin, J.; Meng, H. Comparison and modelling of rutin solubility in supercritical carbon dioxide and subcritical 1,1,1,2-tetrafluoroethane. J. CO2 Util. 2017, 21, 1–8. [Google Scholar] [CrossRef]
- Alshehri, S.; Shakeel, F. Solubility determination, various solubility parameters and solution thermodynamics of sunitinib malate in some cosolvents, water and various (Transcutol + water) mixtures. J. Mol. Liq. 2020, 307, 112970. [Google Scholar] [CrossRef]
- Alanazi, A.; Alshehri, S.; Altamimi, M.; Shakeel, F. Solubility determination and three dimensional Hansen solubility parameters of gefitinib in different organic solvents: Experimental and computational approaches. J. Mol. Liq. 2020, 299, 112211. [Google Scholar] [CrossRef]
- Mani, N.; Jun, H.W.; Beach, J.W.; Nerurkar, J. Solubility of Guaifenesin in the Presence of Common Pharmaceutical Additives. Pharm. Dev. Technol. 2003, 8, 385–396. [Google Scholar] [CrossRef] [PubMed]
- Mishra, S.; Kumar, M.; Pandey, N.K. Aqueous solubility of tri-iso-amyl phosphate (TiAP)—Effect of acidity, temperature and metal ions. Prog. Nucl. Energy 2020, 119, 103166. [Google Scholar] [CrossRef]
- Hrkovac, M.; Kardum, J.P.; Ukrainczyk, N. Influence of NaCl on Granulometric Characteristics and Polymorphism in Batch-Cooling Crystallization of Glycine. Chem. Eng. Technol. 2015, 38, 139–146. [Google Scholar] [CrossRef]
- Jeong, D.-H.; Ullah, H.M.A.; Goo, M.-J.; Ghim, S.-G.; Hong, I.-H.; Kim, A.-Y.; Jeon, S.-M.; Choi, M.-S.; Elfadl, A.K.; Chung, M.-J.; et al. Effects of oral glucosamine hydrochloride and mucopolysaccharide protein in a rabbit model of osteoarthritis. Int. J. Rheum. Dis. 2018, 21, 620–628. [Google Scholar] [CrossRef]
- Xing, R.; Liu, S.; Guo, Z.; Yu, H.; Li, C.; Ji, X.; Feng, J.; Li, P. The antioxidant activity of glucosamine hydrochloride in vitro. Bioorg. Med. Chem. 2006, 14, 1706–1709. [Google Scholar] [CrossRef]
- Du, S.; Pan, Z.; Yu, C.; Lu, J.; Zhang, Q.; Gong, J.; Wang, Y.; Xue, F. Solid-liquid equilibrium of glucosamine hydrochloride in four binary solvents: Experiments, modeling, and molecular simulation. J. Mol. Liq. 2023, 386, 122564. [Google Scholar] [CrossRef]
- Barzegar-Jalali, M.; Jafari, P.; Jouyban, A. Determination and correlation of naproxen solubility in polyethylene glycol dimethyl ether 250 and water mixtures. Phys. Chem. Liq. 2022, 60, 856–870. [Google Scholar] [CrossRef]
- Jia, S.; Gao, Y.; Li, Z.; Zhang, T.; Liu, J.; Wang, J.; Gao, Z.; Gong, J. Process intensification and control strategies in cooling crystallization: Crystal size and morphology optimization of α-PABA. Chem. Eng. Res. Des. 2022, 179, 265–276. [Google Scholar] [CrossRef]
- Liszi, I.; Blickle, T.; Liszi, J. Homogeneous nucleation in cooling crystallization. Cryst. Res. Technol. 1985, 20, 1309–1315. [Google Scholar] [CrossRef]
- Kalam, M.A.; Alshamsan, A.; Alkholief, M.; Alsarra, I.A.; Ali, R.; Haq, N.; Anwer, M.K.; Shakeel, F. Solubility Measurement and Various Solubility Parameters of Glipizide in Different Neat Solvents. ACS Omega 2020, 5, 1708–1716. [Google Scholar] [CrossRef]
- Hussain, A.; Altamimi, M.A.; Alshehri, S.; Imam, S.S. Assessment of solubility and Hansen solubility parameters of rifampicin in various permeation enhancers: Experimental and computational approach. J. Mol. Liq. 2021, 328, 115432. [Google Scholar] [CrossRef]
- Yu, S.; Cheng, Y.; Xing, W.; Xue, F. Solubility determination and thermodynamic modelling of gliclazide in five binary solvent mixtures. J. Mol. Liq. 2020, 311, 113258. [Google Scholar] [CrossRef]
- Ji, W.; Meng, Q.; Li, P.; Yang, B.; Wang, F.; Ding, L.; Wang, B. Measurement and Correlation of the Solubility of p-Coumaric Acid in Nine Pure and Water + Ethanol Mixed Solvents at Temperatures from 293.15 to 333.15 K. J. Chem. Eng. Data 2016, 61, 3457–3465. [Google Scholar] [CrossRef]
- Alshehri, S.; Shakeel, F.; Alam, P.; Peña, Á.; Jouyban, A.; Martinez, F. Effect of temperature and polarity on the solubility and preferential solvation of sinapic acid in aqueous mixtures of DMSO and Carbitol. J. Mol. Liq. 2021, 340, 117268. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, T.; Huang, C.; Wang, H.; Yu, B.; Gong, J. Measurement and Correlation of the Solubility of Maltitol in Different Pure Solvents, Methanol–Water Mixtures, and Ethanol–Water Mixtures. J. Chem. Eng. Data 2016, 61, 1065–1070. [Google Scholar] [CrossRef]
- Lan, G.; Li, X.; Chao, H.; Wang, N.; Wei, C.; Li, Z.; Wang, J. Measurement and Correlation of Solubilities of 3-Nitro-1,2,4-triazol-5-one (NTO) in 10 Pure Solvents and 3 Binary Solvents from 278.15 to 328.15 K. J. Chem. Eng. Data 2021, 66, 3897–3910. [Google Scholar] [CrossRef]
- Noubigh, A. Stearic acid solubility in mixed solvents of (water + ethanol) and (ethanol + ethyl acetate): Experimental data and comparison among different thermodynamic models. J. Mol. Liq. 2019, 296, 112101. [Google Scholar] [CrossRef]
- Yang, C.; Li, Q.; Wu, Y. Thermodynamic models for determination of solid-liquid equilibrium of the 4-methylbenzoic acid in pure and binary organic solvents. J. Mol. Liq. 2017, 238, 432–439. [Google Scholar] [CrossRef]
- Ouyang, J.; Liu, L.; Zhou, L.; Liu, Z.; Li, Y.; Zhang, C. Solubility, dissolution thermodynamics, Hansen solubility parameter and molecular simulation of 4-chlorobenzophenone with different solvents. J. Mol. Liq. 2022, 360, 119438. [Google Scholar] [CrossRef]
- Zhou, G.; Chen, K.; Yang, Z.; Shao, D.; Fan, H. Solubility and Thermodynamic Model Correlation of Zonisamide in Different Pure Solvents from T = (273.15 to 313.15) K. J. Chem. Eng. Data 2020, 65, 3637–3644. [Google Scholar] [CrossRef]
- Shan, Y.; Luo, J.; Shi, C.; Li, J.; Yu, Q. Measurement and Correlation of Solubility Data for Cytosine in Fourteen Pure Solvents from 288.15 to 328.15 K. J. Chem. Eng. Data 2022, 67, 3299–3309. [Google Scholar] [CrossRef]
- Yin, Y.; Bao, Y.; Gao, Z.; Wang, Z.; Liu, D.; Hao, H.; Wang, Y. Solubility of Cefotaxime Sodium in Ethanol + Water Mixtures under Acetic Acid Conditions. J. Chem. Eng. Data 2014, 59, 1865–1871. [Google Scholar] [CrossRef]
- Ye, Y.-j.; Xia, X.-q.; Dai, X.-t.; Huang, C.-h.; Guo, Q. Effects of temperature, salinity, and pH on 222Rn solubility in water. J. Radioanal. Nucl. Chem. 2019, 320, 369–375. [Google Scholar] [CrossRef]
- Huang, W.; Wang, H.; Li, C.; Wen, T.; Xu, J.; Ouyang, J.; Zhang, C. Measurement and correlation of solubility, Hansen solubility parameters and thermodynamic behavior of Clozapine in eleven mono-solvents. J. Mol. Liq. 2021, 333, 115894. [Google Scholar] [CrossRef]
- Li, W.; Wang, L.; Shi, P.; Gao, Z. Determination and Correlation of Solubility of Forms I and II of Imidacloprid in Seven Pure Solvents from 293.15 to 333.15 K. J. Chem. Eng. Data 2020, 65, 5421–5427. [Google Scholar] [CrossRef]
- Mou, M.; Jiang, M. Fast Continuous Non-Seeded Cooling Crystallization of Glycine in Slug Flow: Pure α-Form Crystals with Narrow Size Distribution. J. Pharm. Innov. 2020, 15, 281–294. [Google Scholar] [CrossRef]
- Leng, F.; Zhou, S.; Li, S.; Xu, M.; Zhang, K.; Guo, T.; Chen, T.; Yang, P. Solubility, Crystallization, and Characterization of Cytidine Sulfate. ACS Omega 2023, 8, 25288–25294. [Google Scholar] [CrossRef] [PubMed]
- Constance, E.N.; Mohammed, M.; Mojibola, A.; Egiefameh, M.; Daodu, O.; Clement, T.; Ogundolie, T.; Nwawulu, C.; Aslan, K. Effect of Additives on the Crystal Morphology of Amino Acids: A Theoretical and Experimental Study. J. Phys. Chem. C 2016, 120, 14749–14757. [Google Scholar] [CrossRef]




| Materials | CAS Number | Source | Relative Molecular Mass | Mass Fraction Purity | Analysis Method |
|---|---|---|---|---|---|
| GAH | 66-84-2 | Shanghai Macklin Biochemical Technology Co., Ltd. | 215.63 | ≥0.990 | HPLC a |
| Hydrochloric acid | 7647-01-0 | Sinopharm Chemical Reagent Co., Ltd., China | 36.50 | 0.360–0.380 | HPLC a |
| NaCl | 7647-14-5 | Sinopharm Chemical Reagent Co., Ltd., China | 58.50 | ≥0.995 | HPLC a |
| KCl | 7447-40-7 | Sinopharm Chemical Reagent Co., Ltd., China | 74.50 | ≥0.995 | HPLC a |
| xHCl | |||
|---|---|---|---|
| 278.15 K | |||
| 0.0000 | 21.65 | 21.64 | 21.64 |
| 0.0023 | 19.80 | 19.79 | 19.79 |
| 0.0046 | 18.54 | 18.58 | 18.58 |
| 0.0090 | 15.99 | 15.96 | 15.96 |
| 0.0173 | 12.35 | 12.15 | 12.15 |
| 0.0251 | 9.659 | 9.509 | 9.509 |
| 0.0323 | 7.766 | 7.504 | 7.504 |
| 0.0453 | 5.488 | 5.118 | 5.118 |
| 283.15 K | |||
| 0.0000 | 23.38 | 23.39 | 23.28 |
| 0.0023 | 21.50 | 21.51 | 21.42 |
| 0.0046 | 20.29 | 20.25 | 20.17 |
| 0.0090 | 17.55 | 17.58 | 17.55 |
| 0.0173 | 13.31 | 13.61 | 13.62 |
| 0.0251 | 10.72 | 10.71 | 10.69 |
| 0.0323 | 8.217 | 8.491 | 8.416 |
| 0.0453 | 5.415 | 5.742 | 5.596 |
| 288.15 K | |||
| 0.0000 | 25.16 | 25.25 | 25.25 |
| 0.0023 | 23.40 | 23.33 | 23.33 |
| 0.0046 | 22.10 | 22.03 | 22.03 |
| 0.0090 | 19.47 | 19.32 | 19.32 |
| 0.0173 | 15.09 | 15.19 | 15.20 |
| 0.0251 | 11.71 | 12.03 | 12.04 |
| 0.0323 | 9.321 | 9.593 | 9.593 |
| 0.0453 | 6.150 | 6.465 | 6.457 |
| 293.15 K | |||
| 0.0000 | 27.40 | 27.22 | 27.30 |
| 0.0023 | 25.10 | 25.27 | 25.33 |
| 0.0046 | 23.85 | 23.93 | 23.99 |
| 0.0090 | 20.79 | 21.17 | 21.21 |
| 0.0173 | 17.18 | 16.89 | 16.90 |
| 0.0251 | 13.23 | 13.47 | 13.51 |
| 0.0323 | 11.02 | 10.82 | 10.89 |
| 0.0453 | 7.501 | 7.300 | 7.415 |
| 298.15 K | |||
| 0.0000 | 29.22 | 29.31 | 29.45 |
| 0.0023 | 27.39 | 27.33 | 27.43 |
| 0.0046 | 26.01 | 25.94 | 26.04 |
| 0.0090 | 23.33 | 23.15 | 23.20 |
| 0.0173 | 18.62 | 18.71 | 18.72 |
| 0.0251 | 15.25 | 15.04 | 15.1 |
| 0.0323 | 12.16 | 12.19 | 12.30 |
| 0.0453 | 8.221 | 8.268 | 8.475 |
| 303.15 K | |||
| 0.0000 | 31.46 | 31.53 | 31.69 |
| 0.0023 | 29.58 | 29.51 | 29.63 |
| 0.0046 | 28.11 | 28.08 | 28.20 |
| 0.0090 | 25.33 | 25.25 | 25.31 |
| 0.0173 | 20.75 | 20.66 | 20.67 |
| 0.0251 | 17.02 | 16.75 | 16.82 |
| 0.0323 | 14.13 | 13.71 | 13.84 |
| 0.0453 | 9.826 | 9.387 | 9.643 |
| 308.15 K | |||
| 0.0000 | 33.92 | 33.88 | 34.02 |
| 0.0023 | 31.93 | 31.82 | 31.92 |
| 0.0046 | 30.04 | 30.35 | 30.46 |
| 0.0090 | 27.48 | 27.48 | 27.53 |
| 0.0173 | 22.67 | 22.74 | 22.75 |
| 0.0251 | 18.78 | 18.61 | 18.67 |
| 0.0323 | 15.39 | 15.39 | 15.52 |
| 0.0453 | 10.78 | 10.68 | 10.93 |
| 313.15 K | |||
| 0.0000 | 36.37 | 36.36 | 36.43 |
| 0.0023 | 34.08 | 34.25 | 34.31 |
| 0.0046 | 32.83 | 32.75 | 32.81 |
| 0.0090 | 29.84 | 29.84 | 29.87 |
| 0.0173 | 25.04 | 24.96 | 24.97 |
| 0.0251 | 20.63 | 20.62 | 20.65 |
| 0.0323 | 17.11 | 17.26 | 17.34 |
| 0.0453 | 12.25 | 12.18 | 12.33 |
| 318.15 K | |||
| 0.0000 | 39.08 | 38.98 | 38.93 |
| 0.0023 | 36.86 | 36.82 | 36.79 |
| 0.0046 | 35.68 | 35.29 | 35.27 |
| 0.0090 | 32.45 | 32.34 | 32.32 |
| 0.0173 | 27.39 | 27.32 | 27.31 |
| 0.0251 | 22.78 | 22.80 | 22.77 |
| 0.0323 | 19.36 | 19.33 | 19.30 |
| 0.0453 | 13.76 | 13.92 | 13.87 |
| 323.15 K | |||
| 0.0000 | 41.66 | 41.74 | 41.52 |
| 0.0023 | 39.54 | 39.54 | 39.37 |
| 0.0046 | 37.77 | 37.98 | 37.82 |
| 0.0090 | 34.86 | 34.98 | 34.89 |
| 0.0173 | 29.70 | 29.82 | 29.80 |
| 0.0251 | 24.96 | 25.16 | 25.03 |
| 0.0323 | 21.51 | 21.62 | 21.41 |
| 0.0453 | 15.77 | 15.94 | 15.54 |
| xNaCl | |||
|---|---|---|---|
| 278.15 K | |||
| 0.0000 | 21.65 | 21.64 | 21.40 |
| 0.0091 | 17.89 | 17.79 | 17.44 |
| 0.0181 | 14.02 | 14.05 | 13.98 |
| 0.0356 | 8.708 | 8.891 | 9.164 |
| 0.0441 | 7.146 | 7.310 | 7.509 |
| 0.0580 | 5.376 | 5.526 | 5.617 |
| 0.0845 | 4.047 | 4.097 | 3.860 |
| 283.15 K | |||
| 0.0000 | 23.38 | 23.39 | 23.28 |
| 0.0091 | 19.32 | 19.32 | 19.15 |
| 0.0181 | 15.60 | 15.56 | 15.52 |
| 0.0356 | 10.45 | 10.23 | 10.38 |
| 0.0441 | 8.568 | 8.460 | 8.573 |
| 0.0580 | 6.518 | 6.404 | 6.453 |
| 0.0845 | 4.466 | 4.528 | 4.396 |
| 288.15 K | |||
| 0.0000 | 25.16 | 25.25 | 25.25 |
| 0.0091 | 20.86 | 20.96 | 20.96 |
| 0.0181 | 17.27 | 17.19 | 17.18 |
| 0.0356 | 11.85 | 11.67 | 11.70 |
| 0.0441 | 10.10 | 9.714 | 9.743 |
| 0.0580 | 7.718 | 7.370 | 7.379 |
| 0.0845 | 5.226 | 5.018 | 4.984 |
| 293.15 K | |||
| 0.0000 | 27.40 | 27.22 | 27.30 |
| 0.0091 | 22.56 | 22.75 | 22.87 |
| 0.0181 | 18.85 | 18.93 | 18.95 |
| 0.0356 | 13.26 | 13.21 | 13.14 |
| 0.0441 | 11.17 | 11.07 | 11.02 |
| 0.0580 | 8.372 | 8.426 | 8.398 |
| 0.0845 | 5.719 | 5.576 | 5.627 |
| 298.15 K | |||
| 0.0000 | 29.22 | 29.31 | 29.45 |
| 0.0091 | 24.66 | 24.67 | 24.88 |
| 0.0181 | 20.70 | 20.79 | 20.84 |
| 0.0356 | 14.74 | 14.84 | 14.69 |
| 0.0441 | 12.18 | 12.52 | 12.42 |
| 0.0580 | 9.450 | 9.571 | 9.517 |
| 0.0845 | 6.166 | 6.211 | 6.327 |
| 303.15 K | |||
| 0.0000 | 31.46 | 31.53 | 31.69 |
| 0.0091 | 26.78 | 26.75 | 27.00 |
| 0.0181 | 22.76 | 22.79 | 22.83 |
| 0.0356 | 16.33 | 16.56 | 16.37 |
| 0.0441 | 13.83 | 14.07 | 13.94 |
| 0.0580 | 10.65 | 10.81 | 10.74 |
| 0.0845 | 6.714 | 6.933 | 7.086 |
| 308.15 K | |||
| 0.0000 | 33.92 | 33.88 | 34.02 |
| 0.0091 | 29.23 | 28.99 | 29.21 |
| 0.0181 | 25.07 | 24.91 | 24.95 |
| 0.0356 | 18.26 | 18.36 | 18.18 |
| 0.0441 | 15.75 | 15.72 | 15.59 |
| 0.0580 | 12.13 | 12.14 | 12.07 |
| 0.0845 | 7.666 | 7.754 | 7.907 |
| 313.15 K | |||
| 0.0000 | 36.37 | 36.36 | 36.43 |
| 0.0091 | 31.54 | 31.41 | 31.53 |
| 0.0181 | 27.18 | 27.16 | 27.19 |
| 0.0356 | 20.29 | 20.22 | 20.12 |
| 0.0441 | 17.56 | 17.45 | 17.37 |
| 0.0580 | 13.46 | 13.55 | 13.52 |
| 0.0845 | 8.812 | 8.687 | 8.793 |
| 318.15 K | |||
| 0.0000 | 39.08 | 38.98 | 38.93 |
| 0.0091 | 34.11 | 34.02 | 33.95 |
| 0.0181 | 29.45 | 29.55 | 29.54 |
| 0.0356 | 22.17 | 22.15 | 22.20 |
| 0.0441 | 19.29 | 19.25 | 19.29 |
| 0.0580 | 15.07 | 15.06 | 15.09 |
| 0.0845 | 9.664 | 9.748 | 9.745 |
| 323.15 K | |||
| 0.0000 | 41.66 | 41.74 | 41.52 |
| 0.0091 | 36.56 | 36.83 | 36.47 |
| 0.0181 | 32.11 | 32.09 | 32.02 |
| 0.0356 | 24.23 | 24.13 | 24.41 |
| 0.0441 | 21.19 | 21.14 | 21.35 |
| 0.0580 | 16.84 | 16.66 | 16.79 |
| 0.0845 | 11.08 | 10.95 | 10.77 |
| xKCl | |||
|---|---|---|---|
| 278.15 K | |||
| 0.0000 | 21.65 | 21.64 | 21.4 |
| 0.0099 | 17.83 | 17.98 | 18.02 |
| 0.0196 | 15.35 | 15.49 | 15.34 |
| 0.0385 | 11.87 | 11.64 | 11.40 |
| 0.0477 | 9.852 | 9.804 | 9.873 |
| 0.0570 | 7.864 | 7.945 | 8.399 |
| 0.0741 | 4.765 | 4.997 | 5.994 |
| 283.15 K | |||
| 0.0000 | 23.38 | 23.39 | 23.28 |
| 0.0099 | 19.81 | 19.74 | 19.76 |
| 0.0196 | 17.23 | 17.02 | 16.94 |
| 0.0385 | 12.72 | 12.85 | 12.74 |
| 0.0477 | 11.17 | 11.08 | 11.12 |
| 0.0570 | 9.403 | 9.286 | 9.543 |
| 0.0741 | 6.638 | 6.362 | 6.985 |
| 288.15 K | |||
| 0.0000 | 25.16 | 25.25 | 25.25 |
| 0.0099 | 21.91 | 21.60 | 21.60 |
| 0.0196 | 18.74 | 18.66 | 18.65 |
| 0.0385 | 14.01 | 14.17 | 14.17 |
| 0.0477 | 12.36 | 12.45 | 12.48 |
| 0.0570 | 10.77 | 10.73 | 10.79 |
| 0.0741 | 8.058 | 7.883 | 8.096 |
| 293.15 K | |||
| 0.0000 | 27.40 | 27.22 | 27.30 |
| 0.0099 | 23.59 | 23.56 | 23.54 |
| 0.0196 | 20.41 | 20.42 | 20.47 |
| 0.0385 | 15.38 | 15.61 | 15.71 |
| 0.0477 | 13.72 | 13.94 | 13.95 |
| 0.0570 | 12.34 | 12.27 | 12.16 |
| 0.0741 | 9.809 | 9.524 | 9.337 |
| 298.15 K | |||
| 0.0000 | 29.22 | 29.31 | 29.45 |
| 0.0099 | 25.49 | 25.61 | 25.58 |
| 0.0196 | 22.14 | 22.30 | 22.39 |
| 0.0385 | 17.14 | 17.19 | 17.36 |
| 0.0477 | 15.43 | 15.54 | 15.53 |
| 0.0570 | 13.78 | 13.88 | 13.64 |
| 0.0741 | 11.52 | 11.24 | 10.72 |
| 303.15 K | |||
| 0.0000 | 31.46 | 31.53 | 31.69 |
| 0.0099 | 27.56 | 27.76 | 27.73 |
| 0.0196 | 24.25 | 24.31 | 24.42 |
| 0.0385 | 18.91 | 18.92 | 19.12 |
| 0.0477 | 17.23 | 17.25 | 17.23 |
| 0.0570 | 15.38 | 15.56 | 15.25 |
| 0.0741 | 12.66 | 12.97 | 12.24 |
| 308.15 K | |||
| 0.0000 | 33.92 | 33.88 | 34.02 |
| 0.0099 | 29.97 | 29.99 | 29.97 |
| 0.0196 | 26.53 | 26.46 | 26.56 |
| 0.0385 | 21.14 | 20.81 | 20.99 |
| 0.0477 | 19.46 | 19.08 | 19.05 |
| 0.0570 | 17.34 | 17.28 | 16.98 |
| 0.0741 | 14.01 | 14.66 | 13.93 |
| 313.15 K | |||
| 0.0000 | 36.37 | 36.36 | 36.43 |
| 0.0099 | 32.31 | 32.32 | 32.31 |
| 0.0196 | 28.73 | 28.75 | 28.81 |
| 0.0385 | 23.37 | 22.87 | 22.97 |
| 0.0477 | 21.24 | 21.02 | 21.00 |
| 0.0570 | 19.19 | 19.03 | 18.85 |
| 0.0741 | 15.79 | 16.24 | 15.78 |
| 318.15 K | |||
| 0.0000 | 39.08 | 38.98 | 38.93 |
| 0.0099 | 34.73 | 34.74 | 34.76 |
| 0.0196 | 31.22 | 31.19 | 31.17 |
| 0.0385 | 25.33 | 25.12 | 25.07 |
| 0.0477 | 23.24 | 23.08 | 23.08 |
| 0.0570 | 20.64 | 20.79 | 20.85 |
| 0.0741 | 17.85 | 17.67 | 17.81 |
| 323.15 K | |||
| 0.0000 | 41.66 | 41.74 | 41.52 |
| 0.0099 | 37.38 | 37.25 | 37.30 |
| 0.0196 | 33.79 | 33.78 | 33.64 |
| 0.0385 | 26.95 | 27.58 | 27.28 |
| 0.0477 | 24.86 | 25.26 | 25.28 |
| 0.0570 | 22.63 | 22.55 | 22.99 |
| 0.0741 | 19.53 | 18.89 | 20.02 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pan, Z.; Wang, Y.; Xie, Y.; Tan, J.; Zhang, Q.; Lu, J.; Du, S.; Xue, F. Solubility and Crystallization of Glucosamine Hydrochloride in Water with the Presence of Additives. Crystals 2023, 13, 1326. https://doi.org/10.3390/cryst13091326
Pan Z, Wang Y, Xie Y, Tan J, Zhang Q, Lu J, Du S, Xue F. Solubility and Crystallization of Glucosamine Hydrochloride in Water with the Presence of Additives. Crystals. 2023; 13(9):1326. https://doi.org/10.3390/cryst13091326
Chicago/Turabian StylePan, Zhiying, Yan Wang, Yang Xie, Jie Tan, Qian Zhang, Jianxing Lu, Shichao Du, and Fumin Xue. 2023. "Solubility and Crystallization of Glucosamine Hydrochloride in Water with the Presence of Additives" Crystals 13, no. 9: 1326. https://doi.org/10.3390/cryst13091326
APA StylePan, Z., Wang, Y., Xie, Y., Tan, J., Zhang, Q., Lu, J., Du, S., & Xue, F. (2023). Solubility and Crystallization of Glucosamine Hydrochloride in Water with the Presence of Additives. Crystals, 13(9), 1326. https://doi.org/10.3390/cryst13091326








