Atomic-Level Description of Chemical, Topological, and Surface Morphology Aspects of Oxide Film Grown on Polycrystalline Aluminum during Thermal Oxidation—Reactive Molecular Dynamics Simulations
Abstract
:1. Introduction
2. Materials and Methods
Voronoi Analysis
3. Results and Discussion
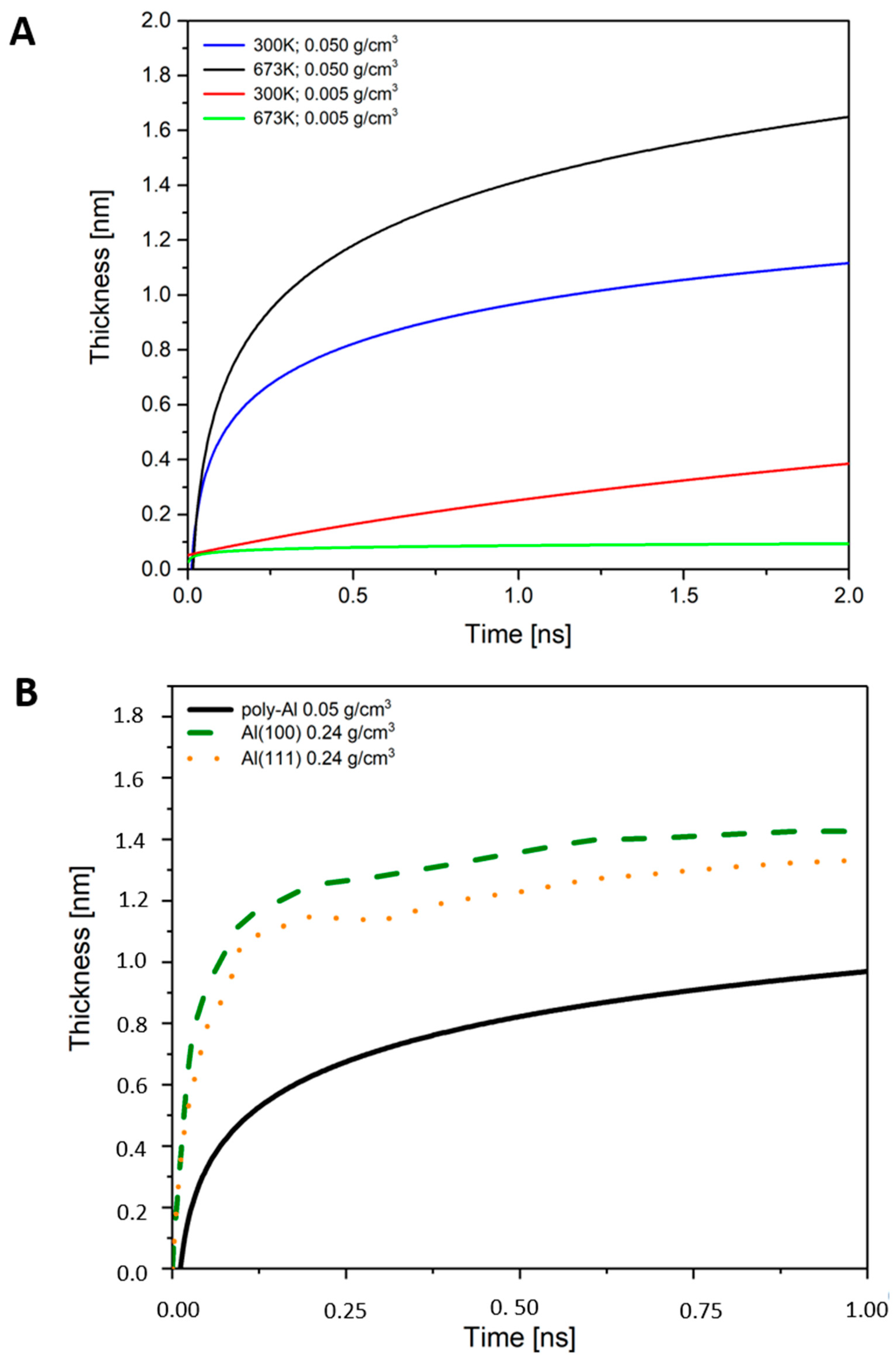
3.1. Thickness of Oxide Films
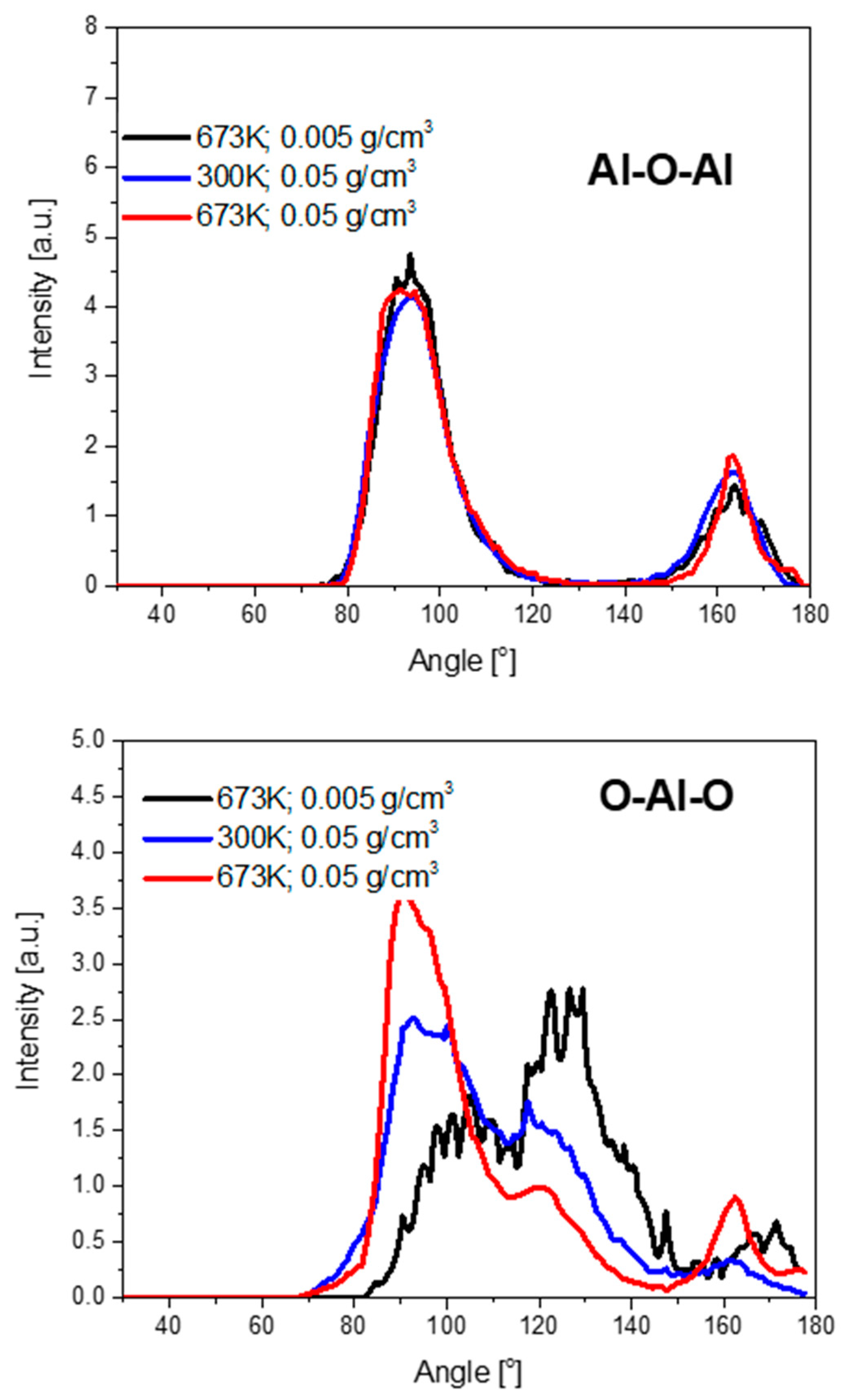
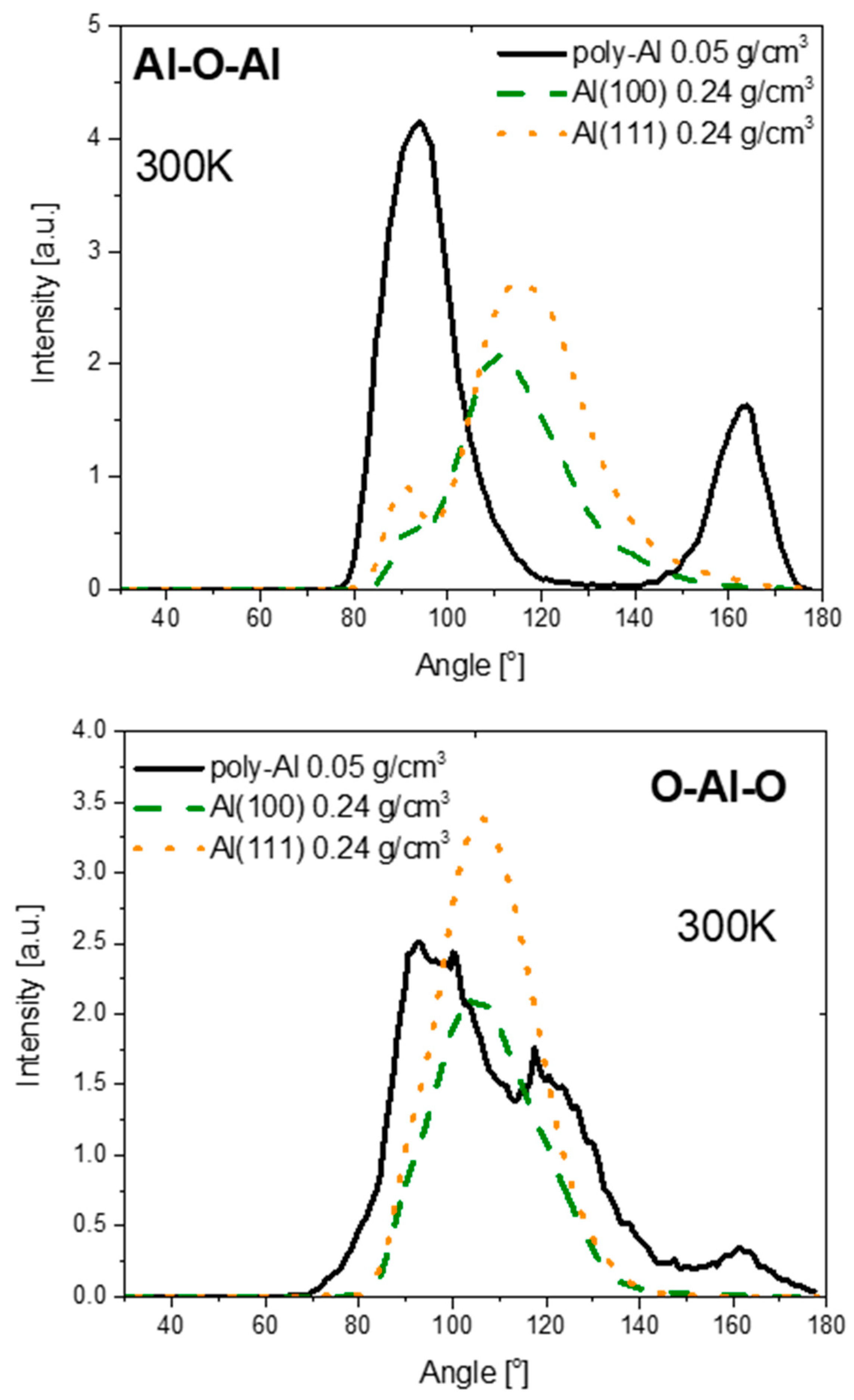
3.2. Structure of Oxide Films
3.3. Topological Aspects of Oxide Films and Al Substrates
3.3.1. Structural Unit Model
3.3.2. Ring Structure Distribution
3.3.3. Voronoi Analysis
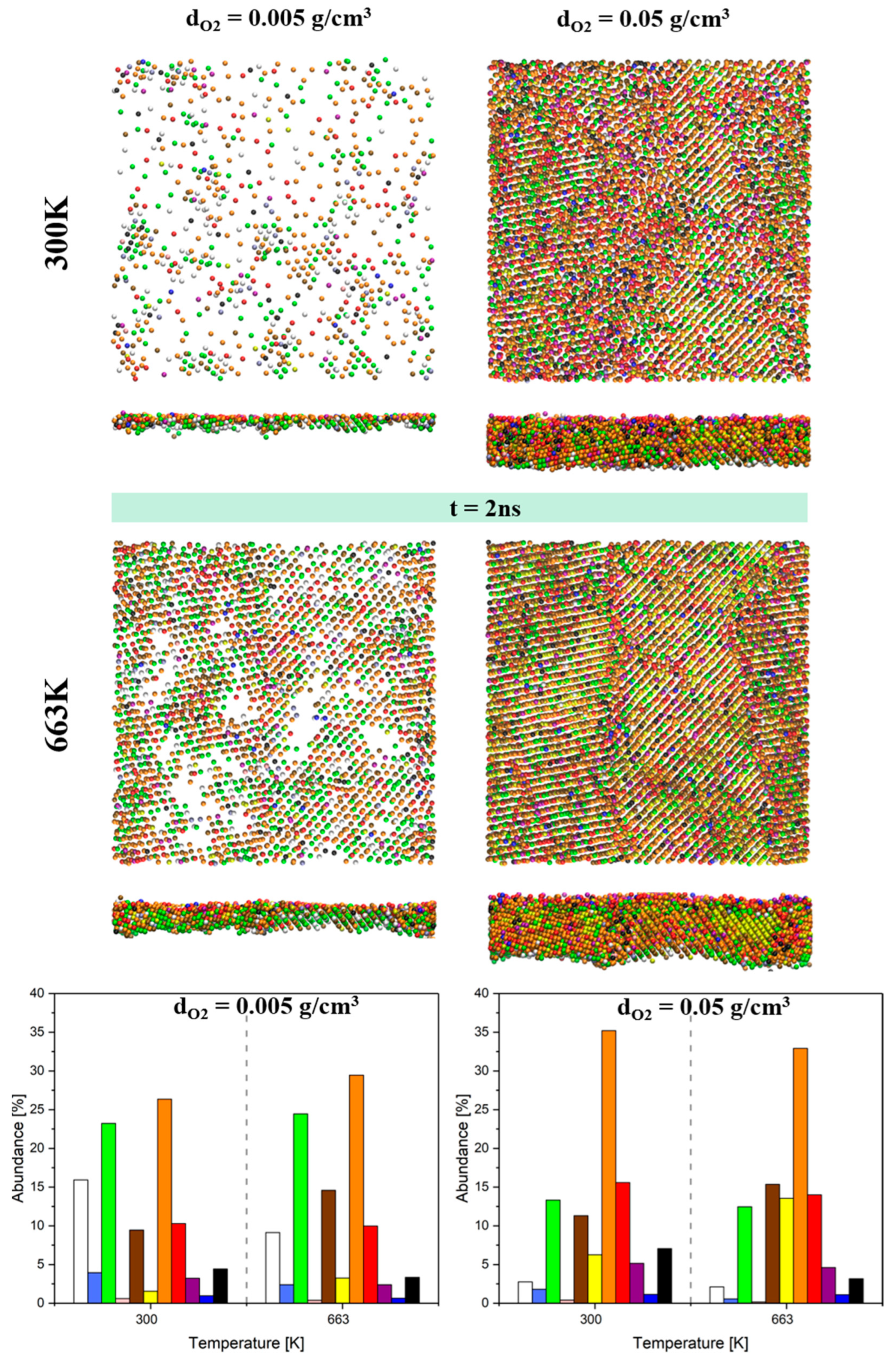
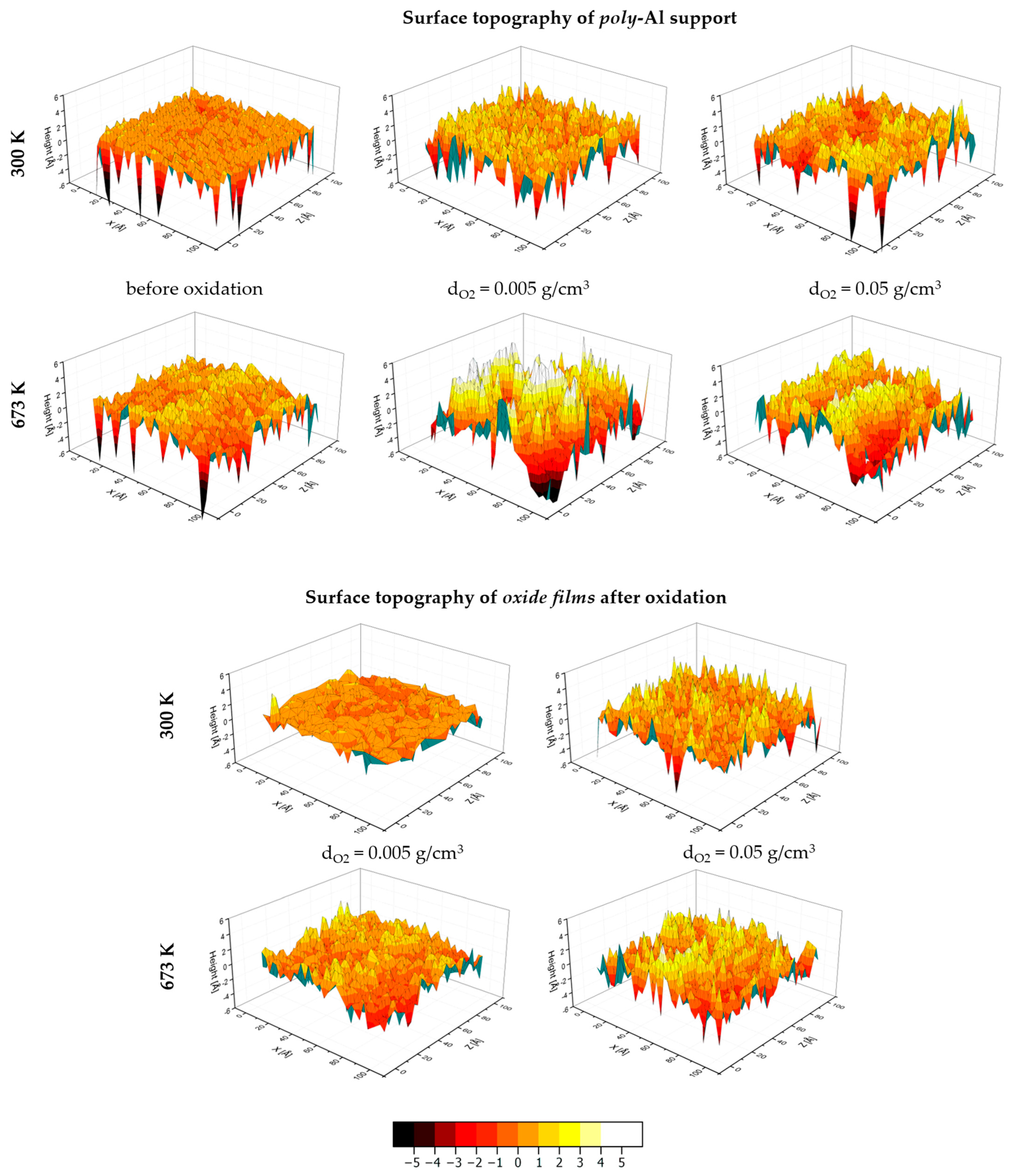
3.4. Surface Topography and Surface Morphology
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Snijders, P.C.; Jeurgens, L.P.H.; Sloof, W.G. Structural Ordering of Ultra-Thin, Amorphous Aluminium-Oxide Films. Surf. Sci. 2005, 589, 98–105. [Google Scholar] [CrossRef]
- Jeurgens, L.P.H.; Sloof, W.G.; Tichelaar, F.D.; Mittemeijer, E.J. Structure and Morphology of Aluminium-Oxide Films Formed by Thermal Oxidation of Aluminium. Thin Solid Films 2002, 418, 89–101. [Google Scholar] [CrossRef]
- Jeurgens, L.P.H.; Sloof, W.G.; Tichelaar, F.D.F.; Mittemeijer, E.J. Growth Kinetics and Mechanisms of Aluminum-Oxide Films Formed by Thermal Oxidation of Aluminum. J. Appl. Phys. 2002, 92, 1649–1656. [Google Scholar] [CrossRef]
- Flötotto, D.; Wang, Z.M.; Jeurgens, L.; Mittemeijer, E.J. Intrinsic Stress Evolution during Amorphous Oxide Film Growth on Al Surfaces. Appl. Phys. Lett. 2014, 104, 091901. [Google Scholar] [CrossRef]
- Reichel, F.; Jeurgens, L.P.H.; Richter, G.; Van Aken, P.A.; Mittemeijer, E.J. The Origin of High-Mismatch Orientation Relationships for Ultra-Thin Oxide Overgrowths. Acta Mater. 2007, 55, 6027–6037. [Google Scholar] [CrossRef]
- Reichel, F.; Jeurgens, L.P.H.; Mittemeijer, E.J. The Effect of Substrate Orientation on the Kinetics of Ultra-Thin Oxide-Film Growth on Al Single Crystals. Acta Mater. 2008, 56, 2897–2907. [Google Scholar] [CrossRef]
- Trybula, M.E.; Korzhavyi, P.A. Temperature Dependency of Structure and Order Evolution in 2D Confined Oxide Films Grown on Al Substrates Using Reactive Molecular Dynamics. Vacuum 2021, 190, 110243. [Google Scholar] [CrossRef]
- Lousada, C.M.; Korzhavyi, P.A. First Stages of Oxide Growth on Al(1 1 0) and Core-Level Shifts from Density Functional Theory Calculations. Appl. Surf. Sci. 2018, 441, 174–186. [Google Scholar] [CrossRef]
- Lanthony, C.; Ducéré, J.M.; Rouhani, M.D.; Hemeryck, A.; Estève, A.; Rossi, C. On the Early Stage of Aluminum Oxidation: An Extraction Mechanism via Oxygen Cooperation. J. Chem. Phys. 2012, 137, 094707. [Google Scholar] [CrossRef]
- Hong, S.; Van Duin, A.C.T. Molecular Dynamics Simulations of the Oxidation of Aluminum Nanoparticles Using the ReaxFF Reactive Force Field. J. Phys. Chem. C 2015, 119, 17876–17886. [Google Scholar] [CrossRef]
- Campbell, T.; Kalia, R.K.; Nakano, A.; Vashishta, P.; Ogata, S.; Rodgers, S. Dynamics of Oxidation of Aluminum Nanoclusters Using Variable Charge Molecular-Dynamics Simulations on Parallel Computers. Phys. Rev. Lett. 1999, 82, 4866–4869. [Google Scholar] [CrossRef]
- Cheng, C.; Ng, K.Y.; Aluru, N.R.; Ngan, A.H.W. Simulation and Experiment of Substrate Aluminum Grain Orientation Dependent Self-Ordering in Anodic Porous Alumina. J. Appl. Phys. 2013, 113, 204903. [Google Scholar] [CrossRef]
- Lyapin, A.; Jeurgens, L.P.H.; Graat, P.C.J.; Mittemeijer, E.J. The Initial, Thermal Oxidation of Zirconium at Room Temperature. J. Appl. Phys. 2004, 96, 7126–7135. [Google Scholar] [CrossRef]
- Panda, E.; Jeurgens, L.P.H.; Mittemeijer, E.J. The Initial Oxidation of Al-Mg Alloys: Depth-Resolved Quantitative Analysis by Angle-Resolved X-ray Photoelectron Spectroscopy and Real-Time in Situ Ellipsometry. J. Appl. Phys. 2009, 106, 114913. [Google Scholar] [CrossRef]
- Trybula, M.E.; Korzhavyi, P.A. Atomistic Simulations of Al(100) and Al(111) Surface Oxidation: Chemical and Topological Aspects of the Oxide Structure. J. Phys. Chem. C 2019, 123, 334–346. [Google Scholar] [CrossRef]
- Jeurgens, L.P.H.; Reichel, F.; Frank, S.; Richter, G.; Mittemeijer, E.J. On the Development of Long-Range Order in Ultra-Thin Amorphous Al2O3 Films upon Their Transformation into Crystalline γ-Al2O3. Surf. Interface Anal. 2008, 40, 259–263. [Google Scholar] [CrossRef]
- Cabrera, N.; Mott, N.F. Theory of the Oxidation of Metals. Rep. Prog. Phys. 1949, 12, 163–184. [Google Scholar] [CrossRef]
- Reichel, F.; Jeurgens, L.P.H.; Richter, G.; Mittemeijer, E.J. Amorphous versus Crystalline State for Ultrathin Al2O3 Overgrowths on Al Substrates. J. Appl. Phys. 2008, 103, 093515. [Google Scholar] [CrossRef]
- Lee, S.K.; Lee, S.B.; Park, S.Y.; Yi, Y.S.; Ahn, C.W. Structure of Amorphous Aluminum Oxide. Phys. Rev. Lett. 2009, 103, 095501. [Google Scholar] [CrossRef]
- Gutierrez, G.; Johansson, B. Molecular Dynamics Study of Structural Properties of Amorphous Al2O3. Phys. Rev. B 2002, 65, 104202. [Google Scholar] [CrossRef]
- Gutierrez, G.; Belonoshko, A.B.; Ahuja, R.; Johansson, B. Structural Properties of Liquid Al2O3: A Molecular Dynamics Study. Phys. Rev. E 2000, 61, 2723–2729. [Google Scholar] [CrossRef]
- Lee, S.K.; Ahn, C.W. Probing of 2 Dimensional Confinement-Induced Structural Transitions in Amorphous Oxide Thin Film. Sci. Rep. 2014, 4, 4200. [Google Scholar] [CrossRef] [PubMed]
- Plimpton, S.J. Fast Parallel Algorithms for Short-Range Molecular Dynamics. J. Comput. Phys. 1995, 117, 1–19. [Google Scholar] [CrossRef]
- Van Duin, A.; Dasgupta, S.; Lorant, F. ReaxFF: A Reactive Force Field for Hydrocarbons. J. Phys. Chem. A 2001, 105, 9396–9409. [Google Scholar] [CrossRef]
- Senftle, T.P.; Hong, S.; Islam, M.M.; Kylasa, S.B.; Zheng, Y.; Shin, Y.K.; Junkermeier, C.; Engel-Herbert, R.; Janik, M.J.; Aktulga, H.M.; et al. The ReaxFF Reactive Force-Field: Development, Applications and Future Directions. Comput. Mater. 2016, 2, 15011. [Google Scholar] [CrossRef]
- Hirel, P. Atomsk: A Tool for Manipulating and Converting Atomic Data Files. Comput. Phys. Commun. 2015, 197, 212–219. [Google Scholar] [CrossRef]
- Verlet, L. Computer “Experiments” on Classical Fluids. I. Thermodynamical Properties of Lennard-Jones Molecules. Phys. Rev. 1967, 159, 98–103. [Google Scholar] [CrossRef]
- Trybula, M.E.; Zydek, A.; Korzhavyi, P.A.; Wojewoda-Budka, J. Structure and Behavior of Oxide-Coated Aluminum in Contact with Acidic and Alkaline Aqueous Solutions—A Reactive Molecular Dynamics Simulation Study. J. Phys. Chem. C 2023, 5, 2493–2507. [Google Scholar] [CrossRef]
- Trybula, M.E. Structure and Transport Properties of the Liquid Al80Cu20 Alloy—A Molecular Dynamics Study. Comput. Mater. Sci. 2016, 122, 341–352. [Google Scholar] [CrossRef]
- Trybula, M.E.; Szafranski, P.W.; Korzhavyi, P.A. Structure and Chemistry of Liquid Al-Cu Alloys: Molecular Dynamics vs Thermodynamics-Based Modelling. J. Mater. Sci. 2018, 53, 8285–8301. [Google Scholar] [CrossRef]
- Żydek, A.; Wermiński, M.; Trybula, M.E. Description of Grain Boundary Structure and Topology in Nanocrystalline Aluminum Using Voronoi Analysis and Order Parameter. Comput. Mater. Sci. 2021, 197, 110660. [Google Scholar] [CrossRef]
- Stukowski, A. Structure Identification Methods for Atomistic Simulations of Crystalline Materials. Model. Simul. Mater. Sci. Eng. 2012, 20, 045021. [Google Scholar] [CrossRef]
- Stukowski, A. Atomic-Scale Modeling of Nanostructured Metals and Alloys. Ph.D. Dissertation, Technical University of Darmstadt, Darmstadt, Germany, 2010. [Google Scholar]
- Lazar, E.A.; Han, J.; Srolovitz, D.J. Topological Framework for Local Structure Analysis in Condensed Matter. Proc. Natl. Acad. Sci. USA 2015, 112, E5769–E5776. [Google Scholar] [CrossRef]
- Lazar, E.A. VoroTop: Voronoi Cell Topology Visualization and Analysis Toolkit. Model. Simul. Mater. Sci. Eng. 2018, 26, 015011. [Google Scholar] [CrossRef]
- Rycroft, C. Voro++: A Three-Dimensional Voronoi Cell Library in C++. Chaos 2009, 19, 041111. [Google Scholar] [CrossRef]
- Sankaranarayanan, S.K.R.S.; Ramanathan, S. On the Low-Temperature Oxidation and Ultrathin Oxide Growth on Zirconium in the Presence of Atomic Oxygen: A Modeling Study. J. Phys. Chem. C 2008, 112, 17877–17882. [Google Scholar] [CrossRef]
- Lamparter, P.; Kniep, R. Structure of Amorphous Al2O3. Phys. B 1997, 234–236, 234–236. [Google Scholar] [CrossRef]




| Density (g/cm3) | T (K) | Chemical Composition (%) | ||
|---|---|---|---|---|
| Al | O | |||
| poly-Al | 0.005 | 673 | 66.4 | 33.6 |
| 0.05 | 300 | 55.2 | 44.8 | |
| 673 | 54.4 | 45.6 | ||
| Phase | O–Al–O Bond Angle (°) | |
|---|---|---|
| Amorphous | Thin film (this work) | Broad peak from 89.0 to 104.7, 117.5, 161.7 |
| Bulk | 93, 171 | |
| α-Al2O3 | 79.53, 86.40, 101.20, 164.13 | |
| γ-Al2O3 | 80.57, 87.78, 86.0, 92.22, 104.53, 109.47, 119.09, 162.34, 180.0 | |
| Liquid | 95, 170 | |
| Phase | Density (g/cm3) | Bond Length (Å) | n-Fold Ring Fraction (%) | ||||
|---|---|---|---|---|---|---|---|
| 2 | 3 | 4 | 5 | 6 | |||
| poly-Al | 4.31 | 1.91 | 34 | 36 | 23 | 6 | 1 |
| Al(100) | 3.45 | 1.83 | 6.5 | 39 | 29.5 | 25 | - |
| Al(111) | 3.48 | 1.83 | 16 | 35 | 27 | 20 | - |
| α-Al2O3 | 3.98 | 1.97 (1.85) | 40 | 60 | - | - | - |
| γ-Al2O3 | 3.66 | 1.941 | 40 | 40 | 18.5 | 1.5 | - |
| am-Al2O3 | 3.3 | 1.76 | 11 | 37 | 41 | 10 | 0.3 |
| Liquid | 3.175 | 1.75 | 13 | 25 | 32 | 23 | 7 |
| O2 Density (g/cm3) | T (K) | Metal/Oxide Interface | Oxide/O2 Interface | |||
|---|---|---|---|---|---|---|
| Ra (Å) | Rz (Å) | Ra (Å) | Rz (Å) | |||
| Before oxidation | 0 | 300 | 0.64 | 4.04 | - | - |
| 673 | 0.85 | 5.19 | - | - | ||
| After oxidation | 0.005 | 300 | 1.27 | 7.84 | 0.61 | 5.71 |
| 673 | 1.76 | 8.83 | 0.90 | 6.67 | ||
| 0.05 | 300 | 1.03 | 6.77 | 0.92 | 6.88 | |
| 673 | 1.49 | 9.17 | 1.11 | 9.06 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trybula, M.E.; Żydek, A.; Korzhvayi, P.A.; Wojewoda-Budka, J. Atomic-Level Description of Chemical, Topological, and Surface Morphology Aspects of Oxide Film Grown on Polycrystalline Aluminum during Thermal Oxidation—Reactive Molecular Dynamics Simulations. Crystals 2023, 13, 1376. https://doi.org/10.3390/cryst13091376
Trybula ME, Żydek A, Korzhvayi PA, Wojewoda-Budka J. Atomic-Level Description of Chemical, Topological, and Surface Morphology Aspects of Oxide Film Grown on Polycrystalline Aluminum during Thermal Oxidation—Reactive Molecular Dynamics Simulations. Crystals. 2023; 13(9):1376. https://doi.org/10.3390/cryst13091376
Chicago/Turabian StyleTrybula, Marcela E., Arkadiusz Żydek, Pavel A. Korzhvayi, and Joanna Wojewoda-Budka. 2023. "Atomic-Level Description of Chemical, Topological, and Surface Morphology Aspects of Oxide Film Grown on Polycrystalline Aluminum during Thermal Oxidation—Reactive Molecular Dynamics Simulations" Crystals 13, no. 9: 1376. https://doi.org/10.3390/cryst13091376








